Abstract
The composition the atmosphere of Venus results from the integration of many processes entering into play over the entire geological history of the planet. Determining the elemental abundances and isotopic ratios of noble gases (He, Ne, Ar, Kr, Xe) and stable isotopes (H, C, N, O, S) in the Venus atmosphere is a high priority scientific target since it could open a window on the origin and early evolution of the entire planet. This chapter provides an overview of the existing dataset on noble gases and stable isotopes in the Venus atmosphere. The current state of knowledge on the origin and early and long-term evolution of the Venus atmosphere deduced from this dataset is summarized. A list of persistent and new unsolved scientific questions stemming from recent studies of planetary atmospheres (Venus, Earth and Mars) are described. Important mission requirements pertaining to the measurement of volatile elements in the atmosphere of Venus as well as potential technical difficulties are outlined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction and Overview
Like all planetary atmospheres, the composition of the atmosphere of Venus informs us about the entire geological history of Earth’s sister planet (e.g. Catling and Kasting 2017). Past space missions have already demonstrated that the elemental and isotopic compositions of volatile elements in the atmosphere of Venus differ greatly from those of other terrestrial planets or reservoirs of volatile elements (solar gas, meteorites, comets) in the Solar System (e.g. Pepin and Porcelli 2002; Baines et al. 2013; Wieler 2002; Chassefière et al. 2012; Avice and Marty 2020). Recent discoveries about the detailed origins and evolution of volatile inventories on Earth, Mars, and in other Solar System reservoirs have raised compelling new questions that motivate the pursuit of improved noble gas and stable isotope measurements in the atmosphere of Venus. Future space missions targeting atmospheric measurements are needed to make critical comparisons of volatile accretion and transport between terrestrial planets.
Noble gases are the best available geochemical tracers of geophysical processes. Noble gases are inert, incompatible during silicate partial melting, and atmophile (Ozima and Podosek 2002). Accordingly, noble gases act as tracers of planetary differentiation, including the coupled evolution of planetary interiors and atmospheres through outgassing. Each noble gas element has at least one stable isotope that is non-radiogenic; these are often referred to as primordial noble gas isotopes, and their budgets are established during accretion. Ratios of primordial noble gas isotopes serve as fingerprints of volatile origins, but these ratios may also be affected by mass-fractionating loss. Each noble gas element also has at least one stable isotope that is produced by nuclear reactions (including radioactive decay) involving non-atmophile reactants. Noble gas abundances are typically so low in planetary solids that their isotopic compositions are very strongly affected by nuclear reactions, even when the reactants are themselves rare in planetary materials. Thus, radioactive decay and other nuclear reactions generate large variations (from a few percent to orders of magnitude) that track planetary differentiation, which fractionates non-atmophile parent to atmophile daughter ratios, on a variety of timescales. Taken together, noble gas elemental abundances and isotopic compositions provide a rich record of planetary volatile origins, and the timing and mechanisms of volatile transport between planetary reservoirs.
Stable isotope ratios of relatively light elements (C, H, O, N, S) have been widely used to put constraints on the budget of volatile elements of terrestrial planets and on the origin and evolution of planetary atmospheres (e.g. Marty 2012; Halliday 2013). These elements do not show the strong nuclear effects seen for noble gases. Nonetheless, various reservoirs in the Solar System show variable mass-dependant and mass-independent isotopic composition. In solar-system materials, isotope variations may have been caused by physico-chemical processes such as photochemistry, partial melting, and partial evaporation. Planetary formation and differentiation may lead to further isotopic variations (Young et al. 2002). The specific case of atmospheric loss may also lead to isotopic fractionation (Jakosky and Pepin 1994). In other words, the isotope ratios of light elements can help track the origin of planetary materials but also place constraints on the evolution of planetary mantles and atmospheres.
The possible sources of Venusian atmophile elements (noble gases and light elements) can be categorized roughly into two types: direct or indirect. Venus could have accreted a significant primary atmosphere directly from the nebula if it accreted as quickly as Mars (Dauphas and Pourmand 2011) and reached near its final size while gas was likely still present in the protoplanetary disk (Weiss et al. 2021). In this case, the isotopic composition of atmophile elements sourced directly from the solar nebula would presumably match those of gases of the proto-Sun. While hydrodynamic escape assisted by later bombardment from small bodies could have eroded away hydrogen and helium, other gases directly accreted from the nebula may have been in-gassed into the mantle of Venus and protected or may have survived in the atmosphere (Olson and Sharp 2019). However, if Venus accreted more slowly and was Mars-sized or smaller only growing to larger sizes after the gaseous protoplanetary disk had dissipated, one may anticipate that a primary atmosphere would have been insignificant. Regardless of the size of its primary atmosphere, Venus also probably accreted volatile-rich building blocks during its growth providing an indirect source of atmophile elements. The isotopic composition of atmophile elements sourced indirectly from accreted building blocks would vary in composition according to the parent-body source region as well as fractionating processes taking place within the parent-body. During the dynamical evolution of the solar system, different reservoirs of planetary building blocks contributed to the growth of the terrestrial planets (O’Brien et al. 2018). Initial accretion in the inner disk near the current location of Venus is presumed to be from volatile-depleted material, although even enstatite and ordinary chondrites, examples of primordial inner solar system material, contain substantial amounts of volatile species like water (e.g. Piani et al. 2020). If not supplied in Venus’s building blocks, most volatiles within Venus could have been delivered from more distant parts of the protoplanetary disk, where volatile abundances in solids were significantly higher. However, this region is vast, encompassing primitive asteroidal and cometary compositions with clear evidence for strong isotopic gradients throughout (Marty et al. 2016). Only with a detailed understanding of the isotopic composition of the Venusian atmosphere will we be able to disentangle the different contributions to the atmosphere of Venus.
Apart from remote observations, and compared to missions involving landing on the surface of Venus, an exhaustive measurement of the elemental and isotopic composition of volatile elements in a sample of Venus’s atmosphere is one of the less risky types of investigations with an astounding scientific outcome. Measuring the elemental and isotopic composition of noble gases and stable isotopes in the atmosphere of Venus is for example one of the main scientific goals of the recently selected NASA DAVINCI mission (Glaze et al. 2017).
This chapter presents an overview of the current state of knowledge and the outlook for future discoveries brought from the study of noble gases and of stable isotopes of C, H, O, N and S regarding the origin and early evolution of the atmosphere of Venus and on the implications for the geological history of the entire planet. Note that other contributions in this journal will cover in detail the role of water in Venus’s history (Salvador et al. 2022) and the complex interactions between the interior and the surface of Venus (Gillmann et al. 2022).
2 Existing Dataset and Current State of Knowledge
The current state of knowledge on the elemental and isotopic compositions of noble gases and stable isotopes of H, C, N and O in the Venus atmosphere has already been reviewed in details elsewhere (von Zahn et al. 1983; Donahue and Pollack 1983; Wieler 2002; Johnson and de Oliveira 2019; Chassefière et al. 2012). This section describes the most important features of the atmosphere of Venus and highlights which scientific data are severely lacking.
2.1 Noble Gases (He, Ne, Ar, Kr, Xe)
Existing data on the elemental abundance and isotopic composition of noble gases in the atmosphere of Venus are reported in Table 1.
2.1.1 Elemental Abundances
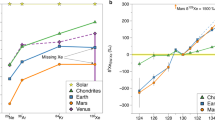
Elemental abundances of Ne, Ar, Kr and Xe in the atmosphere of Venus and in other reservoirs of volatile elements in the Solar System are plotted in Fig. 1 (Dauphas and Morbidelli 2014; Avice and Marty 2020). Chondritic meteorites, especially carbonaceous chondrites, are enriched in heavy noble gases relative to light ones (Porcelli and Ballentine 2002; Wieler 2002). Although Mars is depleted in noble gases relative to Earth by about two orders of magnitude, note that noble gases in these two atmospheres follow globally the same abundance pattern with a similar magnitude of enrichment of Kr relative to Ne relative to Ar. Atmospheric xenon on Earth and Mars presents a depletion relative to a chondritic-like abundance pattern (this is the “missing xenon problem,” see Avice and Marty (2020) for a recent review). For Venus, abundances of Ne and Ar are high compared to Mars and Earth and are similar to those measured in meteorites. Two ranges of estimates exist for the abundance of krypton (see Table 1, Donahue and Pollack (1983) and refs. therein). If there is 25 ppb of 84Kr in the Venus’s atmosphere (Pioneer’s results), krypton would be depleted relative to a chondritic-like abundance pattern, with almost a solar-like 84Kr/36Ar ratio. Conversely, if the abundance is closer to 600 ppb (Venera’s results), the 84Kr/36Ar ratio is close to the chondritic ratio (Fig. 1), suggesting that Ne, Ar and Kr in the Venus’s atmosphere could have been sourced by meteorites. Measuring precisely the abundance of 84Kr would be decisive for understanding the origin of volatile elements in Venus. For xenon, an upper limit of 10 ppb for the abundance of 132Xe has often been cited based on results obtained during the Pioneer mission (e.g. Wieler 2002). However, Istomin et al. (1982, 1983) argued that xenon was detected during the Russian Venera 14 mission and that, according to the sensitivity of the instrument, a detection of 131+132Xe isotopes above background implies a minimum abundance of Xe of 1-2 ppb (Fig. 2). A new precise determination of the abundance of Xe is still pending but considering a 1-10 ppb range suggests that Xe is not more depleted in the Venus atmosphere than atmospheric Xe on Earth and Mars. The fact that Xe in the atmospheres of Earth, Mars and Venus could be depleted in a similar fashion compared to chondritic abundances is striking, especially if the underabundance is due to a selective escape mechanism of xenon ions coupled to hydrogen ions during hydrodynamic escape of hydrogen (Zahnle et al. 2019; Avice and Marty 2020).
Elemental abundances of noble gases in the atmosphere of Venus and in other reservoirs of the Solar System normalized to the abundance of silicium and to the Solar abundances (=1). For Venus, uncertainties on the abundances of Kr and Xe appear with a light purple range (this is an upper limit for Xe). The dark purple range for 130Xe corresponds to another estimate with a minimum of 1 ppb of Xe (see text). The dotted purple line depicts the expected abundances of 130Xe for an Earth-like/Mars-like 130Xe/84Kr ratio. Figure and data modified after Dauphas and Morbidelli (2014). See also references therein
Signal of 131+132Xe detected during the Venera 14 mission. Mass and signal are given without units and the signal at the top of the 136Xe peak is divided by 100. The very high 136Xe peak is due to the presence of calibrant gas (99.99% pure 136Xe). The 131+132Xe could correspond to a minimum abundance of 1-2 ppb of Xe in the atmosphere of Venus. Figure modified after Istomin et al. (1982)
2.1.2 Isotope Ratios
Helium – An upper limit for the 3He/4He ratio of atmospheric helium has been estimated at \(3\times 10^{-4}\) (Hoffman et al. 1980). This leaves a wide range of potential helium isotopic compositions viable for the atmosphere of Venus. The rather imprecise determination could be due to the scarcity of 3He relative to 4He, to the presence of an isobaric interference due to the presence of 1H2D, or could be explained by the fact that droplets of sulfuric acid clogged the inlet port of the Pioneer probe, which interfered with measurements of noble gases (Hoffman et al. 1980). This upper limit is compared to the 3He/4He ratio of other reservoirs in the Solar System in Fig. 3. The atmospheric 3He/4He ratio for Venus seems only marginally lower than the solar value, but since even the order of magnitude of the exact value remains unknown, it is impossible to evaluate the roles of relatively recent (hundreds of millions of years) outgassing of radiogenic 4He and of preferential escape of 3He relative to 4He from the atmosphere of Venus to space.
3He/4He ratio of the atmosphere of Venus and of other reservoirs in the Solar System. Atmospheric escape of helium or degassing of radiogenic 4He leads to a decrease of the 3He/4He ratio. Values are from Porcelli et al. (2002) and refs. therein
Neon – The 20Ne/22Ne ratio in the Venus atmosphere is close to 12 (Table 1, Fig. 4). This ratio is higher than that measured for terrestrial atmospheric neon (9.8, Ozima and Podosek (2002)), which suggests that solar-wind irradiated material (\(^{20}Ne/^{22}Ne=12.8\), Heber et al. (2009)) or solar gas (\(^{20}Ne/^{22}Ne=13.4\), Heber et al. (2012)) contributed volatile elements in greater proportion to the atmosphere of Venus relative to Earth. For the 21Ne/22Ne ratio, only an upper limit of 0.067 has been proposed by Istomin et al. (1983). This value is too imprecise to provide any further constraint on the origin of neon on Venus.
