Abstract
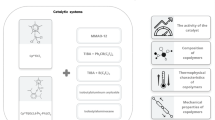
The catalytic activity of the systems based on titanium(iv) alkoxides (Ti(OPri)4, Ti(OPri)2(OCH(CF3)2)2, and Ti(OCH(CF3)2)4) and mixtures of alkylaluminum chlorides (Et2AlCl or Et3Al2Cl3) with dibutylmagnesium in ethylene polymerization and ethylene copolymerization with propylene and 5-ethylidene-2-norbornene was studied. Ultrahigh-molecular-weight polyethylene with the molecular weight reaching 4.9 · 106 Da was found to be formed in the homopolymerization reaction, whereas copolymerization gives ter-copolymers containing propylene (up to 35 mol.%) and 5-ethylidene-2-norbornene (4.3 mol.%) units.
Similar content being viewed by others
Change history
27 February 2023
An Erratum to this paper has been published: https://doi.org/10.1007/s11172-022-3709-6
References
S. M. Kurtz, in UHMWPE Handbook: Ultra-high Molecular Weight Polyethylene in Total Joint Replacement, Elsevier Academic Press, San Diego, 2004.
A. J. Peacock, Handbook of Polyethylene: Structures, Properties and Applications, Marcel Dekker, New York, 2000, ISBN 0471166286.
A. N. Ozerin, S. S. Ivanchev, S. N. Chvalun, V. A. Aulov, N. I. Ivancheva, N. F. Bakeev, Polym. Sci., Ser. A, 2012, 54, 950; DOI: https://doi.org/10.1134/S0965545X12100033.
B. P. Rotzinger, H. D. Chanzy, P. Smith, Polymer, 1989, 30, 1814; DOI: https://doi.org/10.1016/0032-3861(89)90350-9.
S. Ronca, G. Forte, H. Tjaden, S. Rastogi, Ind. Eng. Chem. Res., 2015, 54, 7373; DOI: https://doi.org/10.1021/acs.iecr.5b01469.
A. A. Antonov, K. P. Bryliakov, Eur. Polym. J., 2021, 142, 110162; DOI: https://doi.org/10.1016/j.eurpolymj.2020.110162.
S. Ch. Gagieva, V. A. Tuskaev, R. U. Takazova, A. G. Buyanovskaya, O. V. Smirnova, N. M. Bravaya, B. M. Bulychev, Russ. Chem. Bull., 2019, 68, 2114; DOI: https://doi.org/10.1007/s11172-019-2675-0.
Yu. N. Belokon’, S. Ch. Gagieva, T. A. Sukhova, A. B. Dmitriev, K. A. Lyssenko, N. M. Bravaya, B. M. Bulychev, D. Seebach, Russ. Chem. Bull., 2005, 54, 2348; DOI: https://doi.org/10.1007/s11172-006-0121-6.
V. A. Tuskaev, S. Ch. Gagieva, D. A. Kurmaev, I. V. Fedyanin, S. V. Zubkevich, B. M. Bulychev, Russ. Chem. Bull., 2018, 67, 377; DOI: https://doi.org/10.1007/s11172-018-2084-9.
V. A. Tuskaev, S. Ch. Gagiev.a, D. A. Kurmaev, S. V. Zubkevich, P. V. Dorovatovskii, V. N. Khrustalev, E. S. Mikhaylik, E. K. Golubev, M. I. Buzin, G. G. Nikiforova, V. G. Vasil’ev, T. M. Zvukova, B. M. Bulychev, Inorg. Chim. Acta, 2019, 498, 119159; DOI: https://doi.org/10.1016/j.ica.2019.119159.
V. A. Tuskaev, S. Ch. Gagieva, D. A. Kurmaev, V. N. Khrustalev, P. V. Dorovatovskii, E. S. Mikhaylik, E. K. Golubev, M. I. Buzin, S. V. Zubkevich, G. G. Nikiforova, V. G. Vasil’ev, B. M. Bulychev, K. F. Magomedov, J. Organomet. Chem., 2018, 877, 85; DOI: https://doi.org/10.1016/j.jorganchem.2018.09.014.
