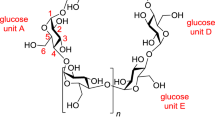
Abstract
This review presents data on advanced research on functionalized cyclodextrins. In particular, metal complexes of cyclodextrin derivatives and their possible applications in chemistry, biology, and medicine are discussed. The advantages and high potential of compounds based on functionalized cyclodextrins are demonstrated.
Similar content being viewed by others
References
H. Dodziuk, Cyclodextrins and their Complexes, Willey-VCH, Warsaw, 2006, 486 p.
T. Endo, Trends Glycosci. Glycotechnol., 2011, 23, 79; DOI: https://doi.org/10.4052/tigg.23.79.
H. Niu, W. Chen, W. Chen, Y. Yun, Q. Zhong, X. Fu, H. Chen, G. Liu, J. Agric. Food Chem., 2019, 67, 12875; DOI: https://doi.org/10.1021/acs.jafc.9b05467.
S. Braga, Biomolecules, 2019, 9, 801; DOI: https://doi.org/10.3390/biom9120801.
M. V. Ambrogio, T. A. Pecorelli, K. Patel, N. M. Khashab, A. Trabolsi, H. A. Khatib, Y. Y. Botros, J. I. Zink, J. F. Stoddart, Org. Lett., 2010, 12, 3304; DOI: https://doi.org/10.1021/ol1101286a.
M. W. Ambrogio, C. R. Thomas, Y.-Li Zhao, J. Zink, J. F. Stoddart, Acc. Chem. Res., 2011, 44, 903; DOI: https://doi.org/10.1021/ar200018x.
K. S. Sharapov, K. V. Zolaeva, V. A. Volynkin, V. T. Panyuskhin, Russ. Chem. Bull., 2019, 68, 507; DOI: https://doi.org/10.1007/s11172-019-2446-y.
R. A. Sharipov, K. S. Sharapov, U. S. Kemelbekov, V. A. Volynkin, V. K. Yu, V. T. Panyushkin, K. D. Praliev, J. Inclusion Phenom. Macrocyclic Chem., 2017, 87, 141; DOI: https://doi.org/10.1007/s10847-016-0685-1.
T. R. Usacheva, D. N. Kabirov, D. A. Alister, M. N. Zavalishin, G. A. Gamov, L. Pham Thi, M. Vu Xuan, D. Nguyen Tuan, Russ. Chem. Bull., 2020, 69, 1692; DOI: https://doi.org/10.1007/s11172-020-2949-6.
S. Fourmentin, G. Crini, E. Lichtfouse, Cyclodextrin Fundamentals, Reactivity and Analysis, Springer, Berlin, 2018, 255 pp.; DOI: https://doi.org/10.1007/978-3-319-76159-6.
D. E. Levy, P. Fuegedi, The Organic Chemistry of Sugars, CRC Press, Boca Raton—London—New York, 2006, 904 pp.
C. J. Bruns, Symmetry, 2019, 11, 1249; DOI: https://doi.org/10.3390/sym11101249.
E. M. M. Del Valle, Process Biochem., 2004, 39, 1033; DOI: https://doi.org/10.1016/s0032-9592(03)00258-9.
S. Immel, J. Brickmann, F. W. Lichtenhaler, Liebigs Ann., 1995, 6, 929; DOI: https://doi.org/10.1002/jlac.1995199506134.
T. Nakagawa, K. Ueno, M. Kashiwa, J. Watanabe, Tetrahedron Lett., 1994, 35, 1921; DOI: https://doi.org/10.1016/S0040-4039(00)73196-0.
D. Ikuta, Y. Hirata, S. Wakamori, H. Shimada, Y. Tomabechi, Y. Kawasaki, K. Ikeuchi, T. Hagimori, S. Matsumoto, H. Yamada, Science, 2019, 364, 674; DOI: https://doi.org/10.1126/science.aaw3053.
M. A. Kapustin, A. S. Chubarova, T. N. Halavatch, V. G. Cigankov, A. M. Bondaruk, V. P. Kurchenko, Tr. Belorus. gos. un-ta [Proc. Belarus. State Univ., Ser. Physiological, Biochemical, and Molecular Biology Sciences], 2016, 11, 73 (in Russian).
