Abstract
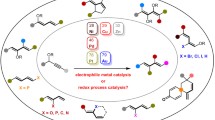
The effect of various factors on the activity and selectivity of palladium N-heterocyclic carbene (NHC) complexes in the telomerization of isoprene with alcohols has been studied. The leaving group of the palladium complex does not affect the results of telomerization of isoprene with methanol, which leads to a predominant formation of the head-to-head product. The use of more fatty alcohols as nucleophiles changes the reaction selectivity toward a preferential formation of the head-to-tail isomers.
Similar content being viewed by others
References
E. J. Smutny, J. Am. Chem. Soc., 1967, 89, 6793; DOI: https://doi.org/10.1021/ja01001a089.
S. Takahashi, T. Shibano, N. Hagihara, Tetrahedron Lett., 1967, 8, 2451; DOI: https://doi.org/10.1016/S0040-4039(00)90830-X.
A. Behr, M. Becker, T. Beckmann, L. Johnen, J. Leschinski, S. Reyer, Angew. Chem., Int. Ed. Eng., 2009, 48, 3598; DOI: https://doi.org/10.1002/anie.200804599.
T. A. Faßbach, A. J. Vorholt, W. Leitner, ChemCatChem, 2019, 11, 1153; DOI: https://doi.org/10.1002/cctc.201801821.
N. Herrmann, D. Vogelsang, A. Behr, T. Seidensticker, ChemCatChem, 2018, 10, 5342; DOI: https://doi.org/10.1002/cctc.201801362.
L. Torrente-Murciano, D. Nielsen, R. Jackstell, M. Beller, K. Cavell, A. Lapkin, Catal. Sci. Technol., 2014, 5, 1206; DOI: https://doi.org/10.1039/C4CY01320D.
R. Jackstell, M. Gómez Andreu, A. Frisch, K. Selvakumar, A. Zapf, H. Klein, A. Spannenberg, D. Röttger, O. Briel, R. Karch, M. Beller, Angew. Chem., Int. Ed. Eng., 2002, 41, 986; DOI: https://doi.org/10.1002/1521-3773(20020315)41:6<986::aid-anie986>3.0.co;2-m.
A. Krotz, F. Vollmüller, G. Stark, M. Beller, Chem. Commun., 2001, 2, 195; DOI: https://doi.org/10.1039/B005885H.
M. S. Viciu, F. K. Zinn, E. D. Stevens, S. P. Nolan, Organometallics, 2003, 22, 3175; DOI: https://doi.org/10.1021/om030337k.
A. Grotevendt, M. Bartolome, D. J. Nielsen, A. Spannenberg, R. Jackstell, K. J. Cavell, L. A. Oro, M. Beller, Tetrahedron Lett., 2007, 48, 9203; DOI: https://doi.org/10.1016/j.tetlet.2007.10.076.
D. Rose, H. Lepper, J. Org. Chem., 1973, 49, 473; DOI: https://doi.org/10.1016/S0022-328X(00)84238-2.
C. U. Pittman, R. M. Hanes, J. J. Yang, J. Mol. Catal., 1982, 15, 377; DOI: https://doi.org/10.1016/0304-5102(82)80030-8.
B. Estrine, S. Bouquillon, F. Hénin, J. Muzart, Appl. Organomet. Chem., 2007, 21, 945; DOI: https://doi.org/10.1002/aoc.1316.
V. Desvergnes-Breuil, C. Pinel, P. Gallezot, Green Chem., 2001, 3, 175; DOI: https://doi.org/10.1039/B103189A.
A. Behr, P. Bahke, B. Klinger, M. Becker, J. Mol. Cat. A: Chem., 2007, 267, 149; DOI: https://doi.org/10.1016/j.molcata.2006.11.043.
N. V. Volchkov, M. B. Lipkind, O. M. Nefedov, Russ. Chem. Bull., 2020, 69, 58; DOI: https://doi.org/10.1007/s11172-020-2724-8.
M. A. Belaya, D. A. Denisov, R. A. Novikov, Yu. V. Tomilov, Russ. Chem. Bull., 2021, 70, 1537; DOI: https://doi.org/10.1007/s11172-021-3249-5.