Three-isotope diagram of neon isotopes (20,21,22Ne). The isotopic composition of Earth atmospheric neon lies on a mixing range between Solar and Meteoritic end-members. The current estimate for Venus atmospheric neon suggests that, similarly to Mars atmospheric neon, Venus could lie outside of this mixing range although the uncertainty on the Ar/Ne ratio in the Venus atmosphere is high. Figure modified after Marty (2012)
Argon – There are two estimates for the 38Ar/36Ar ratio, one with a value of 0.183±0.003 (Istomin et al. 1983), and another with a much less precise value of 0.18±0.02 (Donahue and Pollack 1983). These two values are plotted in Fig. 5. If the conservative error estimate is considered, the 38Ar/36Ar ratio could correspond to any primordial end-member of the Solar System. However, if the value taken for the 38Ar/36Ar ratio is 0.183±0.003, then the primordial isotopes of argon are similar to Solar argon. The 40Ar/36Ar ratio has been measured at 1.11±0.02 (Istomin et al. 1983). Such a low value compared to the isotopic ratio of Earth’s atmospheric argon (≈300, Ozima and Podosek (2002)) has been interpreted as evidence that Venus outgassed only about 25% of the radiogenic 40Ar produced by the radioactive decay of 40K (T\(_{1/2}{=}1.25\) Ga) during 4.56 Ga (Kaula 1999). This estimate for the amount of degassed radiogenic argon is about half the value estimated for Earth (Allègre et al. 1987). Note that estimates for the total outgassing of 40Ar over Venus’s history strongly rely on the assumed K/U ratio for bulk Venus, which remains debated (see the discussions by Lammer et al. (2020) and by Gillmann et al. (2022)). Limited outgassing of radiogenic Ar over 4.56 Ga is the basis for the common view that Venus remained quiescent, in a stagnant-lid tectonic regime, during most of its history (O’Rourke and Korenaga 2015). The high abundance of 36Ar means that a large fraction of argon in the Venus atmosphere (Fig. 1) could be derived from primordial cosmochemical sources (solar gas, meteorites, comets). However, the relative abundances and isotope ratios of Ne and Ar do not provide a simple view of the source of light noble gases to Venus. The primordial Ar/Ne ratio is similar to values measured in meteorites, but the 20Ne/22Ne ratio may be solar-like (see above). The large uncertainty on the determination of the elemental Ar/Ne ratio does not permit evaluation of whether Venus plots on the mixing line between solar and meteoritic end-members (Fig. 4), or if the delivery of gas with an extremely high Ar/Ne ratio (e.g. via comets, Marty et al. (2016)) induced an excess of primordial Ar relative to Ne without changing the 20Ne/22Ne ratio.
Three-isotope diagram of argon isotopes. The thin black line corresponds to the error on the 38Ar/36Ar ratio of Venus reported by Istomin et al. (1983). The purple range represents the uncertainty given by Donahue and Pollack (1983). Values for air are from Ozima and Podosek (2002). Values for Mars are taken from Avice et al. (2018a) and refs. therein. Error bars at 1\(\sigma \)
Krypton and Xenon – Today, there is no available measurement of the isotopic composition of krypton or xenon in the atmosphere of Venus. The isotopic compositions of Kr and Xe in different volatile element reservoirs of the Solar system are plotted in Fig. 6 and briefly discussed here to illustrate the high potential of Kr and Xe isotopic measurements to shed light on volatile origins and loss processes on Venus.
Isotopic composition of krypton (a) and xenon (b) in important reservoirs of the Solar system. Isotopic ratios are expressed in delta values, which correspond to deviations in permil relative to the isotopic composition of the Solar Wind (Meshik et al. 2014, 2020). Data for the Earth’s atmosphere are from Ozima and Podosek (2002). Data for Mars atmospheric Kr are from Conrad et al. (2016) for in-situ measurements and from Swindle et al. (1986) for measurements made on Martian meteorites. Data for Mars atmospheric Xe are from Conrad et al. (2016) for in-situ measurements and from Mathew et al. (1998) for Xe measured in Martian meteorites. Note that the exact values, especially for light Kr and Xe isotopes, remain debated at this time (Avice et al. 2018a). Data for Kr and Xe in the phase Q, the major carrier of heavy noble gases in carbonaceous chondrites, are from Busemann et al. (2000). Error bars at 1\(\sigma \)
The isotopic composition of terrestrial atmospheric krypton is close to the Chondritic end-member. Note that the latter two components are not simply related to one another by mass-dependent fractionation (which would appear as a rotation of the isotopic spectrum centered on 84Kr in Fig. 6). On Earth, mantle Kr is showing a chondritic affinity with a deficit in neutron-rich 86Kr relative to the chondritic composition whose origin remains poorly understood (Péron et al. 2021). The isotopic composition of Mars atmospheric Kr has been measured in-situ (Conrad et al. 2016). Mars Kr might present important excesses in 80Kr and 82Kr isotopes (Fig. 6) which have been attributed to the degassing from the Martian regolith of Kr isotopes produced by neutron capture reactions on bromine (Rao 2002). After correction for these excesses, Mars atmospheric Kr appears to be solar-like (Conrad et al. 2016). However, Mars atmospheric Kr trapped in glass phases of Martian meteorites does not exhibit the excesses measured in situ, and this discrepancy remains unexplained (Conrad et al. 2016; Swindle et al. 1986; Avice et al. 2018a). Note that recent measurements of krypton contained in the Chassigny meteorite reveal that chondritic Kr is present in Mars’s interior (Péron and Mukhopadhyay 2022). This suggests that, contrary to the common view, delivery of chondritic volatile elements can happen before the accretion of solar nebular gases to the Mars atmosphere.
For xenon, the isotopic composition of the Xe in the phase Q component (Q-Xe, Wieler et al. (1992), Busemann et al. (2000)), which is the main carrier of noble gases in carbonaceous chondrites, is enriched in heavy isotopes and depleted in light isotopes, corresponding to a mass-dependent isotopic fractionation of about 1 % per atomic mass unit (or %\(.\mbox{amu}^{-1}\)) compared to the solar composition (Meshik et al. 2020). The origin of phase Q and of the isotopic difference between Q-Xe and Solar-Xe remains debated, but common explanations invoke ionization processes, causing the mass-dependent isotopic fractionation, (e.g. Kuga et al. 2017) accompanied by addition of Xe isotopes either derived from presolar components (Crowther and Gilmour 2013) or produced during radioactive decay of I, U and Pu (Marrocchi et al. 2015).
Xenon on Earth and Mars presents a strong positive (i.e. enrichment in heavy isotopes) isotopic fractionation (3-4 \(\%.u^{-1}\)) relative to primordial end-members in the Solar system. Previous studies argued that Xe had been lost from Earth and Mars during early episodes of hydrodynamic escape (Pepin 1991; Pepin and Porcelli 2002). However, recent studies of paleo-atmospheric xenon trapped in terrestrial rocks of Archean age revealed that the isotopic fractionation of atmospheric xenon was progressive over time (Pujol et al. 2011, 2009; Avice et al. 2017, 2018b; Bekaert et al. 2018; Almayrac et al. 2021) and ceased around the time of the Great Oxidation Event (Avice et al. 2018b; Ardoin et al. 2022). Zahnle et al. (2019) proposed that xenon ions escaped from the Earth’s atmosphere through Coulomb interactions in an ionized hydrogen wind, which caused a progressive depletion and isotopic fractionation of atmospheric Xe on Earth. A similar process was probably operating on Mars (Cassata et al. 2022) although existing data suggest that escape and isotopic fractionation of Xe ceased at least 4.2 Ga ago (Cassata 2017).
Xenon in the Earth’s atmosphere also presents interesting mass-independent features. Even after correction for the mass-dependent fractionation, Earth atmospheric Xe cannot be simply attributed to Solar Xe, Chondritic Xe or a mixture of these two end-member compositions present in the Solar system. Primordial unfractionated xenon is depleted in 134Xe and 136Xe isotopes by several percents relative to Solar or Chondritic Xe. This feature has been recognized decades ago (Takaoka 1972). Interestingly, the severe 134Xe and 136Xe anomalies on Earth relative to other bodies of the solar system correspond to a deficit in r-process Xe isotopes several orders of magnitude higher than other nucleosynthetic deviations reported for refractory nuclides measured in meteorites (e.g., Kleine et al. 2020). Primordial Xe for Earth atmospheric Xe has been named “U-Xe” (Pepin 1991) and has resisted decades of investigation (Pepin 1994). Recent measurements of gases emitted by the comet 67P/Churyumov-Gerasimenko revealed that cometary xenon is also depleted in 134Xe and 136Xe relative to Solar xenon (Marty et al. 2017). This depletion is even more pronounced than for U-Xe. Mass balance calculations suggest that U-Xe could result from a mixing between 78% of Chondritic and 22% of Cometary xenon (Marty et al. 2017). The depletion in 134Xe and 136Xe suggests that comets could be depleted in r-process nuclides and that large-scale nucleosynthetic heterogeneities persisted across the Solar system (Avice et al. 2020).
Radiogenic 129Xe produced by the decay of the short-lived radionuclide 129I (T\(_{1/2}=15.7\) Myr) sheds light on early volatile loss from planetary reservoirs. Radiogenic 129Xe excesses compared to Solar or Chondritic compositions are only generated by I/Xe fractionation within the first ≈100 Myr of Solar System history. On Earth, the mantle exhibits large 129Xe/130Xe excesses compared to chondrites, generally interpreted as evidence for significant early outgassing to generate high I/Xe ratios (e.g. Parai et al. 2019), as the solubility of iodine in silicate melts exceeds that of Xe (Musselwhite et al. 1991). Earth’s atmosphere has a low 129Xe/130Xe ratio relative to the mantle, but exhibits 129Xe excesses compared to a mass-fractionated non-radiogenic composition; an estimated 6.8 +/- 0.3 % of Earth’s atmospheric 129Xe budget is radiogenic (Porcelli and Ballentine 2002). Part of the radiogenic 129Xe in Earth’s atmosphere is derived from mantle outgassing over time: samples of Archean atmosphere show a deficit in 129Xe/130Xe compared to the modern composition after accounting for mass-dependent fractionation, providing a constraint on the rate of mantle Xe outgassing to the atmosphere, which, at the end of the Archean, could have been a least one order of magnitude than today (Avice et al. 2017; Marty et al. 2019). On Mars, the atmospheric radiogenic 129Xe excess relative to a fractionated non-radiogenic composition is much greater than on Earth (Swindle et al. 1986; Garrison and Bogard 1998) (Fig. 6). However, the mantle composition determined from the martian meteorite Chassigny exhibits almost no excess in 129Xe relative to chondrites (Ott 1988; Mathew and Marti 2001); outgassing of the mantle reservoir sampled by Chassigny cannot explain the large radiogenic excess in 129Xe in the martian atmosphere. A distinct reservoir that experienced very strong early degassing (to generate high I/Xe within the lifetime of 129I) must exist on Mars (Musselwhite et al. 1991), and outgassing of this reservoir has added radiogenic 129Xe to the martian atmosphere over time (Mathew and Marti 2001; Cassata 2017).
Although there is no clear determination of the abundance of Xe in the Venus atmosphere, the fact that 131,132Xe isotopes may have been detected during the Venera mission (Fig. 2, Istomin et al. (1983)) but that no 129Xe peak had been identified suggests that the 129Xe/132Xe ratio is not extremely high and is likely lower than on Mars. Taken together, Xe isotopic signatures tell a complex story of the history of a planetary atmosphere. Bulk abundance and relative ratios of primordial isotopes can be particularly diagnostic regarding the nature of accreted volatiles and the mechanism and extent of atmospheric loss to space, such as giant impact erosion or hydrodynamic escape. The presence and abundances of radiogenic isotopes provide further insight regarding the timing and the degree of early volatile loss from mantle reservoirs that have outgassed to the atmosphere. Measurements of Xe in the atmosphere of Venus would thus provide potent insights into volatile origins, major evolutionary events, and transport for both the atmosphere and interior of the planet. These all have important implications for the inventory and history of other volatiles on Venus.
2.2 Stable Isotopes (C, H, O, N, S)
2.2.1 Elemental Abundances
Carbon, hydrogen, oxygen, nitrogen and sulfur in the Venus atmosphere are mainly stored in CO2 (96.5±0.8% by vol.) for carbon and oxygen, H2O (30±15 ppm) for hydrogen, N2 (3.5±0.8%) for nitrogen and SO2 (150±30 ppm) for sulfur (Fegley 2014, and refs. therein).
Estimates for the elemental abundances of carbon and nitrogen in the Venus atmosphere have been reported and discussed by Halliday (2013). Earth and Venus have similar abundances of carbon and nitrogen and the C/N ratio is close to chondritic proportions (Fig. 7). Comparatively, C and N are depleted in abundances on Mars relative to chondrites, Earth and Venus, but the C/N ratio is close to the chondritic value in all three terrestrial planets. However, a near-chondritic C/N ratio should be interpreted with caution considering that strong isotopic fractionation of nitrogen isotopes in the Mars atmosphere demonstrates that Mars suffered from atmospheric escape of nitrogen (Wong et al. 2013, and refs. therein). The low abundance of water in the Venus atmosphere probably results from intense episodes of hydrogen escape (see Sect. 2.2.2 and Salvador et al. (2022)).