M. J. Jozwiakowski, K. A. Connors, Carbohydr. Res., 1985, 143, 51; DOI: https://doi.org/10.1016/S0008-6215(00)90694-3.
A. W. Coleman, I. Nicolis, N. Keller, J. P. Dalbiez, J. Inclusion Phenom. Mol. Recognit. Chem., 1992, 13, 139; DOI: https://doi.org/10.1007/BF01053637.
J. C. de Miranda, T. E. Azevedo Martins, F. Veiga, H. Gomes Ferraz, Braz. J. Pharm. Sci., 2011, 47, 665; DOI: https://doi.org/10.1590/S1984-82502011000400003.
A. V. Astakhova, N. B. Demina, Pharm. Chem. J., 2004, 38, 105; DOI: https://doi.org/10.1023/B:PHAC.0000032490.04705.ba.
M. Sollogoub, Synlett, 2013, 24, 2629; DOI: https://doi.org/10.1055/s-0033-1339877.
N. G. Rambidi, A. V. Berezkin, Fizicheskie i khimicheskie osnovy nanotekhnologiy [Nanotechnologies: Physical and Chemical Foundations], Fizmatlit, Moscow, 2009, 456 pp. (in Russian).
M. K. Grachev, A. V. Edunov, G. I. Kurochkina, I. I. Levina, E. E. Nifantiev, Russ. J. Gen. Chem., 2011, 81, 322; DOI: https://doi.org/10.1134/S1070363211020083.
W. Saenger, J. Jacob, K. Gessler, T. Steiner, D. Hoffmann, H. Sanbe, K. Koizumi, S. M. Smith, T. Takaha, Chem. Rev., 1998, 98, 1787; DOI: https://doi.org/10.1021/cr9700181.
S. Tian, H. Zhu, P. Forgo, V. T. D’Souza, J. Org. Chem., 2000, 65, 2624; DOI: https://doi.org/10.1021/jo991347r.
A.R. Khan, P. Forgo, K. J. Stine, V. T. D’Souza, Chem. Rev., 1998, 98, 1977; DOI: https://doi.org/10.1021/cr970012b.
W. Saenger, M. Noltmeyer, P. C. Manor, B. Hingerty, B. Klar, Bioorg. Chem., 1976, 5, 187; DOI: https://doi.org/10.1016/0045-2068(76)90007-9.
M. K. Grachev, G. I. Kurochkina, A. V. Popkov, Russ. Chem. Bull., 2019, 68, 708; DOI: https://doi.org/10.1007/s11172-019-2477-4.
Modified Cyclodextrins for Chiral Separation, Eds W. Tang, S.-C. Hg, D. Sun, Springer, New York, 2013, 258 pp.
J. Szejtli, Chem. Rev., 1998, 98, 1743; DOI: https://doi.org/10.1021/cr970022c.
B. Brady, Org. Synth., 2000, 77, 220.
H.-S. Byun, O. R. Bittman, Org. Synth., 2000, 77, 225.
H. Law, J. M. Benito, J. M. Garcia Fernandez, L. Jicsinszky, S. Crouzy, J. Defaye, J. Phys. Chem. B, 2011, 115, 7524; DOI: https://doi.org/10.1021/jp2035345.
G. Tripodo, Ch. Wischke, A. T. Neffe, A. Lendlein, Carbohydr. Res., 2013, 381, 59; DOI: https://doi.org/10.1016/j.carres.2013.08.018.
L. D. Melton, K. N. Slessor, Carbohydr. Res., 1971, 18, 29; DOI: https://doi.org/10.1016/S0008-6215(00)80256-6.
M. Popr, S. Hybelbauerova, J. Jindrich, Beilstein J. Org. Chem., 2014, 10, 1390; DOI: https://doi.org/10.3762/bjoc.10.142.
P. Gonil, W. Sajomsang, U. R. Ruktanonchai, N. Pimpha, I. Sramala, O. Nuchuchua, S. Saesoo, Carbohydr. Polym., 2011, 83, 905; DOI: https://doi.org/10.1016/j.carbpol.2010.08.080.
