Abstract
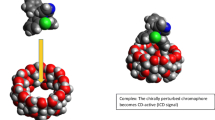
The derivatives 6-Deoxy-6-[1-(2-amino)ethylamino]-β-Cyclodextrin (CDEn), 6-Deoxy-6-[1-(3-amino)propylamino]-β-Cyclodextrin (CDPn) and 6-Deoxy-6-[1-(4-amino)butylamino]-β-Cyclodextrin (CDBn) were assessed with a view to demonstrating that increasing the chain length of the diaminoalkane moiety can affect the chiral selectivity of the metallo-complexes of these materials. It was shown that IR and Raman spectroscopies can be used to characterise these compounds. The results obtained from the electronic absorption spectra suggested the formation of CuCDAm binary complexes and that the derivatives CDEn and CDPn act as bidentate ligands while CDBn acts as a monodentate ligand due to its longer alkane chain. This study also showed that in the ternary complexes with DOPA there is further coordination of the metal ion to the amino nitrogen atom and the hydroxyl oxygen atom of the drug. On the basis of the results of the circular dichroic spectroscopic studies it was suggested that CuCDEn is the better enantioselective material for DOPA and it acts in a multi-site recognition manner, utilising the inclusion properties of the CD cavity in cooperation with the coordination properties of the metal ion.
Similar content being viewed by others
References
Zakaria P., Macka M., Haddad P.P. (2004) J. Chrom. (A) 1031:179
La S., Ahn S., Kim J.H., Goto J., Choi O.K., Kim K.R. (2002) Electrophoresis 23(24):4123
Hoof N.V., Russell N.R., McNamara M., Darcy R. (2000) J. Incl. Phenom. 36:179
Cladrowa-Runge S., Hirz R., Kenndler E., Rizzi A. (1995) J. Chromatogr. (A) 710:339
Poon Y.F., Muderawan I.W., Ng S.C. (2006) J. Chrom. (A) 1101:185
Kitae T., Nakayama T., Kano K. (1998) J. Chem. Soc. Perkin Trans. 2:207
Haskard C.A., Easton C.J., May B.L., Lincoln S.F. (1996) Inorg. Chem. 35:1059
Breslow R., Overman L.E. (1970) J. Am. Chem. Soc. 92:1075
Matsui Y., Yokoi T., Mochida K. (1976) Chem. Lett. 10:1037
Yoichi M., Makota K. (1990) J. Mol. Catal. 61:129
Tabushi I., Shimiza N., Sugimoto T., Shiozuka M., Yamanurra K. (1977) J. Am. Chem. Soc. 99:7100
Bonomo R.P., Cucinotta V., D’Allessandro F., Impellizzeri G., Maccarrone G., Rizzarelli E. (1993). J. Incl. Phenom. Mol. Recog. Chem. 15:167
Brown S.E., Coates J.H., Easton C.J., Lincoln S.F. (1994) J. Chem. Soc. Faraday Trans. 90:739
Brown S.E., Haskard C.A., Easton C.J., Lincoln S.F. (1995) J. Chem. Soc. Faraday Trans. 91:1013
Silverstein R.M., Webster F.X., Kiemle D.J. (2005) Spectrometric Identification of Organic Compounds. John Wiley & Sons, New York
Rodger A., Norden B. (1997) Oxford Chemistry Masters: Circular Dichroism and Linear Dichroism. Oxford University Press, Oxford
Brady B., Lynam N., O’ Sullivan T., Ahern C., Darcy R. (2000) Org. Syn. 77:220
Singh A.P., Cabrer P.R., Alvarez-Parrilla E., Meijide F., Tato J.V. (1999) J. Incl. Phen. 35:335
Schneider H.J., Hacket F., Rüdiger V., Ikeda I. (1998) Chem. Rev. 98:1755
Capretta A., Maharajh R.B., Bell R.A. (1995) Carb. Res. 267:49
Szente L. (1996) Analytical methods for cyclodextrins, cyclodextrin derivatives and cyclodextrin complexes. In: Atwood J.L., Davies J.E.D., MacNicol D.D., Vogtle F. (eds.), Comprehensive Supramolecular Chemistry. 3 Pergamon, Exeter, UK, pp 253–278
Nair B.U., Dismukes G.C. (1983) J. Am. Chem. Soc. 105:124
(a) N.R. Russell and M. McNamara: J. Incl. Phenom. Mol. Recogn. Chem. 7, 455 (1989). (b) M. McNamara and N.R. Russell: J. Incl. Phenom. Mol. Recogn. Chem. 10, 485 (1991). (c) M. McNamara and N.R. Russell: J. Incl. Phenom. Mol. Recogn. Chem. 13, 145 (1992)
(a) J.W. Robinson: Practical Handbook of Spectroscopy, CRC Press, Boston (1991), p. 551. (b) A.T. Tu, J. Lee, and F.F. Milanovich: Carbohydr. Res. 76, 239 (1979)
Lever A.B.P. (1984) Inorganic Electronic Spectroscopy. Elsevier Publishers, New York
Cucinotta V., Giuffrida A., Maccarrone G., Messina M., Puglisi A., Rizzarelli E., Vecchio G. (2005) Dalton Trans. 16:2731
Bonomo R.P., Pedotti S., Vecchio G., Rizzarelli E. (1996) Inorg. Chem. 16:6873
Hathaway B.J., Billing D.E., Nicholls P., Proctor I.M. (1969) J. Chem. Soc. (A) 2:319
Corradini R., Dossena A., Impellizzeri G., Maccarrone G., Marchelli R., Rizzarelli E., Sartor G., Vecchio G. (1994) J. Am. Chem. Soc. 116:10267
G. Maccarone, E. Rizzarelli, and G. Vecchio: Chiral recognition by functionalised cyclodextrin metal complexes. In L. Fabbrizzi and A. Poggi (eds.), Transition Metals in Supramolecular Chemistry, Kluwer Academic Publishers (1994), pp. 351–367
Du Y.Q., Nakamura A., Toda F. (1991) J. Incl. Phenom. Mol. Recogn. Chem. 10:443
Acknowledgments
This work has been carried out (in part) within the structures of the Focas Institute at the Dublin Institute of Technology (DIT), which is funded under the National Development Plan 2000–2006 with assistance from the European Regional Development Fund.
The authors would also like to acknowledge funding from the IT Sector I Postgraduate R & D Skills Programme and DIT.
Elemental analysis and NMR spectra were obtained at the National University of Ireland Dublin.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Potter, C.F., Russell, N.R. & McNamara, M. Spectroscopic Characterisation of Metallo-Cyclodextrins for Potential Chiral Separation of Amino Acids and L/D-DOPA. J Incl Phenom Macrocycl Chem 56, 395–403 (2006). https://doi.org/10.1007/s10847-006-9122-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-006-9122-1