Elemental abundances of noble gases, nitrogen and carbon on bulk Earth, Venus and Mars compared to abundances in CI chondrites. Elemental abundances are normalized to the abundance of silicon and to the Solar composition. Dashed lines depict different levels of depletion compared to volatile elements in CI chondrites. The two very different estimates for the abundance of krypton are also represented (see text). See Halliday (2013) for details. Figure modified after Halliday (2013)
2.2.2 Isotope Ratios
The isotopic composition of hydrogen and nitrogen for Venus are compared to other Solar System reservoirs in Fig. 8. One of the most important feature of the Venus atmosphere is that the D/H ratio (where D\(=^{2}\)H) of hydrogen is extremely high compared to all other reservoirs in the Solar System. The \(\delta \)D\(_{VSMOW}\) value reaches 120,000‰. This very high ratio has been interpreted as evidence that Venus suffered from hydrogen escape episodes during its geological history (Donahue et al. 1982). When hydrogen escapes from an atmosphere, deuterium is more likely to be left behind, as it is twice as heavy (Hunten et al. 1987). The fraction of hydrogen remaining in the atmosphere becomes enriched in deuterium (D) relative to hydrogen (1H). Atmospheric escape was thus an important process in the history of hydrogen on Venus. It must be noted that, since the starting D/H ratio of Venus remains unknown, an elevated \(\delta \)D\(_{SMOW}\) also leaves room for some cometary contribution to the delivery mix of volatile elements to Venus. For nitrogen, the \(\delta ^{15}\)N value has been estimated at 0±200‰. This values is far too imprecise compared to typical values measured for nitrogen in the Solar system (Fig. 8, Füri and Marty (2015)) to draw any conclusion on the origin of Venus nitrogen and to evaluate whether the original ratio has been increased by atmospheric escape processes, as on Mars (McElroy et al. 1976).
Isotopic composition of hydrogen and nitrogen in reservoirs of the solar system. (a) Venus shows an extreme \(\delta \)D value compared to all reservoirs of hydrogen in the solar system. The two dashed lines show the upper and lower bound for the \(\delta ^{15}\)N value. The blue rectangle represents the zoom region for sub-panel (b). Isotope ratios are expressed with the delta notation, which corresponds to the deviation in permil relative to a standard isotope composition (mean Earth’s ocean water for hydrogen, and Earth’s air for nitrogen). Figure adapted from Marty (2012), Füri and Marty (2015), Marty et al. (2016). Data are from McCubbin and Barnes (2019), Marty et al. (2016) and refs. therein
The \(^{12}\mbox{C}/^{13}\mbox{C}\) ratio of carbon in \(\mbox{CO}_{2}\) has been measured at \(89.3\pm 1.6\). This value is identical, within errors, to the isotopic composition of carbon on Earth (Istomin et al. 1980). For oxygen, the \(^{17}\mbox{O}/^{18}\mbox{O}\) ratio remains unknown and the \(^{18}\mbox{O}/^{16}\mbox{O}\) value is \(0.0020\pm 0.0001\) (Hoffman et al. 1980). This estimate is far too imprecise to evaluate if the isotopic composition of oxygen on Venus differs from other oxygen reservoirs in the Solar system (Greenwood and Anand 2020). Finally, there is no isotopic data for sulfur, an important constituent of the atmosphere of Venus.
3 Unsolved and New Questions
There have been no new data acquired in-situ on the elemental abundances and isotopic compositions of noble gases and stable isotopes in the atmosphere of Venus for the last 40 years. However, recent discoveries regarding the origin and evolution of volatile elements Earth and Mars and in other Solar System reservoirs have raised new and critical scientific questions pertaining to Venus, such that previous efforts to define the future of scientific investigations on the atmosphere of Venus (e.g. Chassefière et al. 2012) must be updated. This section presents the most pressing outstanding scientific questions on the origin and early evolution of the atmosphere of Venus.
3.1 Origin of Venus and of Its Atmosphere
Noble gases and stable isotopes are often used to estimate the contributions of volatile elements from different accreted components to planetary atmospheres. In the case of Venus, current estimates of the elemental abundances and isotope ratios of those elements remain too imprecise to draw any firm conclusions, but the existing dataset allows one to pose scientific questions and frame testable hypotheses:
-
To what extent did solar-derived gas contribute to the Venus atmosphere? Although the elemental Ne/Ar ratio of the Venus atmosphere is close to the chondritic value, the 20Ne/22Ne ratio seems higher than the average ratio measured in carbonaceous chondrites and closer to values measured in material irradiated by the Solar wind (e.g. Péron et al. 2018, for a recent review on SW noble gases) or to the ratio measured for solar Ne (Heber et al. 2009). One possible explanation would be that Venus Ne is of solar origin and that some of this Ne escaped from the Venus atmosphere. This would lead to a increase in the Ar/Ne ratio together with a decrease of the 20Ne/22Ne ratio. However, the existing dataset remains imprecise and a mixture between Solar Ne and Meteoritic Ne could also be envisaged (Fig. 4). Better measurements of the elemental abundances and the isotope ratios of Ne and Ar could shed new light on this problem. Precise measurements of the isotopic composition of Kr and Xe, down to the percent level, could also provide answers since the different volatile reservoirs of the Solar system (Solar gas, meteorites and even comets) have distinct Kr and Xe isotopic signatures. These end-members are not simply related by mass-dependent isotopic fractionation, which means that distinction between cosmochemical sources is possible even if the original signature has been modified by atmospheric escape leading to mass-dependent isotopic fractionation. A precise measurement of the \(\delta ^{15}\)N value (to the 5-10‰ precision level) is also desirable since the different classes of meteorites have distinct 15N/14N ratios (Füri and Marty 2015);
-
Did comets contribute volatile elements to Venus? Recent studies of cometary noble gases and comparisons with Earth’s atmospheric noble gases revealed that comets may have contributed a significant portion of Earth’s noble gases, while the contribution to the budget of water would have stayed relatively minor (\(\sim 1\%\)) (Marty et al. 2016, 2017; Bekaert et al. 2020). This cometary contribution might have left its fingerprint in the isotopic composition of Earth atmospheric xenon (Avice et al. 2017; Marty et al. 2017) with a marked depletion in 134Xe and 136Xe isotopes relative to Solar or Meteoritic end-members. Contrary to the case of Earth, Mars Xe could be purely of solar origin (Ott 1988). Measuring precisely the isotopic composition of Venus atmospheric Xe could thus help to evaluate if comets contributed volatile elements to Venus. The Ar/Ne and 20Ne/22Ne ratios are also of interest here. Neon condenses only at very low temperatures (<20K) and comets are thus probably devoid of this element (Bar-Nun and Owen 1998). A significant cometary contribution to the atmosphere of Venus would result in a significant shift to the right in the 20Ne/22Ne vs 36Ar/22Ne space (Fig. 4);
-
Was the early inner Solar system efficiently homogenized for a major element like oxygen? Families of primitive meteorites have widely distinct O isotope compositions. Although the Sun represents more than 99% of the total mass of the Solar System, meteorites and planets such as Earth and Mars are distinct from the Sun in terms of O isotope composition. Earth and Mars show enrichments in17O up to 70‰ at a given 18O/16O ratio, relative to the sun (McKeegan et al. 2011). This ground-breaking observation, pioneered in the 1970’s (Clayton et al. 1973), spurred entire fields of research. Although conflicting interpretations still exist, the current paradigm suggests the starting \(\delta ^{17}\)O-\(\delta ^{18}\)O composition of solids were solar, reflecting an inheritance from the average 16O-rich molecular cloud. Solids subsequently evolved towards non-solar \(\delta ^{17}\)O-\(\delta ^{18}\)O values via interaction with 17O-rich water: they developed positive \(\Delta ^{17}\)O signatures relative to the Sun. Oxygen is a major mineral-forming element and makes up more than 45 wt.% of silicate planets (Javoy et al. 2010). Scientific reasons justifying sample return from Venus for O isotope measurements were recently reviewed (Greenwood and Anand 2020). Briefly, the later stages of terrestrial planet formation are thought to involve collisions with Moon-to-Mars sized planetesimals (Kaib and Cowan 2015). The final \(\Delta ^{17}\)O signature of a planet is the result of the weighted average of the various parent bodies (Young et al. 2016). The Moon has essentially the same \(\Delta ^{17}\)O composition as the Earth for reasons which most likely reflect its mode of formation in a giant impact event (Young et al. 2016). In contrast, Mars and Earth have distinct oxygen isotope \(\Delta ^{17}\)O compositions by +0.3‰ (Clayton and Mayeda 1983). A variable accretionary make-up may explain differences between these two planets. Large random \(\Delta ^{17}\)O variations may have existed among accreting bodies. Mars is only ≈10% of the mass of Earth and sampled a relatively small number of accreting bodies whose weighted average \(\Delta ^{17}\)O may have deviated from the average inner Solar System composition, whereas Earth may have inherited a composition close to the average inner Solar System, with \(\Delta ^{17}\)O differences homogenized away by prolonged planetary growth. Alternatively, the composition of Mars may reflect an inner Solar System poorly homogenized for \(\Delta ^{17}\)O signatures, with a radial gradient in composition. Venus is much larger than Mars, as the second most massive terrestrial planet (after Earth) in our solar system. Whether the Earth and Venus are different in terms of \(\Delta ^{17}\)O would be a test of the potential inhomogeneity of the inner solar system, conceivably recorded by Mars’s composition.
3.2 The Problem of Photochemistry
Photochemistry could prevent a straightforward interpretation of future O and S isotope data in terms of the origin of Venus. Processes associated with photochemistry are known to redistribute 17O between the various O-bearing molecules in the Earth’s atmosphere. Ozone carries a > 100‰ 17O anomaly in air (Thiemens 2006). Isotope exchange between terrestrial CO2 and ozone results in atmospheric CO2 with an anomalous \(\Delta ^{17}\)O relative to its source (Thiemens 2006). In fact, oxygen isotope exchange in modern air, between stratospheric ozone and any O-bearing molecule (SO2, NOx, CO2, etc…) results in 17O signatures skewed towards anomalously positive values for all those oxygen carriers. As a result, stratospheric CO2 on Earth show 17O anomalies up to 15‰ (Thiemens 2006), far from tracing the composition of bulk Earth. Photochemistry is known to occur in the modern atmosphere of Venus (Yung and Demore 1982, and refs. therein), which is a concern for how representative Venusian CO2 may be to the interior of the planet. However, the Earth mechanism described in Thiemens (2006) is unlikely to be translated to Venus. First, a glaring difference is that there is no ozone in the Venusian atmosphere. Second, the Venusian atmospheric composition is crushingly dominated by CO2, in stark contrast with Earth. For instance, the second most abundant O-bearing molecule on Venus is SO2 (150 ppm), followed by water vapor (20 ppm) and carbon monoxide (17 ppm). We suggest that the mass balance is favorable for CO2 to remain mostly unaffected by exotic unidentified chemistry. In other words, it is unclear how any unidentified chemistry involving third-party molecules could alter the 17O of Venus’s CO2 atmosphere, as occurs on Earth.
Sulfur may help to understand the role of photochemistry. It occurs in the nebular gas as H2S (Lauretta et al. 1997; Lodders 2003), which absorbs light and photo-dissociates when irradiated by the deep UV at wavelengths between 160 and 60 nm (Okabe 1978). In theory, photodissociation of H2S produces S0 with 33S and 36S isotopic anomalies (Chakraborty et al. 2013), like what is seen for 17O. However, differentiated meteorites show almost no 33S and 36S variations (Antonelli et al. 2014; Dottin et al. 2018a). This is a major difference with oxygen: only extremely small 33S and 36S variations are anticipated for bulk planets. The similarity for 33S and 36S of Earth and Mars support this suggestion (Franz et al. 2014; Labidi et al. 2013). However, sulfur-bearing molecules such as SO2 are readily photolyzed by the modern Sun’s light, in planetary atmospheres. Volcanic SO2 on both Earth and Mars is known to develop 33S and 36S anomalies, when sent flying to optically thin regions of planetary atmospheres (Baroni et al. 2007; Gautier et al. 2019). 33S and 36S measurements of Venusian SO2 would provide first order constraints on whether any photochemistry is able to modify the composition of Venusian gases, like it does on the surface of Mars (Dottin et al. 2018b; Franz et al. 2014). These measurements will help discussing whether 17O in Venusian CO2 is a pristine measurement of the bulk planet composition, or if atmospheric samples are inevitably skewed by the occurrence of photochemistry.