J. B. Haff, Ch. Bieniarz, J. Org. Chem., 1994, 59, 7511; DOI: https://doi.org/10.1021/jo00103a056.
V. V. Novokshonov, Nguyen Thi Thu Xuan, N. S. Shaglaeva, Russ. J. Org. Chem., 2019, 55, 1616; DOI: https://doi.org/10.1134/S1070428019100245.
V. V. Novokshonov, Nguyen Thi Thu Xuan, S. N. Shaglaeva, T. A. Podgorbunskaya, V. V. Bayandin, Proc. Univ. Appl. Chem. Biotechnol., 2019, 9, 366; DOI: https://doi.org/10.21285/2227-2925-2019-9-3-366-375.
M. D. Mashkovsky, Lekarstvennye sredstva [Medicines] 16th Ed., Vol. 1, Novaya Volna, Moscow, 2019, 1216 pp. (in Russian).
L. Jicsinszky, K. Tuza, G. Cravotto, A. Porcheddu, F. Delogu, E. Colacino, Beilstein J. Org. Chem., 2017, 13, 1893; DOI: https://doi.org/10.3762/bjoc.13.184.
I. I. Stoikov, P. L. Padnya, O. A. Mostovaya, A. A. Vavilova, V. V. Gorbachuk, D. N. Shurpik, G. A. Evtyugin, Russ. Chem. Bull., 2019, 68, 1962; DOI: https://doi.org/10.1007/s11172-019-2655-4.
X. Luo, A. Morrin, A. J. Killard, M. R. Smyth, Electroanalysis, 2006, 18, 319; DOI: https://doi.org/10.1002/elan.200503415.
R. I. Gelb, L. M. Schwartz, D. A. Laufer, Bioorg. Chem., 1982, 11, 274; DOI: https://doi.org/10.1016/0045-2068(82)90003-7.
A. P. Croft, R. A. Bartsch, Tetrahedron, 1983, 39, 1417; DOI: https://doi.org/10.1016/S0040-4020(01)88551-3.
R. L. Vanetten, G. A. Clowes, J. Sebastian, M. L. Bender, J. Am. Chem. Soc., 1967, 89, 3253; DOI: https://doi.org/10.1021/JA00989A028.
S. Tian, P. Forgo, V. T. D’Souza, Tetrahedron Lett., 1996, 37, 8309; DOI: https://doi.org/10.1016/0040-4039(96)01944-2.
J. Jindrich, I. Tislerova, J. Org. Chem., 2005, 70, 9054; DOI: https://doi.org/10.1021/jo051339c.
G. G. Kordopati, G. M. Tsivgoulis, Tetrahedron Lett., 2018, 59, 2447; DOI: https://doi.org/10.1016/j.tetlet.2018.05.039.
A. Miyawaki, Y. Takashima, H. Yamaguchi, A. Harada, Tetrahedron, 2008, 64, 8355; DOI: https://doi.org/10.1016/j.tet.2008.05.040.
P. Zhang, L. Chang-Chun, A. W. Coleman, H. Parrot-Lopez, H. Galons, Tetrahedron Lett., 1991, 32, 2769; DOI: https://doi.org/10.1016/0040-4039(91)85081-F.
G. Benkovics, M. Balint, E. Fenyvesi, E. Varga, S. Beni, K. Yannakopoulou, M. Malanga, Beilstein J. Org. Chem., 2019, 15, 710; DOI: https://doi.org/10.3762/bjoc.15.66.
M. Jouffroy, R. Gramage-Doria, D. Armspach, D. Matt, L. Toupet, Chem. Commun., 2012, 48, 6028; DOI: https://doi.org/10.1039/C2CC31302B.
S. Guieu, M. Sollogoub, in Modern Synthetic Methods in Carbohydrate Chemistry: From Monosaccharides to Complex Glycoconjugates, Eds D. B. Werz, S. Vidal, Wiley-VCH Verlag, Weinheim, 2013, p. 241; DOI: https://doi.org/10.1002/9783527658947.ch9.
M. Guitet, F. Marcelo, S. A. de Beaumais, Y. Zhang, J. Jimenez-Barbero, S. Tilloy, E. Monflier, M. Menand, M. Sollogoub, Eur. J. Org. Chem., 2013, 18, 3691; DOI: https://doi.org/10.1002/ejoc.201300190.
