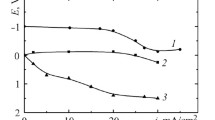
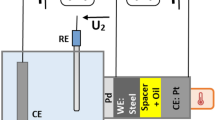
The composition of the working environments was chosen for determining the parameters of hydrogen diffusion through a 20 steel membrane by the Dewanathan–Stahursky method: the anodic cell was filled with a 0.2M KOH+10 g/ dm3 Na2MoO4 solution, and the cathodic cell was filled with a 1M H2SO4 +10 g/ dm3 (NH2)2 CS solution. The effective coefficient of hydrogen diffusion through a membrane made of 20 steel increases in approx. 6 times with an increase in the current density of the cathodic polarization from 0 to 2 A/ dm2 . It takes into account not only the transfer of hydrogen through the membrane but also depends on the types of traps and duration of hydrogen stay in them. The absorption of hydrogen is accompanied by an increase in the defectiveness of the metal structure, which changes the conditions of hydrogen diffusion. The dependence of hydrogen permeability through steel on time was evaluated using the Laplace equation for different probable values of the effective diffusion coefficient and hydrogen concentration. The obtained experimental results agree with theoretical calculations.






Similar content being viewed by others
References
S. K. Yen, and I. B. Huang, “Hydrogen permeation tests in laminates: Application to grain/grain boundary of AISI 430 stainless steel,” Corrosion, 59, Is. 11, 995–1002 (2003). https://doi.org/https://doi.org/10.5006/1.3277523
N. Amokrane, C. Gabrielli, G. Maurin, and L. Mirkova, “Effect of organic additives on hydrogen permeation into an iron membrane studied by frequency analysis techniques,” Electrochim. Acta, 53, Is. 4, 1962–1971(2007). https://doi.org/10.1016/j.electacta.2007.08.053
G. N. Kasatkin, Hydrogen in structural steels [in Russian], Intermet Inzhiniiring, Moscow (2003).
M. C. Khoma, O. E. Narivskyy, V. A. Vynar, N. B. Ratska, R. C. Mardarevych, S. A. Korniy, Ch. B. Vasyliv, and M. R. Chuchman, “Development of new structural elements of gas coolers in atomic and thermal power plants with improved corrosion-mechanic resistance,” Science and Innovation, 17, Is. 6, 50–60 (2021). https://doi.org/10.15407/scine17.06.050
M. A. V. Devanathan, and Z. J. Stachurski, “The mechanism of hydrogen evolution on iron in acid solutions by determination of permeation rates,” Electrochem. Soc., 111, Is.5, 619–623 (1964). https://doi.org/10.1149/1.2426195.
F. Ge, F. Huang, W. Yuan, Z. Peng, J. Liu, and Y. F. Cheng, “Effect of tensile stress on the hydrogen permeation of MS X65 pipeline steel under sulfide films,” Int. J. of Hydrogen Energy, 45, Is. 22, 12419–12431 (2020). https://doi.org/10.1016/j.ijhydene.2020.02.149
C. B. Zheng, H. K. Jiang, and Y. L. Huang, “Hydrogen permeation behavior of X56 steel in simulated atmospheric environment under loading,” Corros. Eng. Sci. Technol., 46, Is. 4, 365–367(2011). https://doi.org/10.1179/147842209X12559428167689
H. M. Nykyforchyn, E. Lunarska, V. I. Kyryliv, and O. V. Maksymiv, “Hydrogen permeability of the surface nanocrystalline structures of carbon steel,” Mater. Sci., 50, No. 5, 67–73 (2015). https://doi.org/https://doi.org/10.1007/s11003-015-9774-3
B. Luo, P. Bai, T. An, S. Zhang, X. Wen, L. Chen, and S. Zheng, “Vapor-deposited iron sulfide films as a novel hydrogen permeation barrier for steel: Deposition condition, defect effect, and hydrogen diffusion mechanism,” Int. J. of Hydrogen Energy, 43, Is. 32, 15564–15574 (2018). https://doi.org/10.1016/j.ijhydene.2018.06.042