3.3 Early Evolution of the Planet
3.3.1 Atmospheric Escape and Xenon
The high D/H ratio of the Venus atmosphere has often been interpreted as evidence that Venus lost its original water (Donahue et al. 1982). Dissociation of water molecules in the upper layers of the Venus atmosphere followed by escape of hydrogen to space, both powered by the strong irradiation from the early Sun, would have resulted in a depletion of water and an increase in the D/H ratio. However, both the initial water content and starting D/H ratio of water on Venus remain unknown. It remains possible that small amounts of water with a high D/H ratio (Altwegg et al. 2015) have been delivered to Venus by cometary bodies. Interestingly, a recent study combined outputs from several modeling approaches (thermochemical model of convection on Venus, atmospheric escape model and N-body simulations of the formation of the solar system) to point out that atmospheric escape on Venus was not able to remove large quantities of water over Venus history (Gillmann et al. 2020). This could imply that Venus was never affected by late accretion of water-rich material. Note however that existing models proposing scenarios for the evolution of water on Venus rely on rather poorly constrained parameters such as the initial water content or the EUV flux from the young Sun. Also, most of the isotopic fractionation of hydrogen, leading to an increase of the D/H ratio, would probably have happened only during the late stages of atmospheric escape of hydrogen. Indeed, when large amounts of hydrogen are lost during intense episodes of escape, deuterium also leaves the atmosphere efficiently and the D/H ratio stays relatively constant (Zahnle et al. 1990). In other words, when the planet suffered from different atmospheric escape regimes in its history, the D/H ratio the present atmosphere brings information on only a modest part of the history of atmospheric escape.
Noble gases hold clues on the extent of mass-fractionating escape processes suffered by Venus. Several models attempted to reproduce the elemental and isotopic composition of neon and argon measured in the atmosphere of Venus (e.g. Pepin 1991; Gillmann et al. 2009; Lammer et al. 2020, 2021). Most models involving atmospheric escape manage to reproduce the data in a consistent way but new measurements are required to really draw firm conclusions on the history of atmospheric escape on Venus. For the time being, the existing dataset only allows to conclude that the early Sun powering atmospheric escape was either a moderate or a slow rotator (Lammer et al. 2020).
Recent studies of ancient terrestrial samples containing paleo-atmospheric gases revealed that the isotopic composition of terrestrial atmospheric Xe evolved during at least 2 Ga after the Earth formed (Pujol et al. 2011, 2009; Avice et al. 2017, 2018b; Bekaert et al. 2018; Ardoin et al. 2022) (Fig. 9). This evolution corresponds to a progressive mass-dependent fractionation of Xe isotopes probably starting from a composition corresponding to a mixture between cometary and meteoritic Xe (Marty et al. 2017) and reaching the modern-like isotopic composition around the time of the Great Oxidation Event ca. 2.3 Ga ago (Avice et al. 2018b; Ardoin et al. 2022). The current favored explanation for explaining this protracted evolution is an escape of xenon ions together with hydrogen ions from the Archean atmosphere to outer space (Zahnle et al. 2019; Catling and Zahnle 2020). The isotopic composition of atmospheric Xe could thus be a tracer of hydrogen escape from planetary atmospheres. Compared to Earth, xenon in the atmosphere of Mars is likely derived from a different source, i.e. solar gas (Garrison and Bogard 1998; Ott 1988) but also presents a mass-dependent fractionation of 3-4 %\(.u^{-1}\) relative to the starting isotopic composition (Swindle et al. 1986). The magnitude of fractionation is similar to the case of Earth atmospheric xenon. Assuming that atmospheric escape of Xe is responsible for both the depletion and the isotopic fractionation of the remaining fraction, this similarity in the extent of isotopic fractionation is intriguing given that the escape process will be governed by parameters (abundance of total H2, irradiation from the Sun, magnetic field etc.) which are likely to have been very distinct for early Earth and Mars. Note that, although present-day isotopic fractionation of atmospheric xenon is similar for Earth and Mars, the fractionation (and maybe escape) of xenon on Mars probably ceased much earlier than for Earth, around 4.2 Ga ago (Cassata 2017; Cassata et al. 2022) (Fig. 9). Collecting data on the abundance and isotopic composition of Xe in the Venus atmosphere is a high priority to determine to what extent “missing and isotopically fractionated xenon” is a common feature of planetary atmospheres of the inner Solar system and to further elucidate if atmospheric escape played a role in shaping the elemental and isotopic composition of atmospheric Xe. Note that potential fissiogenic additions to the budget of atmospheric 131−136Xe isotopes pose a challenge when one wants to determine the fractionation of atmospheric xenon (e.g., Avice et al. 2017). A precise determination of the abundances of less abundant light isotopes such as 124,126,128,130Xe plays then an important role to estimate the degree of fractionation of atmospheric xenon.
Evolution of the isotopic composition of atmospheric xenon on Earth and Mars. Isotopic fractionation is expressed relative the isotopic composition of atmospheric xenon of modern Earth and Mars. Three theoretical scenarios are displayed for Venus Xe: No escape of xenon and an isotopic composition similar to primordial xenon from Solar, Chondritic or Cometary sources; Same fractionation as for Earth and Mars; More escape (and isotopic fractionation) of xenon. Paleo-atmospheric data for Earth are from Avice and Marty (2020) and refs. therein and from Ardoin et al. (2022) and Broadley et al. (2022). Paleo-atmospheric data for Mars are taken from Cassata et al. (2022). Error bars are at 1\(\sigma \)
Further, the isotopic composition of nitrogen may also shed light on atmospheric loss in Venus’ history. It is a reasonable hypothesis that the terrestrial planets formed with a similar nitrogen source, and the 15N/14N ratio places an important constraint on if and when Venus could have lost its putative primordial ocean and on the timing of the runaway greenhouse onset. Nitrogen could have been preferentially lost from Venus’ atmosphere largely during its early geologic history when the atmosphere was presumably relatively thin (Baines et al. 2013, and refs. therein). Once the atmosphere became massive and CO2-rich, further fractionation of isotopes in bulk nitrogen may be less evident due to dilution of N2 by CO2 at the exobase. Therefore, if the 15N/14N ratio is significantly greater than the terrestrial value (thus closer to Mars), this would suggest that intense hydrodynamic escape must have occurred prior to the rise of CO2.
3.3.2 Contributions from Mantle Outgassing and Characterization of Mantle Reservoirs
It is of vital importance to understand the role of mantle outgassing in shaping surface conditions on Venus (Gillmann et al. 2022). Identification of radiogenic 129Xe excesses in the atmosphere of Venus would suggest that at least some portion of the interior experienced early degassing to fractionate I/Xe during the lifetime of 129I, and has subsequently outgassed to affect the Venus atmospheric composition. The magnitude of the radiogenic excess, if any, would provide a lower limit constraint on early outgassing of a Venus mantle reservoir for comparison with terrestrial and martian mantle reservoirs, yielding insights into comparative geodynamics during accretion, and could allow comparisons of the degree of outgassing to the atmosphere. If no radiogenic 129Xe excess is observed in the atmosphere, this would suggest either that the Venus mantle did not experience early outgassing (similar to the Chassigny mantle source on Mars), or that outgassing of the mantle has been very limited over the past 4.45 Ga, pushing any catastrophic early outgassing to explain low atmospheric 40Ar/36Ar to the earliest stages of Venus history. Xe isotopic measurements of the Venus atmosphere are needed to understand the timing and extent of mantle outgassing, and the relationship between early outgassing and atmospheric loss to space (Cassata 2017; Cassata et al. 2022). Note that atmospheric erosion by impacts could also have altered the budget of radiogenic xenon, depending on the timings of outgassing relative to the impacts. Combined, abundances of the radiogenic noble gases 129Xe, 40Ar, and 4He will contribute to the understanding of the relative rates and significance of early, long-term, and recent outgassing, respectively.
4 Recommendations for Future Investigations
The existing dataset on the abundance and isotopic composition of noble gases and stable isotopes in the atmosphere of Venus is partial and imprecise and new investigations are urgent. A list of key measurements of noble gases and of their associated maximal uncertainties required to answer the scientific questions described in Sect. 3 is summarized in Table 1.
Two broad types of science investigation could be envisaged for gathering data on the elemental and isotopic compositions of noble gases and stable isotopes (H, C, N, O, S) in the atmosphere of Venus. One type would be an in-situ mission carrying a scientific payload able to measure the abundances and isotope ratios of the chemical elements of interest. Another one would be a sample return mission during which a portion of the Venus atmosphere would be sampled. The collected sample(s) would then be returned to Earth for characterization with state-of-the-art technologies available in international laboratories. The pros and cons regarding measurements of noble gases and stable isotopes are presented and briefly discussed in the next sections. See also Widemann et al. (2022) of this issue for a detailed discussion of future missions as well as new concepts for exploring Venus.
4.1 In Situ Measurements
To date, almost all data on the abundance and isotopic composition of volatile elements in the atmosphere of Venus (Johnson and de Oliveira 2019) have been collected during in situ investigations by probes plunging through the atmosphere of Venus and carrying mass spectrometers (e.g. Mahaffy 1999). This is the approach that will be used by the recently-selected DAVINCI mission from NASA (Garvin et al. 2022, Fig. 10).
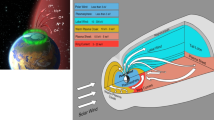
The NASA DAVINCI mission (Garvin et al. 2022) will conduct in situ sampling of the Venus atmosphere during its ≈hour-long descent to the surface, providing critical measurements of noble gases and stable isotopes
One major advantage of in situ investigations is that the sample of interest is taken directly from the well-mixed atmosphere around the probe. In situ investigations at low velocity and well below the homopause ensure that the measured gas is representative of the entire atmosphere and that detected abundances and ratios are unambiguous (Tian 2015). Deep atmosphere sampling also avoids any uncertainties introduced by temporal or spatial variability of the homopause location by species or other upper atmosphere stratification (von Zahn et al. 1980; Mahieux et al. 2012; Gruchola et al. 2019; Peplowski et al. 2020).
Further, in situ sampling greatly minimizes complications of terrestrial contamination, as instrumentation can be cleaned and sealed off prior to launch, particularly for the volatiles. The captured in situ sample will thus greatly exceed any residual terrestrial species and dominate the measured signal. Finally, measurement of the atmosphere during a descent allows for sampling at multiple altitudes for species where there is the potential for vertical distribution that would otherwise complicate interpretation. Variable measurements of the D/H ratio at Venus suggest there may be a gradient (Bertaux et al. 2007), potentially driven by photolysis-induced isotopic fractionation (Liang and Yung 2009), preferential escape, or selective condensation, a process that has been found to be important for fractionating D and H on Mars and Earth (Bertaux and Montmessin 2001).
In situ investigations do present important challenges to be mitigated by each mission. Mass spectrometers on board atmospheric probes are inherently limited compared to ground-based analytical abilities, due to necessary constraints in terms of power consumption, size, mass, measurement duration, and robustness against spaceflight environments (Arevalo et al. 2020). Three key considerations for mass spectrometric measurements of noble gases in particular are sensitivity, selectivity, and dynamic range. Sensitivity and resolution (as a proxy for selectivity) are two commonly limiting factors for flight mass spectrometers and become extremely important when targeted species are rare isotopes (D, 3He, 78,80Kr, 124−128Xe, 33S etc.). The instrument must be sufficiently sensitive to detect a signal corresponding to these rare species. The dynamic range must also be sufficiently large to accurately measure ratios of species whose abundances span orders of magnitude (i.e., Xe vs. Ar). Another complication stems from the fact that isobaric interferences are always present, either in the residual background of the instrument itself or in the sample, even after purification steps (Wieler 2014). For example, for some ionization energies and instruments, doubly ionized \(\mbox{CO}_{2}^{++}\) ions are detected at mass 21.9949 while 22Ne+ ions are detected at mass 21.9914. The mass resolving power of the instrument required to simultaneously measure pure signals of each species if both are present in the instrument is higher than 6,000. Similarly, H35Cl and H37Cl signals are isobaric interferences of 36Ar and 38Ar, respectively (mass resolving power of 3,000). In the absence of high resolution for in situ instruments, the ionization energy can be adjusted to minimize the prevalence of doubly-charged ions. For other interferences, additional preprocessing and gas-cleaning techniques are required.
One way these three challenges have been addressed in previous in situ investigations has been to include an enrichment system to process samples prior to introduction into the instrument (Niemann et al. 1992; Mahaffy et al. 2012). Isobaric interferences can be minimized by significantly increasing the noble gas to interference ratio by working in ultra-clean conditions and by employing efficient purification methods to remove the bulk atmospheric components. Gas enrichment units have been employed (e.g. the SAM experiment onboard the Curiosity rover, Franz et al. (2017)) to artificially increase the partial pressure of noble gases, successfully enabling the measurement of Xe isotopes during a descent at Jupiter (Mahaffy et al. 2000) as well as recently in situ Ar, N2, Kr, and Xe isotopic ratios from the surface of Mars (Atreya et al. 2013; Wong et al. 2013). Note that for Mars data, the N2/Ar ratio was subsequently corrected after taking into account results obtained with calibration cells (Franz et al. 2017). The corrected N2/Ar ratio is now in agreement with results obtained by previous space missions (Owen et al. 1977) and by analyses of SNC meteorites (Avice et al. 2018a). Performing enrichments in a step-wise manner can also address isobaric interferences across targeted species as well expand the effective dynamic range. The improved capabilities are necessarily traded against increases in complexity, size, power consumption, and measurement duration of the experiments.