V. A. Tuskaev, S. Ch. Gagieva, D. A. Kurmaev, E. K. Melnikova, S. V. Zubkevich, M. I. Buzin, G. G. Nikiforova, V. G. Vasil’ev, D. Saracheno, V. S. Bogdanov, V. I. Privalov, B. M. Bulychev, Appl. Organomet. Chem., 2020; e5933; DOI: https://doi.org/10.1002/aoc.5933.
V. A. Tuskaev, S. Ch. Gagieva, A. S. Lyadov, D. A. Kurmaev, G. G. Nikiforova, V. G. Vasil’ev, S. V. Zubkevich, D. Saracheno, A. I. Sizov, V. I. Privalov, B. M. Bulychev, Petrol. Chem., 2020, 60, 329; DOI: https://doi.org/10.1134/S0965544120030226.
V. A. Tuskaev, S. Ch. Gagieva, A. S. Lyadov, D. A. Kurmaev, G. G. Nikiforova, V. G. Vasil’ev, S. V. Zubkevich, D. Saracheno, A. I. Sizov, V. I. Privalov, B. M. Bulychev, Petrol. Chem., 2020, 60, 827; DOI: https://doi.org/10.1134/S0965544120070142.
V. A. Tuskaev, S. Ch. Gagieva, D. A. Kurmaev, V. S. Bogdanov, K. F. Magomedov, Elena S. Mikhaylik, E. K. Golubev, M. I. Buzin, G. G. Nikiforova, V. G. Vasil’ev, V. N. Khrustalev, P. V. Dorovatovskii, A. V. Bakirov, M. A. Shcherbina, P. B. Dzhevakov, B. M. Bulychev, Appl. Organomet. Chem., 2021, e6256; DOI: https://doi.org/10.1002/aoc.6256.
J. Skupiñska, Chem. Rev., 1991, 91, 613; DOI: https://doi.org/10.1021/cr00004a007.
S. M. Pillai, M. Ravindranathan, S. Sivaram, Chem. Rev., 1986, 86, 353; DOI: https://doi.org/10.1021/cr00072a004.
A. W. Al-Sa’doun, Appl. Catal., A, 1993, 105, 1; DOI: https://doi.org/10.1016/0926-860X(93)85131-8.
J.-B. Cazaux, P. Braunstein, L. Magna, L. Saussine, H. Olivier-Bourbigou, Eur. J. Inorg. Chem., 2009, 2942; DOI: https://doi.org/10.1002/ejic.200900322.
B. Zhu, C. Guo, Z. Liu, Y. Yin, J. Appl. Polym. Sci., 2004, 94, 2451; DOI: https://doi.org/10.1002/app.21190.
G. Natta, L. Porri, A. Carbonaro, G. Stoppa, Macromol. Chem., 1964, 77, 114; DOI: https://doi.org/10.1002/macp.1964.020770111.
G. Natta, L. Porri, A. Carbonaro, Macromol. Chem., 1964, 77, 126; DOI: https://doi.org/10.1002/macp.1964.020770112.
D. H. Dawes, C. A. Winkler, J. Polym. Sci., Part A: Polym. Chem., 1964, 2, 3029; DOI: https://doi.org/10.1002/pol.1964.100020703.
L. Oliva, P. Longo, C. Pellecchia, Macromol. Chem. Commun., 1988, 9; DOI: https://doi.org/10.1002/marc.1988.030090201.
A. Mehta, G. Tembe, P. Parikh, G. Mehta, Polym. Int., 2014, 63, 206; DOI: https://doi.org/10.1002/pi.4484.
Y. V. Kissin, L. A. Rishina, S. S. Lalayan, V. G. Krasheninnikov, J. Polym. Sci., Part A: Polym. Chem., 2015, 53, 2124; DOI: https://doi.org/10.1002/pola.27651.
L. A. Rishina, Y. V. Kissin, S. S. Lalayan, V. G. Krasheninnikov, E. O. Perepelitsina, T. I. Medintseva, Polym. Sci., Ser. B, 2016, 58, 152; DOI: https://doi.org/10.1134/S1560090416020056.
V. Prakash Reddy, Organofluorine Chemistry: Synthesis and Applications, Elsevier, Amsterdam, 2020.
A. M. Semenova, M. G. Pervova, M. A. Ezhikova, M. I. Kodess, A. Ya. Zapevalov, A. V. Pestov, Russ. Chem. Bull., 2021, 70, 933; DOI: https://doi.org/10.1007/s11172-021-3169-4.