O. Bistri, T. Lecourt, J.-M. Mallet, M. Sollogoub, P. Sinay, Chem. Biodiversity, 2004, 1, 129; DOI: https://doi.org/10.1002/cbdv.200490004.
T. Lecourt, Y. Bleriot, R. Auzely-Velty, M. Sollogoub, Chem. Commun., 2010, 46, 2238; DOI: https://doi.org/10.1039/b921567k.
J. Liu, B. Wang, C. Przybylski, O. Bistri-Aslanoff, M. Ménand, Y. Zhang, M. Sollogoub, Angew. Chem., Int. Ed., 2021, 60, 12090; DOI: https://doi.org/10.1002/anie.202102182.
E. Engeldinger, D. Armspach, D. Matt, Angew. Chem., Int. Ed., 2001, 40, 2526; DOI: https://doi.org/10.1002/1521-3773(20010702)40:133.0.CO:2-T.
M. Jouffroy, D. Armspach, D. Matt, Dalton Trans., 2015, 44, 12942; DOI: 10.1039/C5DT00667H.
B. Benmerad, P. Clair, D. Armspach, D. Matt, F. Balegroune, L. Toupet, Chem. Commun., 2006, 25, 2678; DOI: https://doi.org/10.1039/B603400D.
D. Armspach, D. Matt, L. Toupet, Angew. Chem., Int. Ed., 2009, 48, 4555; DOI: https://doi.org/10.1002/anie.200901200.
J. Li, X. J. Loh, Adv. Drug Delivery Rev., 2008, 60, 1000; DOI: https://doi.org/10.1016/j.addr.2008.02.011.
E. Rizzarelli, G. Vecchio, Coord. Chem. Rev., 1999, 188, 343; DOI: https://doi.org/10.1016/S0010-8545(99)00059-4.
S. V. Chilajwar, P. P. Pednekar, K. R. Jadha, G. J. C. Gupta, V. J. Kadam, Expert Opin. Drug Delivery, 2014, 11, 111; DOI: https://doi.org/10.1517/17425247.2014.865013.
F. I. Donati, in Cyclodextrins in Pharmaceutics, Cosmetics, and Biomedicine: Current and Future Industrial Applications, Ed. E. Bilensoy, John Wiley&Sons, Hoboken, 2011, Ch. 19, p. 363.
S. A. Kedik, A. V. Panov, V. S. Tyukova, M. S. Zolotareva, Razrabotka i registratsiya lekarstv. sredstv [Drug Development & Registration] 2016, No. 3, 68 (in Russian).
F. Manakker, T. Vermonden, C. F. Nostrum, W. E. Hennink, Biomacromolecules, 2009, 10, 3157; DOI: https://doi.org/10.1021/bm901065f.
R. Mejia-Ariza, L. Grana-Suarez, W. Verboom, J. Huskens, J. Mater. Chem. B, 2017, 5, 36; DOI: https://doi.org/10.1039/c6tb02776h.
S. I. Dikhtyarev, M. V. Shteyngard, L. A. Chayka, Itogi nauki i tekhniki. Ser. Mikrobiologiya [Advances in Science and Technology, Ser. Microbiology], 1988, 20, 97 (in Russian).
M. V. Papezhuk, V. A. Volynkin, T. A. Stroganova, G. D. Krapivin, T. R. Usacheva, L. P. Thi, Macroheterocycles, 2020, 13, 64; DOI: https://doi.org/10.6060/mhc191281v.
M. A. Malenkovskaya, D. A. Shipilov, M. K. Grachev, Russ. J. Bioorg. Chem., 2020, 46, 71; DOI: https://doi.org/10.1134/S1068162020010045.
T. Loftsson, M. Brewster, J. Pharm. Sci., 1996, 85, 1017; DOI: https://doi.org/10.1021/js950534b.
T. Loftsson, D. Duchene, Int. J. Pharm., 2007, 329, 1; DOI: https://doi.org/10.1016/j.ijpharm.2006.10.044.
T. Loftsson, D. Hreinsdóttir, M. Masson, Int. J. Pharm., 2005, 302, 18; DOI: https://doi.org/10.1016/j.jpharm.2005.05.042.
D. A. Shipilov, G. I. Kurochkina, A. A. Sergievich, M. K. Grachev, Macroheterocycles, 2017, 10, 238; DOI: https://doi.org/10.6060/mhc170510s.