Measurements of stable isotopes are also challenged by the presence of isobaric interferences within a mass spectrometer (e.g. 12C16O signal interfering with 28N2 signal). Additional steps of chromatography can partially solve this problem since it allows to introduce the chemical species at distinct times into the mass spectrometer. Alternatively, unlike noble gases, many of the volatile species that carry isotopes of interest have strong spectral features and thus can be measured with high sensitivity and specificity using Tunable Laser Spectroscopy (TLS) (Tarsitano and Webster 2007). Isotopic ratios of carbon, oxygen, and hydrogen have been measured using TLS on Mars (Franz et al. 2020), and similar technique could be adapted to in situ measurements at Venus even on a descent probe.
Finally, given the harsh conditions encountered by a probe plunging in the Venus atmosphere, all the scientific tasks must be achieved very rapidly, on the order of the hour, and the scientific data must be transferred either to an orbiting spacecraft or directly to Earth as soon as possible. Long-lived surface platforms, or aerial platforms at more temperate altitudes, could enable repeatable measurements with longer processing and integration times. However, these are less technically mature, more costly and the discussion of their inherent challenges is beyond the scope of this contribution.
4.2 Sample Return
There are many scientific motivations for mission concepts proposing to return a sample from the Venus atmosphere and several mission scenarios have already been envisaged (e.g. Rodgers et al. 2000; Sweetser et al. 2003; Greenwood et al. 2018). In most cases, the sequence of atmospheric sampling is part of a larger complex mission involving a descent stage, ground operations by a lander etc. One technical challenge is then to find a method to launch the atmospheric samples from Venus ground to orbit (e.g. Rodgers et al. 2000). One alternative scenario for a sample return mission scenario would be a probe launched from Earth, plunging at high velocity through the Venus atmosphere, sampling the atmosphere (Sotin et al. 2018) and returning the sample back to Earth. Such a mission can be achieved with a free-return ballistic trajectory (Sweetser et al. 2003). One important advantage of this type of scenario is that the scientific payload for this type of mission could be very simple with only few instruments to characterize the sampled gas such as pressure gauges. Most of the payload would consist in the sampling system (pipes, valves and sampling cylinders). This simple approach would certainly save mission costs and reduce the risk of this type of mission. Importantly, there would be no stringent constraint on the delay between sampling and transfer in new containers followed by scientific analyses if the sampling cylinders present very low leak rates and very low degassing rates of unwanted background gases (e.g. H2) that could compromise the original chemical composition of the sample. Finally, and this is probably the most relevant argument here, sampled gas could be measured on Earth with state-of-the-art mass spectrometry & spectroscopy techniques, some of them being non-destructive such as Tunable Laser Spectroscopy (TLS) (e.g. Crosson et al. 2002; Tarsitano and Webster 2007). This non-destructive technique would allow to make a thoughtful characterization of the sample with, for example, measurements of the isotope ratio of H, O, C. High precision noble gas mass spectrometry could be done on very small amounts of the collected gas (Wieler 2014), especially if high-sensitive methods are used such as resonance ionization mass spectrometry (Gilmour et al. 1994). Although many of the scientific questions raised in Sect. 3 could be, at least partially, answered with an in-situ sampling and characterization mission, returning an atmospheric sample from Venus would bring the scientific output of any mission to Venus to another level. For example, determination of the \(\Delta ^{15}\mbox{N}^{15}\mbox{N}\) value of Venus atmospheric nitrogen, which quantifies the excess in the 15N15N molecule relative to a random distribution of N atoms in N2, would allow to investigate the photochemistry of atmospheric nitrogen on Venus and to evaluate the amount of exchanges between Venus interior and its atmosphere (Yeung et al. 2017; Labidi et al. 2020; Gillmann et al. 2022). Similarly, applying very sensitive isotope determination techniques to a sample from the Venus atmosphere would allow detection and precise measurement of the abundances of minor isotopes of noble gases (3He but also 78Kr and 124,126Xe) in order to better evaluate the delivered mix of volatile elements to the Venus atmosphere but also to put constraints on atmospheric escape processes or on interior-surface interactions.
However, a sample return mission faces important challenges. In the case of a free-return ballistic trajectory, the sample will be collected at high velocity (\(>10~\mbox{km}\,\mbox{s}^{-1}\)) and the gas in the front of the probe will be shocked, brought to high temperatures, and eventually turned into a plasma (Sweetser et al. 2003). Gas will then be fed into the sampling system via an inlet port and will have to travel through pipes and valves until a final expansion step into the sampling cylinder. All the steps described above could alter the chemical and isotopic integrity of the sample. The presence of a plasma implies that molecules will be dissociated. Cooling of the plasma will eventually lead to a recombination of the atoms into new molecules not originally present in the atmosphere of Venus. Noble gas atoms will also be ionized in the plasma and will be implanted on the surfaces of the probe. Numerous studies pointed out that the isotopic composition of noble gas trapped in the upper layers of solid exposed to ion implantation is mass-dependently fractionated relative to the starting composition (Bernatowicz and Hagee 1987; Kuga et al. 2017). Transfer of the gas from the atmosphere to the cylinder could also induce chemical and/or isotopic fractionation of gaseous species depending on the parameters of the gas flow. The effects of high velocity sampling on the chemical and isotopic composition of the sampled gas are currently studied via numerical simulations using a Direct Simulation Monte Carlo (DSMC) approach (Rabinovitch et al. 2019) and analog experiments for high velocity sampling are currently under development.
Returning an atmospheric sample from Venus requires important technical developments for the sampling system and the sample containers, and a full curation procedure for the sample(s) must be carefully prepared. Technologies for storing gas samples have already been employed in previous space missions (Allen et al. 2011; Moeller et al. 2021). A sample container must fit important criteria including: (i) a proper seal with a helium leak-rate on the order of 10−10 scc/s or below (Moeller et al. 2021); (ii) minimal interactions between sampled gas and container material; (iii) a sufficiently robust mechanical structure to resist Earth return methods (capture on orbit or direct re-entry). Sample curation is an extremely complex and costly task (e.g. McCubbin et al. 2019). Furthermore, curation of an atmospheric sample from another planet would be a novelty and would rest on very different principles compared to most existing setups for curating extraterrestrial rocks. For example, part of the modern sample curation process for samples recovered from asteroid Itokawa by the Japanese Hayabusa mission (JAXA) involves high-vacuum chambers and the use of pure gases in order to preserve the sample from any terrestrial contamination (Yada et al. 2014). Preserving and handling a gas sample from the Venus atmosphere would, of course, rely on different principles (no direct pumping nor gas injection), which remain to be defined by the international community.
Interestingly, a mission to Venus returning atmospheric samples to Earth would likely fit in the “Unrestricted Category V” of the classification scheme established by the Committee on Space Research (COSPAR 2020). If the classification is not revisited in the future, it would mean that such a sample would be subject to less stringent restrictions regarding its potential bearing of traces of life compared to samples from Mars, Europa or Enceladus. This would certainly relax the constraints for sample handling, preliminary characterization and preparation for long-term curation. However, some studies pointed out that Venus may have presented habitable surface conditions until as little as 700 Ma ago (Way et al. 2016) and that life forms could be hosted in the lower cloud layer on present-day Venus (Limaye et al. 2018). See also Westall et al. (this journal). It is plausible that, in the near future, Venus will join Mars, Europa and Enceladus in the list of objects for which a special care must be taken if a sample return is planned.
5 Conclusions
Knowing the elemental abundances and isotopic compositions of noble gases and H, C, N, O, S in the Venus atmosphere enables setting important constraints on the origin and early evolution of Earth’s sister planet. Existing data are incomplete, but important differences compared to Earth and Mars have already been pointed out by previous studies. Venus is enriched in light noble gases compared to Earth and Mars. The Ar/Ne ratio is close to chondritic values whereas the isotopic composition of neon may be solar-like. There is little radiogenic 40Ar in the atmosphere of Venus compared to Earth, suggesting that Venus is less degassed compared to Earth. Data on the abundance and isotope composition of krypton and xenon are cruelly lacking; these data are needed to put new constraints on the delivery mix of volatile elements to the entire planet and on the age and history of the atmosphere. For stable isotopes, the very high D/H ratio suggests that Venus suffered from hydrogen escape although the starting abundance and isotopic composition of Venus water remains unknown. A precise determination of the isotopic composition of nitrogen could help constrain the extent of elemental and isotopic fractionation of hydrogen and nitrogen by thermal and non-thermal atmospheric escape processes. Measurements of the isotopic composition of xenon will determine if, like Earth and Mars, Venus suffered from coupled hydrogen-xenon escape processes. About future investigations, the scientific community and institutional decision makers can opt for two types of investigations offering interesting complimentary perspectives: in-situ measurements or sample return. There is a strong and useful heritage of space technologies designed for in-situ measurements with an impressive list of outstanding scientific results obtained on diverse objects in the Solar System. Base on this approach, the recently selected DAVINCI (NASA) mission will provide a wealth of new data on the elemental and isotopic composition of noble gases and stable isotopes contained in the atmosphere of Venus. Sample return concepts present important but surmountable technical, scientific and organisational challenges. It may be within reach in the coming decades. Returning a sample from the atmosphere of Venus would provide, with a careful curation of the returned sample, enough analysable material to achieve high-precision measurements and would certainly lead to scientific breakthroughs.
References
Allègre CJ, Staudacher T, Sarda P (1987) Rare gas systematics: formation of the atmosphere, evolution and structure of the Earth’s mantle. Earth Planet Sci Lett 81(2):127–150. https://doi.org/10.1016/0012-821X(87)90151-8
Allen C, Allton J, Lofgren G, Righter K, Zolensky M (2011) Curating NASA’s extraterrestrial samples—past, present, and future. Geochemistry 71(1):1–20. https://doi.org/10.1016/j.chemer.2010.12.003
Almayrac MG, Broadley MW, Bekaert DV, Hofmann A, Marty B (2021) Possible discontinuous evolution of atmospheric xenon suggested by Archean barites. Chem Geol 581:120,405. https://doi.org/10.1016/j.chemgeo.2021.120405
Altwegg K, Balsiger H, Bar-Nun A, Berthelier JJ, Bieler A, Bochsler P, Briois C, Calmonte U, Combi M, De Keyser J, Eberhardt P, Fiethe B, Fuselier S, Gasc S, Gombosi TI, Hansen KC, Hässig M, Jackel A, Kopp E, Korth A, LeRoy L, Mall U, Marty B, Mousis O, Neefs E, Owen T, Reme H, Rubin M, Semon T, Tzou CY, Waite H, Wurz P (2015) 67P/Churyumov-Gerasimenko, a Jupiter family comet with a high D/H ratio. Science 347(6220):3. https://doi.org/10.1126/science.1261952
Antonelli MA, Kim ST, Peters M, Labidi J, Cartigny P, Walker RJ, Lyons JR, Hoek J, Farquhar J (2014) Early inner solar system origin for anomalous sulfur isotopes in differentiated protoplanets. Proc Natl Acad Sci 111(50):17,749–17,754. https://doi.org/10.1073/pnas.1418907111
Ardoin L, Broadley M, Almayrac M, Avice G, Byrne D, Tarantola A, Lepland A, Saito T, Komiya T, Shibuya T, Marty B (2022) The end of the isotopic evolution of atmospheric xenon. Geochem Perspect Lett 20:43–47. https://doi.org/10.7185/geochemlet.2207
Arevalo R, Ni Z, Danell RM (2020) Mass spectrometry and planetary exploration: a brief review and future projection. J Mass Spectrom 55(1):e4454. https://doi.org/10.1002/jms.4454
Atreya SK, Trainer MG, Franz HB, Wong MH, Manning HLK, Malespin CA, Mahaffy PR, Conrad PG, Brunner AE, Leshin LA, Jones JH, Webster CR, Owen TC, Pepin RO, Navarro-Gonzalez R (2013) Primordial argon isotope fractionation in the atmosphere of Mars measured by the SAM instrument on Curiosity and implications for atmospheric loss. Geophys Res Lett 40(21):5605–5609. https://doi.org/10.1002/2013GL057763
Avice G, Marty B (2020) Perspectives on atmospheric evolution from noble gas and nitrogen isotopes on Earth, Mars & Venus. Space Sci Rev 216(3):36. https://doi.org/10.1007/s11214-020-00655-0.
