Abstract
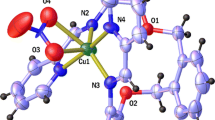
The solution state of a new antituberculosis drug 1,6-bis(hydrazidomethylsulflnyl)hexane (L) and its complexation with copper(II) were investigated by spectrophotometry, pH-potentiometry (T = 25 °C; at variable ionic strength), and mathematical modeling. The dissociation constants of the protonated and imidol forms of compound L were determined. Complexation reactions in the Cu2+—L system result in mononuclear complexes [CuL]2+ and [CuL2]2+ with the amide form of the ligand provided that the redox reactions are excluded. The most probable (optimized) structures of 1,6-bis(hydrazidomethylsulfinyl)hexane and its complexes were obtained from quantum chemical calculations within the framework of the density functional theory (PBE/6-311G (d) method). Compound L adopts a folded conformation. The coordination site of the 1:1 complex includes six oxygen atoms from two carbonyl groups, two sulfoxide groups, and two water molecules. In the 1:2 complex, each ligand molecule is coordinated in bidentate mode through the carbonyl oxygen atom and the primary amino nitrogen of a hydrazide fragment.
Similar content being viewed by others
References
P. Madaan, V. K. Tyagi, J. Oleo Sci., 2008, 57, 197.
Global Tuberculosis Report 2017, World Health Organization, 2017, Ch. 4, p. 63; https://www.who.int/tb/publications/global_report/gtbr2017_main_text.pdf.
V. V. Neklyudov, G. A. Boos, M. M. Shulaeva, G. A. Chmutova, Yu. I. Bagina, Yu. I. Salnikov, R. R. Amirov, Russ. Chem. Bull., 2017, 66, 628.
V. V. Neklyudov, G. A. Boos, G. A. Chmutova, M. M. Shulaeva, Yu. I. Bagina, R. R. Amirov, Russ. J. Gen. Chem., 2018, 88, 1672.
Patent RU 2591256; Byull. Izobret. [Inventions & Utility Models], 2016, No. 20 (in Russian).
V. V. Neklyudov, G. A. Boos, S. G. Fattakhov, G. A. Chmutova, M. M. Shulaeva, Yu. I. Salnikov, Russ. Chem. Bull., 2014, 63, 1113.
Yu. I. Salnikov, A. N. Glebov, F. V. Devyatov, Poliyadernye kompleksy v rastvorakh [Polynuclear Complexes in Solutions], Izd-vo Kazanskogo Gos. Un-ta, Kazan, 1989, 287 pp. (in Russian).
V. P. Vasiliev, Termodinamicheskie svojstva rastvorov elektrolitov [Thermodynamic Properties of Electrolyte Solutions], Vysshaya Shkola, Moscow, 1982, 320 pp. (in Russian).
Yu. Yu. Lurie, Spravochnik po analiticheskoy khimii [A Handbook of Analytical Chemistry], Khimiya, Moscow, 1989, 448 pp. (in Russian).
Ja. Bjerrum, Metal Amine Formation in Aqueous Solution. Theory of Reversible Step Reactions, Univ. Copenhagen, Copenhagen, 1957.
E. S. Shcherbakova, I. P. Gol’dshtein, E. N. Gur’yanova, K. A. Kocheshkov, Bull. Acad. Sci. USSR. Div. Chem. Sci., 1975, 24, 1165.
F. R. Hartley, C. Burgess, R. M. Alcock, Solution in Equilibria, Ellis Horwood, New York, 1980, 361 p.
O. V. Ardashov, A. M. Genaev, I. V. Il’ina, D. V. Korchagina, K. P. Volcho, N. F. Salakhutdinov, Russ. J. Org. Chem., 2010, 46, 1786.
G. Imre, I. Jakli, A. Kalaszi, O. Farkas, 1st European Chemistry Congress (Budapest, Hungary, August 27–31, 2006), Budapest, 2006.
D. N. Laikov, Chem. Phys. Lett., 1997, 281, 151.
D. N. Laikov, Yu. A. Ustynyuk, Russ. Chem. Bull., 2005, 54, 820.
J. P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865.
V. V. Neklyudov, G. A. Boos, S. G. Fattakhov, G. A. Chmutova, M. M. Shulaeva, Yu. I. Sal’nikov, Russ. J. Gen. Chem., 2014, 84, 562.
V. V. Neklyudov, G. A. Boos, S. G. Fattakhov, G. A. Chmutova, M. M. Shulaeva, Yu. I. Salnikov, Butlerovskie soobshcheniya, 2016, 46, 29 [Butlerov Commun. (Engl. Transl.), 2016, 46] (in Russian).
A. D. Ahmed, P. K. Mandal, N. Ray Chaudhuru, J. Inorg. Nucl. Chem., 1966, 28, 2951.
G. V. Afanasyeva, Candidate of Science (Chem.) Thesis, Kazanskiy gos. un-t, Kazan, 2008, 240 pp. (in Russian).
P. N. Buev, N. I. Pechurova, Zh. Neorgan. Khim., 1981, 26, 1953 [J. Inorg. Chem. USSR (Engl. Transl.), 1981, 26] (in Russian).
V. V. Neklyudov, G. A. Boos, S. G. Fattakhov, G. A. Chmutova, M. M. Shulaeva, Yu. I. Sal’nikov, Russ. J. Gen. Chem., 2013, 83, 1369.
Yu. I. Salnikov, G. A. Boos, Kh. V. Gibadullina, Izv. Vuz. Khim. Khim. Tekhnol., 1991, 34, 20 (in Russian).
F. Umland, A. Janssen, D. Thierig, G. Wuensch, Theorie und praktische Anwendung von Komplexbildnern, Akademische Verlagsgesellschaft, Frankfurt am Main, 1971.
M. Beck, I. Nagypal, Chemistry of Complex Equilibria, Akademia Kiado, Budapest, 1989.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This work was financially supported by the Ministry of Education and Science of the Russian Federation through research funding to Kazan Federal University within the framework of the State Assignment (Scientific Research Project No. 4.727.2014/K).
Russian Chemical Bulletin, International Edition, Vol. 69, No. 10, pp. 1907–1915, October, 2020
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 10, pp. 1907–1915, October, 2020.
Rights and permissions
About this article
Cite this article
Neklyudov, V.V., Boos, G.A., Shulaeva, M.M. et al. 1,6-Bis(hydrazidomethylsulfinyl)hexane: the solution state and complexation with copper(II). Russ Chem Bull 69, 1907–1915 (2020). https://doi.org/10.1007/s11172-020-2977-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-020-2977-2