A. V. Edunov, G. I. Kurochkina, M. K. Grachev, I. I. Levina, T. A. Batalova, E. E. Nifant’ev, Russ. J. Org. Chem., 2011, 47, 981; DOI: https://doi.org/10.1134/S1070428011070037.
D. A. Shipilov, M. A. Malenkovskaya, N. V. Kutyasheva, G. I. Kurochkina, A. A. Sergievich, M. K. Grachev, Russ. Chem. Bull., 2019, 68, 862; DOI: https://doi.org/10.1007/s11172-019-2497-0.
M. K. Grachev, A. V. Edunov, G. I. Kurochkina, N. O. Soboleva, L. K. Vasyanina, E. E. Nifant’ev, Russ. Chem. Bull., 2012, 61, 181; DOI: https://doi.org/10.1007/s11172-012-0025-6.
K. Kaminski, M. Kujdowicz, M. Kajta, M. Nowakowska, K. Szczubiaika, Eur. J. Pharm. Biopharm., 2015, 91, 111; DOI: https://doi.org/10.1016/j.ejpb.2015.02.002.
R. Matten, T. Hoare, Int. J. Pharm., 2014, 472, 315; DOI: https://doi.org/10.1016/j.ijpharm.2014.06.046.
C. M. Hsu, S. C. Yu, F. J. Tsai, Y. Tsai, Colloids Surf., B, 2019, 180, 68; DOI: https://doi.org/10.1016/j.colsurfb.2019.04.020.
V. J. Stella, R. A. Rajewski, Int. J. Pharm., 2020, 583, 119396; DOI: https://doi.org/10.1016/j.iipharm.2020.119396.
D. R. Luke, K. Tomaszewski, B. Damle, H. T. Schlamm, J. Pharm. Sci., 2010, 99, 3291; DOI: https://doi.org/10.1002/jps.22109.
C. S. Magnolim, C. Moriwaki, A. C. Nogueira, F. Sato, M. L. Baesso, A. M. Neto, G. Matioli, Food Chem., 2014, 153, 361; DOI: https://doi.org/10.1016/j.foodchem.2013.12.067.
T. R. Usacheva, D. N. Kabirov, D. A. Beregova, G. A. Gamov, V. A. Sharnin, M. Biondi, L. Mayol, C. Giancola, J. Therm. Anal. Calorim., 2019, 138, 417; DOI: https://doi.org/10.1007/s10973-019-08136-5.
D. Kabirov, T. Silvestri, M. Niccoli, T. Usacheva, L. Mayol, M. Biondi, C. Giancola, J. Therm. Anal. Calorim., 2020; DOI: https://doi.org/10.1007/s10973-020-10381-y.
J. D. Heidel, Cold Spring Harbor Protoc., 2011, 11, 1392; DOI: https://doi.org/10.1101/pdb.prot066654.
W.-F. Lai, H.-S. Jung, PLOS ONE, 2014, 9, e100258; DOI: https://doi.org/10.1371/journal.pone.0100258.
J. Szejtli, L. Szente, Proc. 8th Int. Symp. on Cyclodextrins, Budapest, Hungary, March, 31–April, 2, 1996, p. 163.
M. E. Skold, G. D. Thyne, J. W. Drexler, J. E. McCray, J. Contam. Hydrol., 2009, 107, 108; DOI: https://doi.org/10.1016/j.jconhyd.2009.04.006.
X. Hu, G. Xu, H. Zhang, M. Li, Y. Tu, X. Xie, Y. Zhu, L. Jiang, X. Zhu, X. Ji, Y. Li, A. Li, ACS Appl. Mater. Interfaces, 2020, 12, 12165; DOI: https://doi.org/10.1021/acsami.0c00597.
X. Liao, Q. Zhang, ACS Appl. Polym. Mater., 2019, 1, 2089; DOI: https://doi.org/10.1021/acsapm.9b00399.
A. Alsbaiee, B. J. Smith, L. Xiao, Y. Ling, D. E. Helbling, W. R. Dichtel, Nature, 2016, 529, 190; DOI: https://doi.org/10.1038/nature16185.