Avice G, Marty B, Burgess R (2017) The origin and degassing history of the Earth’s atmosphere revealed by Archean xenon. Nat Commun 8:15,455. https://doi.org/10.1038/ncomms15455
Avice G, Bekaert DV, Chennaoui Aoudjehane H, Marty B (2018a) Noble gases and nitrogen in Tissint reveal the composition of the Mars atmosphere. Geochem Perspect Lett 6:11–16. https://doi.org/10.7185/geochemlet.1802
Avice G, Marty B, Burgess R, Hofmann A, Philippot P, Zahnle K, Zakharov D (2018b) Evolution of atmospheric xenon and other noble gases inferred from Archean to Paleoproterozoic rocks. Geochim Cosmochim Acta 232:82–100. https://doi.org/10.1016/j.gca.2018.04.018
Avice G, Moreira M, Gilmour JD (2020) Xenon isotopes identify large-scale nucleosynthetic heterogeneities across the solar system. Astrophys J 889(1):68. https://doi.org/10.3847/1538-4357/ab5f0c
Baines KH, Atreya SK, Bullock MA, Grinspoon DH, Mahaffy P, Russell CT, Schubert G, Zahnle K (2013) The atmospheres of the terrestrial planets: clues to the origins and early evolution of Venus, Earth, and Mars. In: Comparative climatology of terrestrial planets. University of Arizona Press, Tucson, pp 1–28. http://muse.jhu.edu/books/9780816599752/9780816599752-13.pdf
Bar-Nun A, Owen T (1998) Trapping of gases in water ice and consequences to comets and the atmospheres of the inner planets. In: Solar system ices. Springer, Dordrecht, pp 353–366. https://doi.org/10.1007/978-94-011-5252-5_15
Baroni M, Thiemens MH, Delmas RJ, Savarino J (2007) Mass-independent sulfur isotopic compositions in stratospheric volcanic eruptions. Science 315(5808):84–87. https://doi.org/10.1126/science.1131754
Bekaert DV, Broadley MW, Delarue F, Avice G, Robert F Marty B (2018) Archean kerogen as a new tracer of atmospheric evolution: implications for dating the widespread nature of early life. Sci Adv 4(2):eaar2091. https://doi.org/10.1126/sciadv.aar2091
Bekaert DV, Broadley MW, Marty B (2020) The origin and fate of volatile elements on Earth revisited in light of noble gas data obtained from comet 67P/Churyumov-Gerasimenko. Sci Rep 10(1):5796. https://doi.org/10.1038/s41598-020-62650-3
Bernatowicz TJ, Hagee BE (1987) Isotopic fractionation of Kr and Xe implanted in solids at very low energies. Geochim Cosmochim Acta 51(6):1599–1611. https://doi.org/10.1016/0016-7037(87)90341-3
Bertaux JL, Montmessin F (2001) Isotopic fractionation through water vapor condensation: the Deuteropause, a cold trap for deuterium in the atmosphere of Mars. J Geophys Res, Planets 106(E12):32,879–32,884. https://doi.org/10.1029/2000JE001358
Bertaux JL, Vandaele AC, Korablev O, Villard E, Fedorova A, Fussen D, Quémerais E, Belyaev D, Mahieux A, Montmessin F, Muller C, Neefs E, Nevejans D, Wilquet V, Dubois JP, Hauchecorne A, Stepanov A, Vinogradov I, Rodin A, the SPICAV/SOIR team (2007) A warm layer in Venus’ cryosphere and high-altitude measurements of HF, HCl, H2O and HDO. Nature 450(7170):646–649. https://doi.org/10.1038/nature05974
Broadley M, Byrne D, Ardoin L, Almayrac M, Bekaert D, Marty B (2022) High precision noble gas measurements of hydrothermal quartz reveal variable loss rate of Xe from the Archean atmosphere. Earth Planet Sci Lett 588:117,577. https://doi.org/10.1016/j.epsl.2022.117577
Busemann H, Baur H, Wieler R (2000) Primordial noble gases in “phase Q” in carbonaceous and ordinary chondrites studied by closed-system stepped etching. Meteorit Planet Sci 35(5):949–973. https://doi.org/10.1111/j.1945-5100.2000.tb01485.x
Cassata WS (2017) Meteorite constraints on Martian atmospheric loss and paleoclimate. Earth Planet Sci Lett 479:322–329. https://doi.org/10.1016/j.epsl.2017.09.034
Cassata WS, Zahnle KJ, Samperton KM, Stephenson PC, Wimpenny J (2022) Xenon isotope constraints on ancient Martian atmospheric escape. Earth Planet Sci Lett 580:117,349. https://doi.org/10.1016/j.epsl.2021.117349
Catling DC, Kasting JF (2017) Atmospheric evolution on inhabited and lifeless worlds. Cambridge University Press, Cambridge. https://doi.org/10.1017/9781139020558
Catling DC, Zahnle KJ (2020) The Archean atmosphere. Sci Adv 6(9):eaax1420. https://doi.org/10.1126/sciadv.aax1420
Chakraborty S, Jackson TL, Ahmed M, Thiemens MH (2013) Sulfur isotopic fractionation in vacuum UV photodissociation of hydrogen sulfide and its potential relevance to meteorite analysis. Proc Natl Acad Sci 110(44):17,650–17,655. https://doi.org/10.1073/pnas.1213150110
Chassefière E, Wieler R, Marty B, Leblanc F (2012) The evolution of Venus present state of knowledge and future exploration. Planet Space Sci 63–64(C):15–23. https://doi.org/10.1016/j.pss.2011.04.007
Clayton RN, Mayeda TK (1983) Oxygen isotopes in eucrites, shergottites, nakhlites, and chassignites. Earth Planet Sci Lett 62(1):1–6. https://doi.org/10.1016/0012-821X(83)90066-3
Clayton RN, Grossman L, Mayeda TK (1973) A component of primitive nuclear composition in carbonaceous meteorites. Science 182(4111):485–488. https://doi.org/10.1126/science.182.4111.485
Conrad PG, Malespin CA, Franz HB, Pepin RO, Trainer MG, Schwenzer SP, Atreya SK, Freissinet C, Jones JH, Manning H, Owen T, Pavlov AA, Wiens RC, Wong MH, Mahaffy PR (2016) In situ measurement of atmospheric krypton and xenon on Mars with Mars Science Laboratory. Earth Planet Sci Lett 454:1–9. https://doi.org/10.1016/j.epsl.2016.08.028
COSPAR (2020) COSPAR policy on planetary protection. Space Res Today 208:10–22. https://doi.org/10.1016/j.srt.2020.07.009
Crosson ER, Ricci KN, Richman BA, Chilese FC, Owano TG, Provencal RA, Todd MW, Glasser J, Kachanov AA, Paldus BA, Spence TG, Zare RN (2002) Stable isotope ratios using cavity ring-down spectroscopy: determination of 13C/12C for carbon dioxide in human breath. Anal Chem 74(9):2003–2007. https://doi.org/10.1021/ac025511d
Crowther SA, Gilmour JD (2013) The Genesis solar xenon composition and its relationship to planetary xenon signatures. Geochim Cosmochim Acta 123(C):17–34. https://doi.org/10.1016/j.gca.2013.09.007
Dauphas N, Morbidelli A (2014) Geochemical and planetary dynamical views on the origin of Earth’s atmosphere and oceans. In: Treatise on geochemistry, 2nd edn. Elsevier, Oxford, pp 1–35. http://www.sciencedirect.com/science/article/pii/B9780080959757013012
Dauphas N, Pourmand A (2011) Hf–W–Th evidence for rapid growth of Mars and its status as a planetary embryo. Nature 473(7348):489–492. https://doi.org/10.1038/nature10077
Donahue TM, Pollack JB (1983) Origin and evolution of the atmosphere of Venus. In: Venus. University of Arizona Press, Tucson, pp 1003–1036
Donahue TM, Hoffman JH, Hodges RR, Watson AJ (1982) Venus was wet: a measurement of the ratio of deuterium to hydrogen. Science 216(4546):630–633. https://doi.org/10.1126/science.216.4546.630
Dottin JW, Farquhar J, Labidi J (2018a) Multiple sulfur isotopic composition of main group pallasites support genetic links to IIIAB iron meteorites. Geochim Cosmochim Acta 224:276–281. https://doi.org/10.1016/j.gca.2018.01.013
Dottin JW, Labidi J, Farquhar J, Piccoli P, Liu MC, McKeegan KD (2018b) Evidence for oxidation at the base of the nakhlite pile by reduction of sulfate salts at the time of lava emplacement. Geochim Cosmochim Acta 239:186–197. https://doi.org/10.1016/j.gca.2018.07.029
Fegley B (2014) Venus. In: Treatise on geochemistry. Elsevier, Amsterdam, pp 127–148. https://doi.org/10.1016/B978-0-08-095975-7.00122-4
Franz HB, Kim ST, Farquhar J, Day JMD, Economos RC, McKeegan KD, Schmitt AK, Irving AJ, Hoek J, Iii JD (2014) Isotopic links between atmospheric chemistry and the deep sulphur cycle on Mars. Nature 508(7496):364–368. https://doi.org/10.1038/nature13175
Franz HB, Trainer MG, Malespin CA, Mahaffy PR, Atreya SK, Becker RH, Benna M, Conrad PG, Eigenbrode JL, Freissinet C, Manning HLK, Prats BD, Raaen E, Wong MH (2017) Initial SAM calibration gas experiments on Mars: quadrupole mass spectrometer results and implications. Planet Space Sci 138:44–54. https://doi.org/10.1016/j.pss.2017.01.014
Franz HB, Mahaffy PR, Webster CR, Flesch GJ, Raaen E, Freissinet C, Atreya SK, House CH, McAdam AC, Knudson CA, Archer PD, Stern JC, Steele A, Sutter B, Eigenbrode JL, Glavin DP, Lewis JMT, Malespin CA, Millan M, Ming DW, Navarro-González R, Summons RE (2020) Indigenous and exogenous organics and surface–atmosphere cycling inferred from carbon and oxygen isotopes at Gale crater. Nat Astron 4(5):526–532. https://doi.org/10.1038/s41550-019-0990-x
Füri E, Marty B (2015) Nitrogen isotope variations in the Solar System. Nat Geosci 8(7):1–8. https://doi.org/10.1038/ngeo2451
Garrison DH, Bogard DD (1998) Isotopic composition of trapped and cosmogenic noble gases in several Martian meteorites. Meteorit Planet Sci 33(4):721–736. https://doi.org/10.1111/j.1945-5100.1998.tb01678.x
Garvin JB, Getty SA, Arney GN, Johnson NM, Kohler E, Schwer KO, Sekerak M, Bartels A, Saylor RS, Elliott VE, Goodloe CS, Garrison MB, Cottini V, Izenberg N, Lorenz R, Malespin CA, Ravine M, Webster CR, Atkinson DH, Aslam S, Atreya S, Bos BJ, Brinckerhoff WB, Campbell B, Crisp D, Filiberto JR, Forget F, Gilmore M, Gorius N, Grinspoon D, Hofmann AE, Kane SR, Kiefer W, Lebonnois S, Mahaffy PR, Pavlov A, Trainer M, Zahnle KJ, Zolotov M (2022) Revealing the mysteries of Venus: the DAVINCI mission. Planet Sci J 3(5):117. https://doi.org/10.3847/PSJ/ac63c2
Gautier E, Savarino J, Hoek J, Erbland J, Caillon N, Hattori S, Yoshida N, Albalat E, Albarede F, Farquhar J (2019) 2600-years of stratospheric volcanism through sulfate isotopes. Nat Commun 10(1):127. https://doi.org/10.1038/s41467-019-08357-0
Gillmann C, Chassefière E, Lognonné P (2009) A consistent picture of early hydrodynamic escape of Venus atmosphere explaining present Ne and Ar isotopic ratios and low oxygen atmospheric content. Earth Planet Sci Lett 286(3–4):503–513. https://doi.org/10.1016/j.epsl.2009.07.016
Gillmann C, Golabek GJ, Raymond SN, Schönbächler M, Tackley PJ, Dehant V, Debaille V (2020) Dry late accretion inferred from Venus’s coupled atmosphere and internal evolution. Nat Geosci 13:265–269. https://doi.org/10.1038/s41561-020-0561-x
Gillmann C, Way MJ, Avice G, Golabek GJ, Höning D, Breuer D, Krissansen-Totton J, Lammer H, Plesa AC, Persson M, O’Rourke JG, Scherf M Zolotov MY (2022, this journal) The long-term evolution of the atmosphere of Venus: processes and feedback mechanisms. Space Sci Rev https://doi.org/10.1007/s11214-022-00924-0
Gilmour JD, Lyon IC, Johnston WA, Turner G (1994) RELAX: an ultrasensitive, resonance ionization mass spectrometer for xenon. Rev Sci Instrum 65(3):617–625. https://doi.org/10.1063/1.1145127
Glaze LS, Garvin JB, Robertson B, Johnson NM, Amato MJ, Thompson J, Goodloe C, Everett D (2017) DAVINCI: deep atmosphere Venus investigation of noble gases, chemistry, and imaging. In: 2017 IEEE aerospace conference. IEEE, Big Sky, pp 1–5. https://doi.org/10.1109/AERO.2017.7943923
