Abstract
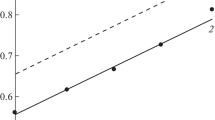
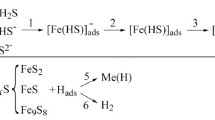
The effect of thiourea (0.5–10 mM) on the kinetics of the hydrogen evolution reaction (HER) at iron and the hydrogen transport through a steel membrane out of ethylene glycol (containing 2 and 10 wt % H2O) and aqueous solutions containing HCl (0.1–0.99 M) with a constant ionic strength equal to unity is studied in parallel experiments. The presence of 0.5 mM of thiourea in the solutions raises the overvoltage of hydrogen evolution, while a subsequent increase in its concentration does not effect the HER kinetics. The dependence of the flux of hydrogen diffusion through the membrane on the thiourea content passes through a maximum.
Similar content being viewed by others
REFERENCES
Vigdorovich, V.I., D'yachkova, T.P., and Alekhina, O.V., Vestn. Tambov. Univ.: Est. Khim. Nauki, 2003, vol. 8, p. 791.
Fil'ko, A.I., in: Ingibitory korrozii metallov (Inhibitors of Metal Corrosion), Moscow: Mosk. Gos. Ped. Inst., 1960, p. 63.
Balezin, S.A. and Solovei, D.Ya., Uch. Zap. Mosk. Gos. Ped. Inst., 1951, vol. 63, p. 151.
Vigdorovich, V.I. and Tsygankova, L.E., Zh. Fiz. Khim., 1976, vol. 50, p. 2968.
Tsygankova, L.E., Vigdorovich, V.I., and Berdnikova, G.G., Zh. Fiz. Khim., 1998, vol. 72, p. 841.
Kardash, N.V. and Batrakov, V.V., Zashch. Met., 1995, vol. 31, p. 441.
Vigdorovich, V.I., D'yachkova, T.P., and Tsygankova, L.E., Elektrokhimiya, 2001, vol. 37, p. 1437.
Tsygankova, L.E., Vigdorovich, V.I., and Danilova, T.S., Khim. Khim. Tekhnol., 1976, vol. 19, p. 1557.
Antropov, L.I., Teoreticheskaya elektrokhimiya (Theoretical Electrochemistry), Moscow: Vysshaya Shkola, 1984.
Iyer, R.N., Pickering, H.W., and Zamanzadeh, M., J.Electrochem. Soc., 1989, vol. 136, p. 2463.
Pickering, H.W. and Iyer, R.N, J. Electrochem. Soc., 1990, vol. 137, p. 3512.
Iyer, R.N., Zamanzadeh, M., and Pickering, H.W., Corrosion, 1990, vol. 46, no.6, p. 46.
Abd Elhamid, M.H., Ateya, B.G., and Pickering, H.W., J. Electrochem. Soc., 1997, vol. 144, p. L58.
Abd Elhamid, M.H., Ateya, B.G., and Pickering, H.W., J. Electrochem. Soc., 2000, vol. 147, p. 2258.
Abd Elhamid, M.H., Ateya, B.G., and Pickering, H.W., J. Electrochem. Soc., 2000, vol. 147, p. 2959.
Khoriuti, D. and Toiya, T., in Solid State Surface Science, Green, M., Ed., New York: Marcel Dekker, 1969.
Toya, T., Ito, T., and Ishi, Sh., Elektrokhimiya, 1978, vol. 14, p. 703.
Vigdorovich, M.V. and Kuznetsov, A.M., Trudy X mezh-region. nauchnotekh. konf. “Problemy khimii i khimicheskoi tekhnologii” (Proc. X Interregion Conf. “Problems in Chemistry and Chemical Technology), Tambov, 2003, pp. 14–18.
Author information
Authors and Affiliations
Corresponding author
Additional information
__________
Translated from Elektrokhimiya, Vol. 41, No. 10, 2005, pp. 1178–1184.
Original Russian Text Copyright © 2005 by Vigdorovich, Tsygankova, Alekhina, D'yachkova.
Rights and permissions
About this article
Cite this article
Vigdorovich, V.I., Tsygankova, L.E., Alekhina, O.V. et al. Effect of Thiourea on the Kinetics of the Hydrogen Evolution Reaction at Iron and the Transport of Hydrogen through a Steel Membrane in Solutions of C2H4(OH)2-H2O-HCl. Russ J Electrochem 41, 1046–1052 (2005). https://doi.org/10.1007/s11175-005-0179-8
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11175-005-0179-8