Z. Wang, P. Zhang, F. Hu, Y. Zhao, L. Zhu, Carbohydr. Polym., 2017, 177, DOI: https://doi.org/10.1016/j.carbpol.2017.08.059.
R. Breslow, S. D. Dong, Chem. Rev., 1998, 98, 1997; DOI: https://doi.org/10.1021/cr970011j.
R. Breslow, L. E. Overman, J. Am. Chem. Soc., 1970, 92, 1075; DOI: https://doi.org/10.1021/ja00707a062.
R. Breslow, P. J. Duggan, J. P. Light, J. Am. Chem. Soc., 1992, 114, 3982; DOI: https://doi.org/10.1021/ja00036a059.
M. Rezac, R. Breslow, Tetrahedron Lett., 1997, 38, 5763; DOI: https://doi.org/10.1016/S0040-4039(97)01289-6.
M. Sawamura, K. Kitayama, Y. Ito, Tetrahedron: Asymmetry, 1993, 4, 1829; DOI: https://doi.org/10.1016/S0957-4166(00)80423-1.
M. T. Reetz, J. Rudolph, Tetrahedron: Asymmetry, 1993, 4, 2405; DOI: https://doi.org/10.1016/S0957-4166(00)82210-7.
E. E. Nifantiev, M. K. Grachev, S. Yu. Burmistrov, Chem. Rev., 2000, 100, 3755; DOI: https://doi.org/10.1021/cr9601371.
S. V. Kurkov, T. Loftsson, Int. J. Pharm., 2013, 453, 167; DOI: https://doi.org/10.1016/j.iipharm.2012.06.055.
H. Aghahosseini, A. Ramazani, Curr. Org. Chem., 2016, 20, 2817; DOI: https://doi.org/10.2174/1385272820666160328201207.
X. Wang, M. L. Brusseau, Environ. Sci. Technol., 1995, 29, 2632; DOI: https://doi.org/10.1021/es00010a026.
M. L. Brusseau, X. Wang, W. J. Wang, Environ. Sci. Technol., 1997, 31, 1087; DOI: https://doi.org/10.1021/es960612c.
P. Zhang, C. Tugny, J. M. Suárez, M. Guitet, E. Derat, N. Vanthuyne, Y. Zhang, O. Bistri, V. Mouriès-Mansuy, M. Ménand, S. Roland, L. Fensterbank, M. Sollogoub, Chem, 2017, 3, 174; DOI: https://doi.org/10.1016/j.chempr.2017.05.009.
G. Xu, S. Leloux, P. Zhang, J. M. Suárez, Y. Zhang, E. Derat, M. Ménand, O. Bistri-Aslanoff, S. Roland, T. Leyssens, O. Riant, M. Sollogoub, Angew. Chem., Int. Ed., 2020, 59, 7591; DOI: https://doi.org/10.1002/anie.202001733.
C. F. Potter, N. R. Russell, M. McNamara, J. Inclusion Phenom. Macrocyclic Chem., 2006, 56, 395; DOI: https://doi.org/10.1007/s10847-006-9122-1.
Y. Matsui, Y. Yokoi, K. Mochida, Chem. Lett., 1976, 10, 1037; DOI: https://doi.org/10.1246/cl.1976.1037.
I. A. Ananyeva, E. N. Shapovalova, S. A. Lopatin, O. A. Shpigun, V. P. Varlamov, V. A. Davankov, Vestn. MGU. Ser. 2. Khimiya [Moscow Univ. Bull. Ser. Khim. (Engl. Transl.)], 2001, 42, 273 (in Russian).
H. Zhang, T. Tan, C. Hetenyi, Y. Lv, D. van der Spoel, J. Phys. Chem. C, 2014, 118, 7163; DOI: https://doi.org/10.1021/jp412041d.
E. Cho, S. Jung, Molecules, 2015, 20, 19620; DOI: https://doi.org/10.3390/molecules201019620.
Y. Liu, C. C. You, B. Li, Chem. Eur. J., 2001, 7, 1281; DOI: https://doi.org/10.1002/1521-3765(20010316)7:6<1281::AID-CHEM1281>3.0.CO;2-H.