N. Yoshimura, Applied Homogeneous Catalysis with Organometallic Compounds. A Comprehensive Handbook in Two Volumes., VCH, Weinheim, 1996.
P. W. N. M. van Leeuwen, N. D. Clément, M. J.-L. Tschan, Coord. Chem. Rev., 2011, 255, 1499; DOI: https://doi.org/10.1016/j.ccr.2010.10.009.
B. Cornils, J. Mol. Cat. A: Chem., 1999, 143, 1; DOI: https://doi.org/10.1016/S1381-1169(98)00357-4.
F. Benvenuti, C. Carlini, A. M. Raspolli Galletti, G. Sbrana, M. Marchionna, R. Patrini, J. Mol. Cat. A: Chem., 1999, 137, 49; DOI: https://doi.org/10.1016/S1381-1169(98)00074-0.
K. Kaneda, H. Kurosaki, M. Terasawa, T. Imanaka, S. Teranishi, J. Org. Chem., 1981, 46, 2356; DOI: https://doi.org/10.1021/jo00324a029.
R. Benn, P. W. Jolly, R. Mynott, G. Schenker, Organometallics, 1985, 4, 1136; DOI: https://doi.org/10.1021/om00125a034.
R. Benn, P. W. Jolly, R. Mynott, B. Raspel, G. Schenker, K. P. Schick, G. Schroth, Organometallics, 1985, 4, 1945; DOI: https://doi.org/10.1021/om00130a005.
P. W. Jolly, R. Mynott, B. Raspel, K. P. Schick, Organometallics, 1986, 5, 473; DOI: https://doi.org/10.1021/om00134a014.
L. Völkl, S. Recker, M. Niedermaier, S. Kiermaier, V. Strobel, D. Maschmeyer, D. Cole-Hamilton, W. Marquardt, P. Wasserscheid, M. Haumann, J. Catal., 2015, 329, 547; DOI: https://doi.org/10.1016/j.jcat.2015.06.004.
U. Asghar, A. Masoom, A. Javed, A. Abbas, Am. J. Chem. Eng., 2020, 8, 63; DOI: https://doi.org/10.11648/j.ajche.20200803.12.
R. Jackstell, A. Grotevendt, D. Michalik, L. El Firdoussi, M. Beller, J. Organomet. Chem., 2007, 692, 4737; DOI: https://doi.org/10.1016/j.jorganchem.2007.06.039.
R. R. Aysin, S. S. Bukalov, Russ. Chem. Bull., 2021, 70, 706; DOI: https://doi.org/10.1007/s11172-021-3140-4.
V. A. Glushkov, D. N. Babentsev, M. V. Dmitriev, I. A. Borisova, M. S. Denisov, Russ. Chem. Bull., 2021, 70, 122; DOI: https://doi.org/10.1007/s11172-021-3065-y.
R. C. Nunes, M. H. Araujo, E. N. dos Santos, Cat. Commun., 2007, 8, 1798; DOI: https://doi.org/10.1016/j.catcom.2007.01.033.
S. Harkal, R. Jackstell, F. Nierlich, D. Ortmann, M. Beller, Org. Lett., 2005, 7, 541; DOI: https://doi.org/10.1021/o1048025g.
G. A. Chesnokov, A. A. Ageshina, A. V. Maryanova, S. A. Rzhevskiy, P. S. Gribanov, M. A. Topchiy, M. S. Nechaev, A. F. Asachenko, Russ. Chem. Bull., 2020, 69, 2370; DOI: https://doi.org/10.1007/s11172-020-3028-8.
S. A. Rzhevskiy, M. A. Topchiy, K. A. Lyssenko, A. N. Philippova, M. A. Belaya, A. A. Ageshina, M. V. Bermeshev, M. S. Nechaev, A. F. Asachenko, J. Organomet. Chem., 2020, 912, 121140; DOI: https://doi.org/10.1016/j.jorganchem.2020.121140.
P. B. Dzhevakov, M. A. Topchiy, A. A. Ageshina, L. I. Minaeva, S. A. Rzhevskiy, M. S. Nechaev, S. N. Osipov, A. F. Asachenko, Russ. Chem. Bull., 2020, 69, 2307; DOI: https://doi.org/10.1007/s11172-020-3038-6.