C. Campbell, S. G. Bott, R. Larsen, W. G. Van Der Sluys, Inorg. Chem., 1994, 33, 4950; DOI: https://doi.org/10.1021/ic00100a019.
J. Fisher, W. G. Van DerSluys, J. C. Huffman, J. Sears, Synth. React. Inorg. Met. Org. Chem., 1993, 23, 479; DOI: https://doi.org/10.1080/15533179308016651.
G. Leone, G. Zanchin, R. Di Girolamo, F. De Stefano, C. Lorber, C. De Rosa, G. Ricci, F. Bertini, Macromolecules 2020, 53, 5881. DOI: https://doi.org/10.1021/acs.macromol.0c01123.
M. Mitani, J. Mohri, Y. Yoshida, J. Saito, S. Ishii, K. Tsuru, S. Matsui, R. Furuyama, T. Nakano, H. Tanaka, S. Kojoh, T. Matsugi, N. Kashiwa, T. Fujita, Living J. Am. Chem. Soc., 2002, 124, 3327; DOI: https://doi.org/10.1021/ja0117581.
M. Mitani, R. Furuyama, J. Mohri, J. Saito, S. Ishii, H. Terao, T. Nakano, H. Tanaka, T. Fujita, J. Am. Chem. Soc., 2003, 125, 4293; DOI: https://doi.org/10.1021/ja029560j.
S. Ishii, R. Furuyama, N. Matsukawa, J. Saito, M. Mitani, H. Tanaka, T. Fujita, Macromol. Rapid Commun., 2003, 24, 452; DOI: https://doi.org/10.1002/marc.200390072.
M. C. W. Chan, S. C. F. Kui, J. M. Cole, G. J. McIntyre, S. Matsui, N. Zhu, K.-H. Tam, Chem. — Eur. J., 2006, 12, 2607; DOI: https://doi.org/10.1002/chem.200501054.
P. Cossee, J. Catal., 1964, 3, 80; DOI: https://doi.org/10.1016/0021-9517(64)90095-8.
A. J. Gordon, R. A. Ford, The Chemist’s companion, Wiley, New-York, London, Sydney, Toronto, 1972.
B. Wunderlich, C. M. Cormier, J. Polym. Sci., Part A-2:Polym. Phys., 1967, 5, 987; DOI: https://doi.org/10.1002/pol.1967.160050514.
F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 73; DOI: https://doi.org/10.1002/wcms.81.
F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2017, 8, e1327; DOI: https://doi.org/10.1002/wcms.1327.
C. Adamo, V. Barone, J. Chem. Phys., 1999, 110, 6158; DOI: https://doi.org/10.1063/1.478522.
F. Weigend, R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297; DOI: https://doi.org/10.1039/B508541A.
J. P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865; DOI: https://doi.org/10.1103/PhysRevLett.77.3865.
J. P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett., 1997, 78, 1396; DOI: https://doi.org/10.1103/PhysRevLett.78.1396.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Academician of the Russian Academy of Sciences O. M. Nefedov on the occasion of his 90th birthday.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 1, pp. 76–82, January, 2022.
This was financially carried out by the Russian Science Foundation (Project No. 18-13-00375). The NMR spectroscopic studies of the copolymers were performed in terms of state assignment “Substances and Materials for Providing Safety, Reliability, and Energy Effi ciency” (Project No. AAAA- A21- 121011590086-0). The DSC studies were carried out on the equipment of the Center of Investigation of Structure of Molecules at the A. N. Nesmeyanov Institute of Organoelement Compounds (Russian Academy of Sciences) supported by the Ministry of Science and Higher Education of the Russian Federation. The catalytic activity of titanium alkoxides on the model reaction of ethylene polymerization and the properties of the prepared polyethylene samples were studied in the framework of the Program of Development of the Moscow University Interdisciplinary Scientifi c Educational School “Future of the Planet and Global Environmental Changes.”
No human or animal subjects were used in this study.
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Tuskaev, V.A., Bogdanov, V.S., Gagieva, S.C. et al. New catalytic systems based on fluorine-containing titanium(iv) alkoxides for the synthesis of ultrahigh-molecular-weight polyethylene and olefin elastomers. Russ Chem Bull 71, 76–82 (2022). https://doi.org/10.1007/s11172-022-3379-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-022-3379-4