A. P. Singh, P. R. Cabrer, E. Alvarez-Parrilla, F. Meijide, J. V. J. Tato, J. Inclusion Phenom. Macrocyclic Chem., 1999, 35, 335; DOI: https://doi.org/10.1021/la9817359.
D. Dong, D. Baigl, Y. Cui, P. Sinay, M. Sollogoub, Y. Zhang, Tetrahedron, 2007, 63, 2973; DOI: https://doi.org/10.1016/j.tet.2007.01.065.
Y. Liu, Y. Chen, Acc. Chem. Res., 2006, 39, 953; DOI: https://doi.org/10.1021/ar0502275.
Y. Liu, Y. Chen, B. Li, T. Wada, Y. Inoue, Chem. Eur. J., 2001, 7, 2528; DOI: https://doi.org/10.1002/1521-3765(20010618)7:12<2528::aid-chem25280>3.0.co;2-9.
Y. Liu, Y. W. Yang, L. Li, Y. Chen, Org. Biomol. Chem., 2004, 2, 1542; DOI: https://doi.org/10.1039/b402841d.
Y. Liu, L. Li, H. Y. Zhang, Y. Song, J. Org. Chem., 2003, 68, 527; DOI: https://doi.org/10.1021/jo025919a.
Y. Liu, Y. L. Zhao, Y. Chen, F. Ding, G. S. Chen, Bio-conjugate Chem., 2004, 15, 1236; DOI: https://doi.org/10.1021/bc049870m.
Y. Liu, Y. Song, Y. Chen, X. Q. Li, F. Ding, R. Q. Zhong, Chem. Eur. J., 2004, 10, 3685; DOI: https://doi.org/10.1002/chem.200305724.
Y. Dai, S. Wang, J. Zhou, J. Tang, W. Tang, Electrophoresis, 2013, 34, 833; DOI: https://doi.org/10.1002/elps.201200473.
D. A. Shipilov, G. I. Kurochkina, A. K. Akhlebinin, N. V. Kutyasheva, M. K. Grachev, Macroheterocycles, 2019, 12, 94; DOI: https://doi.org/10.6060/mhc181110s.
S. Kumar, R. Rao, J. Inclusion Phenom. Macrocyclic Chem., 2019, 94, 11; DOI: https://doi.org/10.1007/s10847-019-00903-z.
F. Trotta, M. Zanetti, R. Cavalli, Beilstein J. Org. Chem., 2012, 8, 2091; DOI: https://doi.org/10.3762/bjoc.8.235.
S. Kumar, P. Dalal, R. Rao, in Colloid Science in Pharmaceutical Nanotechnology, Ed. S. Karakuş, IntechOpen, 2020, 24 pp.; DOI: https://doi.org/10.5772/intechopen.90365.
S. Anandam, S. Selvamuthukumar, J. Porous Mater., 2014, 21, 1015; DOI: https://doi.org/10.1007/s10934-014-9851-2.
S. Swaminanthan, P. R. Vavia, F. Trotta, R. Covalli, S. Tumbiolo, L. Bertinetti, S. Coluccia, J. Inclusion Phenom. Macrocyclic Chem., 2013, 76, 201; DOI: https://doi.org/10.1007/s10847-012-0192-y.
S. S. Darandale, P. R. Vavia, J. Inclusion Phenom. Macrocyclic Chem., 2013, 75, 315; DOI: https://doi.org/10.1007/s10847-012-086-9.
M. R. Rao, C. Shirsath, AAPS PharmSciTech, 2017, 18, 1728; DOI: https://doi.org/10.1208/s12349-016-0636-6.
S. Kumar, S. Pooja, F. Trotta, R. Rao, Pharmaceutics, 2018, 10, 169; DOI: https://doi.org/10.3390/pharmaceutics10040169.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no competing interests.
Additional information
This work was financially supported by the Ministry of Science and Education of the Russian Federation (Project No. FZEN-2020-0022).
No human or animal subjects were used in this research.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 3, pp. 430–442, March, 2022.
Rights and permissions
About this article
Cite this article
Papezhuk, M.V., Volynkin, V.A. & Panyushkin, V.T. The structure and properties of functionalized cyclodextrins and complex compounds based on them. Russ Chem Bull 71, 430–442 (2022). https://doi.org/10.1007/s11172-022-3430-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-022-3430-5