H. Addach, P. Berçot, M. Rezrazi, and J. Takadoum, “Study of the electrochemical permeation of hydrogen in iron,” Corros. Sci., 51, Is. 2, 263–267 (2009). https://doi.org/10.1016/j.corsci.2008.10.024
X. Wen, P. Bai, B. Luo, S. Zheng, and C. Chen, “Review of recent progress in the study of corrosion products of steels in a hydrogen sulfide environment,” Corros. Sci., 139, 124–140 (2018). https://doi.org/https://doi.org/10.1016/j.corsci.2018.05.002
Y. Qi, H. Luo, S. Zheng, C. Chen, and D. Wang, “Effect of immersion time on the hydrogen content and tensile properties of A350LF2 steel exposed to hydrogen sulphide environments,” Corros. Sci., 69, 164–174 (2013). https://doi.org/https://doi.org/10.1016/j.corsci.2012.11.038
S. J. Kim, H. G. Jung, and K. Y. Kim, “Effect of tensile stress in elastic and plastic range on hydrogen permeation of high-strength steel in sour environment,” Electrochim. Acta, 78, 139–146 (2012). https://doi.org/https://doi.org/10.1016/j.electacta.2012.05.147
C. Plennevaux, J. Kittel, M. Frégonèse, B. Normand, F. Ropital, F. Grosjean, and T. Cassagne, “Contribution of CO2 on hydrogen evolution and hydrogen permeation in low alloy steels exposed to H2S environment,” Electrochemistry Communications, 26, Is. 1, 17–20 (2013). https://doi.org/https://doi.org/10.1016/j.elecom.2012.10.010
M. S. Khoma, V. A. Vynar, N. B. Ratska, I. O. Patsai, V. I. Pokhmurskii, and R. M. Yurkevych, “Corrosion and tribocorrosion of 07Kh16N6 steel in hydrogen-sulfide media,” Mater. Sci., 58, No. 3, 281–289 (2022). https://doi.org/https://doi.org/10.1016/j.corsci.2008.10.024
M. S. Khoma, Kh. B., Vasyliv, and M. R. Chuchman, “Influence of the Hydrogen Sulfide Concentration on the Corrosion and Hydrogenation of Pipe Steels (A Survey),” Mater. Sci., 57, No. 3, 308–318 (2021). https://doi.org/10.1007/s11003-021-00546-x
S. H. Wang, W. C. Luu, K. F. Ho, and J. K. Wu, “Hydrogen permeation in a submerged arc weldment of TMCP steel,” Mat. Chemistry and Phys., 77, Is. 2, 447–454 (2003). https://doi.org/10.1016/S0254-0584(02)00100-1
Method of Measurement of Hydrogen Permeation and Determination of Hydrogen Uptake and Transport in Metals by an Electrochemical Technique, ISO 17081:2004 (2004).
W. Siegl, Hydrogen Trapping in Iron and Iron-Based Alloys, Dissertation, Montan Universität Leoben, Montan (2020).
J. J. M. Jebaraj, D. J. Morrison, and I. I. Suni, “Hydrogen diffusion coefficients through Inconel 718 in different metallurgical conditions,” Corros. Sci., 80, 517–522 (2014). https://doi.org/https://doi.org/10.1016/j.corsci.2013.11.002
M. S. Khoma, V. R. Ivashkiv, S. A. Halaichak, M. R. Chuchman, and Kh. B. Vasyliv, “Influence of the structure of carbon steels on the corrosion, hydrogenation, and corrosion cracking in hydrogen-sulfide media,” Mater. Sci., 55, No. 2, 272–276 (2019). https://doi.org/https://doi.org/10.1007/s11003-019-00299-8
M. M. Shved, Changes in the Operational Properties of Iron and Steel under the Influence of Hydrogen [in Russian], Naukova Dumka, Kyiv (1985).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Fizyko-Khimichna Mekhanika Materialiv, Vol. 59, No. 4, 51–58, July–August, 2023
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khoma, M.S., Ivashkiv, V.R., Chuchman, M.R. et al. Methodological Features of the Study of Hydrogen Permeation Through a Steel Membrane from an Acid Environment. Mater Sci 59, 434–442 (2023). https://doi.org/10.1007/s11003-024-00795-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11003-024-00795-6