Greenwood RC, Anand M (2020) What is the oxygen isotope composition of Venus? The scientific case for sample return from Earth’s “Sister” planet. Space Sci Rev 216(4):52. https://doi.org/10.1007/s11214-020-00669-8
Greenwood RC, Barrat JA, Miller MF, Anand M, Dauphas N, Franchi IA, Sillard P, Starkey NA (2018) Oxygen isotopic evidence for accretion of Earth’s water before a high-energy Moon-forming giant impact. Sci Adv 4(3):eaao5928. https://doi.org/10.1126/sciadv.aao5928
Gruchola S, Galli A, Vorburger A, Wurz P (2019) The upper atmosphere of Venus: model predictions for mass spectrometry measurements. Planet Space Sci 170:29–41. https://doi.org/10.1016/j.pss.2019.03.006
Halliday AN (2013) The origins of volatiles in the terrestrial planets. Geochim Cosmochim Acta 105(C):146–171. https://doi.org/10.1016/j.gca.2012.11.015
Heber VS, Wieler R, Baur H, Olinger C, Friedmann TA, Burnett DS (2009) Noble gas composition of the solar wind as collected by the Genesis mission. Geochim Cosmochim Acta 73(24):7414–7432. https://doi.org/10.1016/j.gca.2009.09.013
Heber VS, Baur H, Bochsler P, McKeegan KD, Neugebauer M, Reisenfeld DB, Wieler R, Wiens RC (2012) Isotopic mass fractionation of solar wind: evidence from fast and slow solar wind collected by the Genesis mission. Astrophys J 759(121):1–13. https://doi.org/10.1088/0004-637X/759/2/121
Hoffman JH, Hodges RR, Donahue TM, McElroy MB (1980) Composition of the Venus lower atmosphere from the Pioneer Venus Mass Spectrometer. J Geophys Res Space Phys 85(A13):7882–7890. https://doi.org/10.1029/JA085iA13p07882
Hunten DM, Pepin RO, Walker JC (1987) Mass fractionation in hydrodynamic escape. Icarus 69(3):532–549. https://doi.org/10.1016/0019-1035(87)90022-4
Istomin V, Grechnev K, Kotchnev V (1980) Mass spectrometer measurements of the composition of the lower atmosphere of Venus. In: COSPAR colloquia series, vol 20. Elsevier, Amsterdam, pp 215–218. https://doi.org/10.1016/S0964-2749(13)60044-X
Istomin VG, Grechnev KV, Kochnev VA (1982) Preliminary results of mass-spectrometric measurements on the descent “Venera 13” and “Venera 14” space probes. Pis’ma Astron Zh 8(7):391–398
Istomin VG, Grechnev KV, Kochnev VA (1983) Venera 13 and Venera 14: mass spectrometry of the atmosphere. Kosm Issled 21:410–420
Jakosky BM, Pepin RO (1994) Mars atmospheric loss and isotopic fractionation by solar-wind-induced sputtering and photochemical escape. Icarus 111:271–288
Javoy M, Kaminski E, Guyot F, Andrault D, Sanloup C, Moreira M, Labrosse S, Jambon A, Agrinier P, Davaille A, Jaupart C (2010) The chemical composition of the Earth: enstatite chondrite models. Earth Planet Sci Lett 293(3–4):259–268. https://doi.org/10.1016/j.epsl.2010.02.033
Johnson NM, de Oliveira MRR (2019) Venus atmospheric composition in situData: a compilation. Earth Space Sci 6(7):1299–1318. https://doi.org/10.1029/2018EA000536
Kaib NA, Cowan NB (2015) The feeding zones of terrestrial planets and insights into Moon formation. Icarus 252(C):161–174. https://doi.org/10.1016/j.icarus.2015.01.013
Kaula WM (1999) Constraints on Venus evolution from radiogenic argon. Icarus 139(1):32–39. https://doi.org/10.1006/icar.1999.6082
Kleine T, Budde G, Burkhardt C, Kruijer TS, Worsham EA, Morbidelli A, Nimmo F (2020) The non-carbonaceous–carbonaceous meteorite dichotomy. Space Sci Rev 216(4):55. https://doi.org/10.1007/s11214-020-00675-w
Kuga M, Cernogora G, Marrocchi Y, Tissandier L, Marty B (2017) Processes of noble gas elemental and isotopic fractionations in plasma-produced organic solids: cosmochemical implications. Geochim Cosmochim Acta 217:219–230. https://doi.org/10.1016/j.gca.2017.08.031
Labidi J, Cartigny P, Moreira M (2013) Non-chondritic sulphur isotope composition of the terrestrial mantle. Nature 501(7466):208–211. https://doi.org/10.1038/nature12490
Labidi J, Barry PH, Bekaert DV, Broadley MW, Marty B, Giunta T, Warr O, Sherwood Lollar B, Fischer TP, Avice G, Caracausi A, Ballentine CJ, Halldórsson SA, Stefánsson A, Kurz MD, Kohl IE, Young ED (2020) Hydrothermal 15N15N abundances constrain the origins of mantle nitrogen. Nature 580(7803):367–371. https://doi.org/10.1038/s41586-020-2173-4
Lammer H, Leitzinger M, Scherf M, Odert P, Burger C, Kubyshkina D, Johnstone C, Maindl T, Schäfer C, Güdel M, Tosi N, Nikolaou A, Marcq E, Erkaev N, Noack L, Kislyakova K, Fossati L, Pilat-Lohinger E, Ragossnig F, Dorfi E (2020) Constraining the early evolution of Venus and Earth through atmospheric Ar, Ne isotope and bulk K/U ratios. Icarus 339:113,551. https://doi.org/10.1016/j.icarus.2019.113551
Lammer H, Brasser R, Johansen A, Scherf M, Leitzinger M (2021) Formation of Venus, Earth and Mars: constrained by isotopes. Space Sci Rev 217(1):7. https://doi.org/10.1007/s11214-020-00778-4
Lauretta DS, Lodders K, Fegley B (1997) Experimental simulations of sulfide formation in the solar nebula. Science 277(5324):358–360. https://doi.org/10.1126/science.277.5324.358
Liang MC, Yung YL (2009) Modeling the distribution of H \(_{\textrm{2}}\) O and HDO in the upper atmosphere of Venus. J Geophys Res 114:E00B28. https://doi.org/10.1029/2008JE003095
Limaye SS, Mogul R, Smith DJ, Ansari AH, Słowik GP, Vaishampayan P (2018) Venus’ spectral signatures and the potential for life in the clouds. Astrobiology 18(9):1181–1198. https://doi.org/10.1089/ast.2017.1783
Lodders K (2003) Solar system abundances and condensation temperatures of the elements. Astrophys J 591(2):1220. https://doi.org/10.1086/375492
Mahaffy P (1999) Mass spectrometers developed for planetary missions. In: Ehrenfreund P, Krafft C, Kochan H, Pirronello V (eds) Laboratory astrophysics and space research. Astrophysics and space science library, vol 236. Springer, Dordrecht, pp 355–376. https://doi.org/10.1007/978-94-011-4728-6_13
Mahaffy PR, Niemann HB, Alpert A, Atreya SK, Demick J, Donahue TM, Harpold DN, Owen TC (2000) Noble gas abundance and isotope ratios in the atmosphere of Jupiter from the Galileo Probe Mass Spectrometer. J Geophys Res, Planets 105(E6):15,061–15,071. https://doi.org/10.1029/1999JE001224
Mahaffy PR, Webster CR, Cabane M, Conrad PG, Coll P, Atreya SK, Arvey R, Barciniak M, Benna M, Bleacher L, Brinckerhoff WB, Eigenbrode JL, Carignan D, Cascia M, Chalmers RA, Dworkin JP, Errigo T, Everson P, Franz H, Farley R, Feng S, Frazier G, Freissinet C, Glavin DP, Harpold DN, Hawk D, Holmes V, Johnson CS, Jones A, Jordan P, Kellogg J, Lewis J, Lyness E, Malespin CA, Martin DK, Maurer J, McAdam AC, McLennan D, Nolan TJ, Noriega M, Pavlov AA, Prats B, Raaen E, Sheinman O, Sheppard D, Smith J, Stern JC, Tan F, Trainer M, Ming DW, Morris RV, Jones J, Gundersen C, Steele A, Wray J, Botta O, Leshin LA, Owen T, Battel S, Jakosky BM, Manning H, Squyres S, Navarro-González R, McKay CP, Raulin F, Sternberg R, Buch A, Sorensen P, Kline-Schoder R, Coscia D, Szopa C, Teinturier S, Baffes C, Feldman J, Flesch G, Forouhar S, Garcia R, Keymeulen D, Woodward S, Block BP, Arnett K, Miller R, Edmonson C, Gorevan S, Mumm E (2012) The sample analysis at Mars investigation and instrument suite. Space Sci Rev 170(1–4):401–478. https://doi.org/10.1007/s11214-012-9879-z
Mahieux A, Vandaele AC, Robert S, Wilquet V, Drummond R, Montmessin F, Bertaux JL (2012) Densities and temperatures in the Venus mesosphere and lower thermosphere retrieved from SOIR on board Venus Express: carbon dioxide measurements at the Venus terminator. J Geophys Res, Planets 117(E07001):1–15. https://doi.org/10.1029/2012JE004058
Marrocchi Y, Avice G, Estrade N (2015) Multiple carriers of Q noble gases in primitive meteorites. Geophys Res Lett 42:2093–2099. https://doi.org/10.1002/(ISSN)1944-8007
Marty B (2012) The origins and concentrations of water, carbon, nitrogen and noble gases on Earth. Earth Planet Sci Lett 313–314:56–66. https://doi.org/10.1016/j.epsl.2011.10.040
Marty B, Avice G, Sano Y, Altwegg K, Balsiger H, Hässig M, Morbidelli A, Mousis O, Rubin M (2016) Origins of volatile elements (H, C, N, noble gases) on Earth and Mars in light of recent results from the ROSETTA cometary mission. Earth Planet Sci Lett 441:91–102
Marty B, Altwegg K, Balsiger H, Bar-Nun A, Bekaert DV, Berthelier JJ, Bieler A, Briois C, Calmonte U, Combi M, De Keyser J, Fiethe B, Fuselier SA, Gasc S, Gombosi TI, Hansen KC, Hässig M, Jackel A, Kopp E, Korth A, Le Roy L, Mall U, Mousis O, Owen T, Reme H, Rubin M, Semon T, Tzou CY, Waite JH, Wurz P (2017) Xenon isotopes in 67P/Churyumov-Gerasimenko show that comets contributed to Earth’s atmosphere. Science 356(6342):1069–1072. https://doi.org/10.1126/science.aal3496
Marty B, Bekaert DV, Broadley MW, Jaupart C (2019) Geochemical evidence for high volatile fluxes from the mantle at the end of the Archaean. Nature 575(7783):485–488. https://doi.org/10.1038/s41586-019-1745-7
Mathew KJ, Marti K (2001) Early evolution of Martian volatiles: nitrogen and noble gas components in ALH84001 and Chassigny. J Geophys Res 106(E1):1401–1422. https://doi.org/10.1029/2000JE001255
Mathew KJ, Kim JS, Marti K (1998) Martian atmospheric and indigenous components of xenon and nitrogen in SNC meteorites. Meteorit Planet Sci 33:655–664
McCubbin FM, Barnes JJ (2019) Origin and abundances of H2O in the terrestrial planets, Moon, and asteroids. Earth Planet Sci Lett 526:115,771. https://doi.org/10.1016/j.epsl.2019.115771
McCubbin FM, Herd CDK, Yada T, Hutzler A, Calaway MJ, Allton JH, Corrigan CM, Fries MD, Harrington AD, McCoy TJ, Mitchell JL, Regberg AB, Righter K, Snead CJ, Tait KT, Zolensky ME, Zeigler RA (2019) Advanced curation of astromaterials for planetary science. Space Sci Rev 215(8):48. https://doi.org/10.1007/s11214-019-0615-9
McElroy MB, Yung YL, Nier AO (1976) Isotopic composition of nitrogen: implications for the past history of Mars’ atmosphere. Science 194(4260):70–72. https://doi.org/10.1126/science.194.4260.70
McKeegan KD, Kallio APA, Heber VS, Jarzebinski G, Mao PH, Coath CD, Kunihiro T, Wiens RC, Nordholt JE, Moses RW, Reisenfeld DB, Jurewicz AJG, Burnett DS (2011) The oxygen isotopic composition of the Sun inferred from captured solar wind. Science 332(6037):1528–1532. https://doi.org/10.1126/science.1204636
Meshik A, Hohenberg C, Pravdivtseva O, Burnett D (2014) Heavy noble gases in solar wind delivered by Genesis mission. Geochim Cosmochim Acta 127(C):326–347. https://doi.org/10.1016/j.gca.2013.11.030
Meshik A, Pravdivtseva O, Burnett D (2020) Refined composition of Solar Wind xenon delivered by Genesis NASA mission: comparison with xenon captured by extraterrestrial regolith soils. Geochim Cosmochim Acta 276:289–298. https://doi.org/10.1016/j.gca.2020.03.001
Moeller RC, Jandura L, Rosette K, Robinson M, Samuels J, Silverman M, Brown K, Duffy E, Yazzie A, Jens E, Brockie I, White L, Goreva Y, Zorn T, Okon A, Lin J, Frost M, Collins C, Williams JB, Steltzner A, Chen F, Biesiadecki J (2021) The sampling and caching subsystem (SCS) for the scientific exploration of Jezero crater by the Mars 2020 perseverance rover. Space Sci Rev 217(1):5. https://doi.org/10.1007/s11214-020-00783-7
