Abstract
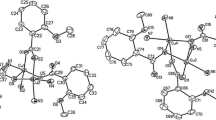
The syntheses and crystal structures of four new divalent transition metal complexes of the types [Cu2(dien)2(nic)](ClO4)3 · MeOH (nic = anion of nicotinic acid; dien = diethylenetriamine), 1; [Cu(dien)(nic)]2(nic)2, 2; [Cu(dien)(nic)]2(BF4)2 · 2MeOH, 3 and [Ni(dien)(nic)(H2O)]4(NO3)4 · 2MeOH, 4, are reported, which were prepared by the reactions of diethylenetriamine and nicotinic acid with Cu(ClO4)2 · 6H2O, Cu(OAc)2 · H2O, Cu(BF4)2 · 6H2O and Ni(NO3)2 · 6H2O in MeOH, respectively. These complexes were characterized by single-crystal X-ray diffraction method and elemental analyses. In the cation of complex 1, one nicotinate ligand bridges two Cu(II) metal centers through the pyridyl nitrogen atom and one of the carboxylate oxygen atoms. The cations of complexes 2 and 3 form the twelve-membered metallocycles, involving two Cu(II) ions that are bridged by two nicotinate ligands. The cation of complex 4 forms a tetranuclear cage with the four Ni(II) metal centers bridged by four nicotinate ligands and each Ni(II) metal center adopts the distorted octahedral geometry. Their thermal properties have been investigated by using differential scanning calorimetry (DSC) and thermogravimetric analyses (TGA).




Similar content being viewed by others
References
(a) Zhou Y, Bi W, Chen J, Cao R, Hong M (2003) Acta Crystallogr E59:m356. (b) Broderick WE, Pressprich MR, Geiser UG, Willet, RD, Legg, JI (1986) Inorg Chem 25:3372. (c) Batten SR, Harris AR (2001)Acta Crystallogr E57:m9. (d) Liang Y, Li W, Guo B-J (2005) Acta Crystallogr E61:ml782. (e) Cingi MB, Dominano P, Guastini C, Mussati A, Nardelli M (1971) Gazz Chim Ital 101:455. (f) Kenar A, Arici C, Atakol O, Ülkü D (1999) Anal Sci 15:399. (g) Jia H-B, Yu J-H, Xu J-Q, Ye L, Ding H, Jing W-J, Wang T-G, Xu J-N, Li Z-C (2002) J Mol Struct 61:23
(a) Bacchi A, Chiusoli GP, Costa M, Giacobbi E (2003) Eur J Inorg Chem 1523. (b) Song R, Kim KM, Sohn YS (2000) Inorg Chim Acta 304:156. (c) Palicova M, Segla P, Miklos D, Kopcova M, Melnil M, Dudova B, Hudecova D, Glowiak T (2000) Polyhedron 19:2689. (d) Sang R-L, Xu L (2006) Eur J Inorg Chem 1260. (e) Zhang H-X, Kang B-S, Chen Z-N, Su C-Y, Yu K-B (2003) Bull Chem Soc Jpn 76:2307
(a) Chapman ME, Ayyappan P, Foxman BM, Yee GT, Lin W (2001) Cryst Growth Des 1:159. (b) Tsao T-B, Lee G-H, Yeh C-Y, Peng S-M (2003) Dalton Trans 1465. (c) Kutasi AM, Batten SR, Harris AR, Moubaraki B, Murrar KS (2002) Cryst Eng Commun 4:202. (d) Kall P-O, Grins J, Fahlman F, Soderlind F (2001) Polyhedron 20:2747. (e) Yeh C-W, Suen M-C, Hu H-L, Chen J-D, Wang J-C (2004) Polyhedron 23:1947
(a) Lin W, Ayyappan P (2003) Polyhedron 22:3037. (b) Lu F-C, Zeng Y-F, Li J-R, Bu X-H, Zhang H-J, Ribas (2005) J Inorg Chem 44:7298. (c) Song R, Kim KM, Sohn YS (2003) Inorg Chem 42:821. (d) Abu-Youssef MAM (2005) Polyhedron 24:1829. (e) Lin W, Chapman ME, Wang Z, Lee GT (2000) Inorg Chem 39:4169. (f) Lin W, Evans OR, Xiong R-G, Wang Z (1998) J Am Chem Soc 120:13272
(a) Lin W, Evans OR, Ayyappan P (2001) Inorg Chem 40:4627. (b) Madalan AM, Paraschiv C, Sutter J-P, Schmidtmann M, Müller A, Andruh M (2005) Cryst Growth Des 5:707. (c) Chen W, Yuan H-M, Wang J-Y, Liu Z-Y, Xu J-J, Yang M, Chen J-S (2003) J Am Chem Soc 125:9266. (d) Rather B, Moulton B, Walsh RDB, Zaworotko M (2002) J Chem Comm 694; (e) Luo J, Jiang F, Wang LH, Lin Z, Cao R, Hong M (2004) J Mol Struct 707:211
(a) SMART/SAINT/ASTRO, Release, 4.03 (1995) Siemens Energy and Automation, Inc., Madison, WI, (b) XSCANS, Release, 2.1 (1995) Siemens Energy and Automation, Inc., Madison, WI
SHELXTL 5.10 (1997) Bruker Analytical X-ray Instruments Inc., Karlsruche, Germany
(a) Miki, K, Kaida, S, Saeda, M, Yamatoya, K, Kasai, N, Sato, M, Nakaya, J-I (1986) Acta Crystallogr C42:1004. (b) Castillo, O, Muga, I, Luque, A, Gutierrez-zorilla JM, Sertucha, J, Vitoria, P, Roman P (1999) Polyhedron 18:1235
Wasson AE, LaDuca RL (2007) Polyhedron 26:1001
Acknowledgments
We are grateful the National Science Council of the Republic of China for support. This research was also supported by the project of the specific fields in Chung Yuan Christian University, Taiwan, R. O. C. under grant CYCU-95-CR-CH.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, C., Chan, ZK., Yeh, CW. et al. Synthesis, structures and thermal properties of Cu(II) and Ni(II) complexes containing diethylenetriamine and nicotinate ligands. Struct Chem 19, 87–94 (2008). https://doi.org/10.1007/s11224-007-9256-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-007-9256-9