S. A. Rzhevskiy, A. N. Philippova, G. A. Chesnokov, A. A. Ageshina, L. I. Minaeva, M. A. Topchiy, M. S. Nechaev, A. F. Asachenko, Chem. Commun., 2021, 57, 5686; DOI: https://doi.org/10.1039/D1CC01837J.
S. A. Rzhevskiy, M. A. Topchiy, V. N. Bogachev, L. I. Minaeva, I. R. Cherkashchenko, K. V. Lavrov, G. K. Sterligov, M. S. Nechaev, A. F. Asachenko, Mendeleev Commun., 2021, 31, 409; DOI: https://doi.org/10.1016/j.mencom.2021.04.042.
G. A. Kichigina, P. P. Kushch, D. P. Kiryukhin, Russ. Chem. Bull., 2021, 70, 1640; DOI: https://doi.org/10.1007/s11172-021-3265-5.
E. V. Bermesheva, A. I. Voznyak, M. V. Bermeshev, A. F. Asachenko, M. A. Topchiy, M. S. Nechaev, M. P. Filatova, A. P. Khrychikova, Polymer Sci., Series B, 2020, 62, 319; DOI: https://doi.org/10.1134/S1560090420030021.
M. A. Topchiy, S. A. Rzhevskiy, A. A. Ageshina, N. Y. Kirilenko, G. K. Sterligov, D. Y. Mladentsev, D. Y. Paraschuk, S. N. Osipov, M. S. Nechaev, A. F. Asachenko, Mendeleev Commun., 2020, 30, 717; DOI: https://doi.org/10.1016/j.mencom.2020.11.009.
S. A. Rzhevskiy, M. A. Topchiy, V. N. Bogachev, A. A. Ageshina, L. I. Minaeva, G. K. Sterligov, M. S. Nechaev, A. F. Asachenko, Mendeleev Commun., 2021, 31, 478; DOI: https://doi.org/10.1016/j.mencom.2021.07.013.
P. S. Gribanov, E. M. Atoian, A. N. Philippova, M. A. Topchiy, A. F. Asachenko, S. N. Osipov, Eur. J. Org. Chem., 2021, 2021, 1378; DOI: https://doi.org/10.1002/ejoc.202001620.
A. A. Ageshina, G. K. Sterligov, S. A. Rzhevskiy, M. A. Topchiy, G. A. Chesnokov, P. S. Gribanov, E. K. Melnikova, M. S. Nechaev, A. F. Asachenko, M. V. Bermeshev, Dalton Trans., 2019, 48, 3447; DOI: https://doi.org/10.1039/C9DT00216B.
I. Maluenda, M.-T. Chen, D. Guest, S. Mark Roe, M. L. Turner, O. Navarro, Catal. Sci.Technol., 2015, 5, 1447; DOI: https://doi.org/10.1039/C5CY00058K.
I. Maluenda, M.-T. Chen, D. Guest, S. Roe, M. Turner, O. Navarro, Catal. Sci. Technol., 2015, 5; DOI: https://doi.org/10.1039/C5CY00058K.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 5, pp. 940–945, May, 2022.
This work was financially supported by the Russian Science Foundation (Project No. 19-73-10185) using the equipment of the Center for Collective Use “Analytical Center for Problems of Deep Oil Refining and Petrochemistry” of the A. V. Topchiev Institute of Petrochemical Synthesis of the Russian Academy of Sciences (IPCS RAS). Part of the work (development and validation of protocols for determining the content of telomers by GC) was carried out by S. A. Rzhevskiy and M. S. Nechaev within the framework of the Russian state assignment to the IPCS RAS.
No human or animal subjects were used in this research.
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Topchiy, M.A., Ageshina, A.A., Rzhevskiy, S.A. et al. Solvent-free telomerization of isoprene with alcohols catalyzed by palladium(ɪɪ) carbene complexes. Russ Chem Bull 71, 940–945 (2022). https://doi.org/10.1007/s11172-022-3494-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-022-3494-2