Musselwhite DS, Drake MJ, Swindle TD (1991) Early outgassing of Mars supported by differential water solubility of iodine and xenon. Nature 352(6337):697–699. https://doi.org/10.1038/352697a0
Niemann HB, Harpold DN, Atreya SK, Carignan GR, Hunten DM (1992) Galileo probe mass spectrometer experiment. Space Sci Rev 60:111–142
O’Brien DP, Izidoro A, Jacobson SA, Raymond SN, Rubie DC (2018) The delivery of water during terrestrial planet formation. Space Sci Rev 214(1):47. https://doi.org/10.1007/s11214-018-0475-8
Okabe H (1978) Photochemistry of small molecules. Wiley, New York
Olson PL, Sharp ZD (2019) Nebular atmosphere to magma ocean: a model for volatile capture during Earth accretion. Phys Earth Planet Inter 294:106294. https://doi.org/10.1016/j.pepi.2019.106294
O’Rourke JG, Korenaga J (2015) Thermal evolution of Venus with argon degassing. Icarus 260:128–140. https://doi.org/10.1016/j.icarus.2015.07.009
Ott U (1988) Noble gases in SNC meteorites: Shergotty, Nakhla, Chassigny. Geochim Cosmochim Acta 52:1937–1948. https://doi.org/10.1016/0016-7037(88)90017-8
Owen T, Biemann K, Rushneck DR, Biller JE, Howarth DW, Lafleur AL (1977) The composition of the atmosphere at the surface of Mars. J Geophys Res 82(28):4635–4639. https://doi.org/10.1029/JS082i028p04635
Ozima M, Podosek FA (2002) Noble gas geochemistry, 2nd edn. Cambridge University Press, Cambridge
Parai R, Mukhopadhyay S, Tucker JM, Pető MK (2019) The emerging portrait of an ancient, heterogeneous and continuously evolving mantle plume source. Lithos 346–347:105,153. https://doi.org/10.1016/j.lithos.2019.105153
Pepin RO (1991) On the origin and early evolution of terrestrial planet atmospheres and meteoritic volatiles. Icarus 92(1):2–79. https://doi.org/10.1016/0019-1035(91)90036-s
Pepin RO (1994) The hunt for U-xenon. Meteoritics 29:568–569
Pepin RO, Porcelli D (2002) Origin of noble gases in the terrestrial planets. Rev Mineral Geochem 47(1):191–246. https://doi.org/10.2138/rmg.2002.47.7
Peplowski PN, Lawrence DJ, Wilson JT (2020) Chemically distinct regions of Venus’s atmosphere revealed by measured N2 concentrations. Nat Astron 4:947–950. https://doi.org/10.1038/s41550-020-1079-2
Péron S, Mukhopadhyay S (2022) Krypton in the Chassigny meteorite shows Mars accreted chondritic volatiles before nebular gases. Science 377:320–324. https://doi.org/10.1126/science.abk1175
Péron S, Moreira M, Agranier A (2018) Origin of light noble gases (He, Ne, and Ar) on Earth: a review. Geochem Geophys Geosyst 461(4):1227. https://doi.org/10.1002/2017GC007388
Péron S, Mukhopadhyay S, Kurz MD, Graham DW (2021) Deep-mantle krypton reveals Earth’s early accretion of carbonaceous matter. Nature 600(7889):462–467. https://doi.org/10.1038/s41586-021-04092-z
Piani L, Marrocchi Y, Rigaudier T, Vacher LG, Thomassin D, Marty B (2020) Earth’s water may have been inherited from material similar to enstatite chondrite meteorites. Science 369(6507):1110–1113. https://doi.org/10.1126/science.aba1948
Porcelli D, Ballentine CJ (2002) Models for distribution of terrestrial noble gases and evolution of the atmosphere. Rev Mineral Geochem 47(1):411–480. https://doi.org/10.2138/rmg.2002.47.11
Porcelli D, Ballentine CJ, Wieler R (2002) An overview of noble gas geochemistry and cosmochemistry. Rev Mineral Geochem 47(1):1–19. https://doi.org/10.2138/rmg.2002.47.1
Pujol M, Marty B, Burnard P, Philippot P (2009) Xenon in Archean barite: weak decay of 130Ba, mass-dependent isotopic fractionation and implication for barite formation. Geochim Cosmochim Acta 73(22):6834–6846. https://doi.org/10.1016/j.gca.2009.08.002
Pujol M, Marty B, Burgess R (2011) Chondritic-like xenon trapped in Archean rocks: a possible signature of the ancient atmosphere. Earth Planet Sci Lett 308(3–4):298–306. https://doi.org/10.1016/j.epsl.2011.05.053
Rabinovitch J, Borner A, Gallis MA, Sotin C (2019) Hypervelocity noble gas sampling in the upper atmosphere of Venus. In: AIAA aviation 2019 forum. American Institute of Aeronautics and Astronautics, Dallas. https://doi.org/10.2514/6.2019-3223
Rao M (2002) Neutron capture isotopes in the Martian regolith and implications for Martian atmospheric noble gases. Icarus 156(2):352–372. https://doi.org/10.1006/icar.2001.6809
Rodgers D, Gilmore M, Sweetser T, Cameron J, Chen G-S, Cutts J, Gershman R, Hall J, Kerzhanovich V, McRonald A, Nilsen E, Petrick W, Sauer C, Wilcox B, Yavrouian A, Zimmerman W (2000) Venus sample return. A hot topic. In: 2000 IEEE aerospace conference. Proceedings (Cat. No. 00TH8484), vol 7. IEEE, Big Sky, pp 473–484. https://doi.org/10.1109/AERO.2000.879315
Salvador A, Avice G, Breuer D, Gillmann C, Jacobson SA, Lammer H, Marcq E, Raymond SN, Sakuraba H, Scherf M, Way MJ (2022, this journal) Magma ocean, water, and the early atmosphere of Venus. Space Sci Rev
Sotin C, Avice G, Baker J, Freeman T, Madzunkov SM, Stevenson T, Arora N, Darrach MR, Lightsey G, Marty B (2018) Cupid’s arrow: a small satellite to measure noble gases in Venus atmosphere. In: 49th lunar and planetary science conference
Sweetser T, Peterson C, Nilsen E, Gershman B (2003) Venus sample return missions—a range of science, a range of costs. Acta Astronaut 52(2–6):165–172. https://doi.org/10.1016/S0094-5765(02)00153-4
Swindle TD, Caffee MW, Hohenberg CM (1986) Xenon and other noble gases in shergottites. Geochim Cosmochim Acta 50(6):1001–1015. https://doi.org/10.1016/0016-7037(86)90381-9
Takaoka N (1972) An interpretation of general anomalies of xenon and the isotopic composition of primitive xenon. J Mass Spectrom Soc Jpn 20(4):287–302. https://doi.org/10.5702/massspec1953.20.287
Tarsitano CG, Webster CR (2007) Multilaser Herriott cell for planetary tunable laser spectrometers. Appl Opt 46(28):6923. https://doi.org/10.1364/AO.46.006923
Thiemens MH (2006) History and applications of mass-independent isotope effects. Annu Rev Earth Planet Sci 34(1):217–262. https://doi.org/10.1146/annurev.earth.34.031405.125026
Tian F (2015) Atmospheric escape from solar system terrestrial planets and exoplanets. Annu Rev Earth Planet Sci 43:459–476. https://doi.org/10.1146/annurev-earth-060313-054834
von Zahn U, Fricke KH, Hunten DM, Krankowsky D, Mauersberger K, Nier AO (1980) The upper atmosphere of Venus during morning conditions. J Geophys Res 85(A13):7829–7840
von Zahn U, Kumar S, Niemann H, Prinn R (1983) Composition of the Venus atmosphere. In: Venus. The University of Arizona Press, Tucson, p 299
Way MJ, Del Genio AD, Kiang NY, Sohl LE, Grinspoon DH, Aleinov I, Kelley M, Clune T (2016) Was Venus the first habitable world of our solar system? Geophys Res Lett 43:8376–8383. https://doi.org/10.1002/(ISSN)1944-8007
Weiss BP, Bai XN, Fu RR (2021) History of the solar nebula from meteorite paleomagnetism. Sci Adv 7(1):eaba5967. https://doi.org/10.1126/sciadv.aba5967
Widemann T, Smrekar SE, Garvin JB, Straume-Lindner AG, Ocampo AC, Voirin T, Hensley S, Darby Dyar M, Whitten JL, Nunes DC, Getty SA, Arney GN, Johnson NM, Kohler E, Spohn T, O’Rourke JG, Wilson C, Way MJ, Ostberg C, Westall F, Höning D, Jacobson S, Salvador A, Avice G, Carter L, Gilmore M, Ghail R, Helbert J, Byrne P, Herrick RR, Izenberg N, Marcq E, Rolf T, Weller M, Gillmann C, Korablev O, Zelenyi L, Zasova L, Gorinov D (2022) Venus evolution through time: key science questions, selected mission concepts and future investigations. Space Sci Rev
Wieler R (2002) Noble gases in the solar system. Rev Mineral Geochem 47(1):21–70. https://doi.org/10.2138/rmg.2002.47.2
Wieler R (2014) Noble gas mass spectrometry. In: Treatise on geochemistry, 2nd edn. Elsevier, Oxford, pp 355–373. https://doi.org/10.1016/B978-0-08-095975-7.01428-5
Wieler R, Anders E, Baur H, Lewis RS, Signer P (1992) Characterisation of Q-gases and other noble gas components in the Murchison meteorite. Geochim Cosmochim Acta 56(7):2907–2921. https://doi.org/10.1016/0016-7037(92)90367-R
Wong MH, Atreya SK, Mahaffy PN, Franz HB, Malespin C, Trainer MG, Stern JC, Conrad PG, Manning HLK, Pepin RO, Becker RH, McKay CP, Owen TC, Navarro-González R, Jones JH, Jakosky BM, Steele A (2013) Isotopes of nitrogen on Mars: atmospheric measurements by Curiosity’s mass spectrometer. Geophys Res Lett 40(23):6033–6037. https://doi.org/10.1002/2013GL057840
Yada T, Fujimura A, Abe M, Nakamura T, Noguchi T, Okazaki R, Nagao K, Ishibashi Y, Shirai K, Zolensky ME, Sandford S, Okada T, Uesugi M, Karouji Y, Ogawa M, Yakame S, Ueno M, Mukai T, Yoshikawa M, Kawaguchi J (2014) Hayabusa-returned sample curation in the Planetary Material Sample Curation Facility of JAXA: Hayabusa return sample curation in JAXA. Meteorit Planet Sci 49(2):135–153. https://doi.org/10.1111/maps.12027
Yeung LY, Li S, Kohl IE, Haslun JA, Ostrom NE, Hu H, Fischer TP, Schauble EA, Young ED (2017) Extreme enrichment in atmospheric 15N15N. Sci Adv 3(11):eaao6741. https://doi.org/10.1126/sciadv.aao6741
Young ED, Galy A, Nagahara H (2002) Kinetic and equilibrium mass-dependent isotope fractionation laws in nature and their geochemical and cosmochemical significance. Geochim Cosmochim Acta 66(6):1095–1104. https://doi.org/10.1016/S0016-7037(01)00832-8
Young ED, Kohl IE, Warren PH, Rubie DC, Jacobson SA, Morbidelli A (2016) Oxygen isotopic evidence for vigorous mixing during the Moon-forming giant impact. Science 351(6272):493–496. https://doi.org/10.1126/science.aad0525
Yung YL, Demore W (1982) Photochemistry of the stratosphere of Venus: implications for atmospheric evolution. Icarus 51(2):199–247. https://doi.org/10.1016/0019-1035(82)90080-X
Zahnle K, Kasting JF, Pollack JB (1990) Mass fractionation of noble gases in diffusion-limited hydrodynamic hydrogen escape. Icarus 84(2):502–527. https://doi.org/10.1016/0019-1035(90)90050-J
Zahnle KJ, Gacesa M, Catling DC (2019) Strange messenger: a new history of hydrogen on Earth, as told by xenon. Geochim Cosmochim Acta 244:56–85. https://doi.org/10.1016/j.gca.2018.09.017
Acknowledgements
G.A. acknowledges the French Centre National d’Etudes Spatiales (CNES) for its funding support for Venus related studies. We warmly thank Tilman Spohn and members of the team at ISSI (International Space Science Institute) in Bern for their hospitality. We also thank the two anonymous reviewers for their comments and the editors for their careful handling of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Venus: Evolution Through Time
Edited by Colin F. Wilson, Doris Breuer, Cédric Gillmann, Suzanne E. Smrekar, Tilman Spohn and Thomas Widemann
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Avice, G., Parai, R., Jacobson, S. et al. Noble Gases and Stable Isotopes Track the Origin and Early Evolution of the Venus Atmosphere. Space Sci Rev 218, 60 (2022). https://doi.org/10.1007/s11214-022-00929-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11214-022-00929-9