Abstract
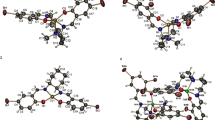
A potentially hexadentate N4O2 Schiff base ligand, L, has been synthesized by condensation of an aromatic diamine with 2-pyridinecarbaldehyde. The complexes [MLNO3]NO3 (M=Cu, Ni, Cd or Zn) and [MlCl2] (M=Co or Mn) were synthesized by the reactions of L with metal salts in methanol. Both free L and its complexes were characterized by physicochemical and spectroscopic methods. In addition, the crystal structures of [CuLNO3]NO3 and [ZnLNO3]NO3 have been determined by single-crystal X-ray diffraction. In both complexes, the ligand L is coordinated via pyridine and azomethine nitrogen atoms to give a distorted octahedral geometry. These complexes have antibacterial activities against three Gram-positive and three Gram-negative bacteria, which in most cases exceed those of tobramycin and tetracycline as standards.



Similar content being viewed by others
References
Chaviara ATh, Cox PJ, Repana KH, Papi RM, Papazisis KT, Zambouli D, Kortsaris AH, Kyriakidis DA, Bolos CA (2004) J Inorg Biochem 98:1271–1283
Shankera K, Rohinia R, Ravindera V, Reddyb PM, Hob Y (2009) Spectrochim Acta Part A 73:205–211
Bala S, Uppal G, Kamboj S, Saini V, Prasad DN (2012) Med Chem Res 21:1–13
Wu H, Jia F, Kou F, Liu B, Yuan J, Bai Y (2011) Trans Met Chem 36:847–853
Wang M, Wang LF, Li YZ, Li QX, Xu ZD, Qu DM (2001) Trans Met Chem 26:307–310
Guo Z, Xing R, Liu S, Zhong Z, Ji X, Wang L, Li P (2007) Carbohydr Res 342:1329–1332
Jarrahpour A, Khalili D, De Clercq E, Salmi C, Brunel JM (2007) Molecules 12:1720–1730
Sriram D, Yogeeswari P, Myneedu NS, Saraswat V (2006) Bioorg Med Chem Lett 16:2127–2129
Metzler CM, Cahill A, Metzler DE (1980) J Am Chem Soc 102:6075–6082
Dudek GO, Dudek EP (1965) Chem Commun 19:464–466
Keypour H, Shayesteh M, Sharifi-Rad A, Salehzadeh S, Khavasi H, Valencia L (2008) J Organomet Chem 693:3179–3187
Keypour H, Shayesteh M, Golbedaghi R, Chehregani A, Blackman AG (2012) J Coord Chem 65:1004–1016
Keypour H, Shayesteh M, Rezaeivala M, Chalabian F, Valencia L (2013) Spectrochim Acta A 101:59–66
Keypour H, Shayesteh M, Rezaeivala M, Chalabian F, Elerman Y, Buyukgungor O (2013) J Mol Struct 1032:62–68
Keypour H, Shooshtari A, Rezaeivala M, Kup FO, Rudbari HA (2015) Polyhedron 97:75–82
Sahin D, Hayvali Z (2012) J Incl Phenom Macro Chem 72:289–297
Fenton DE, Mattews RW, McPartlin M, Murphy BP, Scowen IJ, Tasker PA (1996) Dalton Trans 16:3421–3427
X-AREA (2005) Version 1.30, program for the acquisition and analysis of data. Stoe & Cie GmbH, Darmstadt
X-RED (2005) Version 1.28b, program for data reduction and absorption correction. Stoe & Cie GmbH, Darmstadt
X-SHAPE (2004) Version 2.05, program for crystal optimization for numerical absorption correction. Stoe & Cie GmbH, Darmstadt
Burla MC, Caliandro R, Camalli M, Carrozzini B, Cascarano GL, De Caro L, Giacovazzo C, Polidori G, Spagna R (2005) J Appl Crystallogr 38:381–388
Sheldrick GM (2008) Acta Crystallogr A 64:112–122
Forbes BA, Sahm DF, Weissfeld AS, Trevino EA (1990) Methods for testing antimicrobial effectiveness. In: Baron EJ, Peterson LR, Finegold SM (eds) Bailey & Scott’s diagnostic microbiology, 8th ed. Mosby Co, St Louis, pp 171–194
Russel AD, Furr JR (1977) J Appl Bacteriol 43:23–25
Chehregani A, Mohsenzadeh F, Mirazi N, Hajisadeghian S, Baghali S (2010) Pharm Biol 48:1280–1284
Performance standards for antimicrobial susceptibility testing; Ninth informational supplement (2008) NCCLS document M100-S9. National Committee for Clinical Laboratory Standards, Wayne
Khan MR, Omosto AD (2003) Fitoterapia 74:4494–4497
Dyke SF, Floyd AJ, Sainsbury M, Theobald RS (1978) Organic spectroscopy—an introduction. Longman, New York
Liu H, Wang H, Gao F, Niu D, Lu Z (2007) J Coord Chem 60:2671–2678
Patil SA, Unki SN, Kulkarni AD, Naik VH, Badami PS (2011) Spectrochim Acta A 79:1128–1136
Anan NA, Hassan SM, Saad EM, Butler IS, Mostafa SI (2011) Carbohydr Res 346:775–793
Banerjee S, Ray A, Sen S, Mitra S, Hughes DL, Butcher RJ, Batten SR, Turner DR (2008) Inorg Chim Acta 361:2692–2700
Abou-Hussein AA, Linert W (2015) Spectrochim Acta A 141:223–232
Montazerozohori M, Khani S, Tavakol H, Hojjati A, Kazemi M (2011) Spectrochim Acta Part A 81:122–127
Khandar AA, Cardin C, Hosseini-Yazdi SA, McGrady J, Abedi M, Zarei SA, Gan Y (2010) Inorg Chim Acta 363:4080–4087
Khandar AA, White J, Taghvaee-Yazdeli T, Hosseini-Yazdi SA, McArdle P (2013) Inorg Chim Acta 400:203–209
Acknowledgments
We are grateful to the Faculty of Chemistry of Bu-Ali Sina University for financial supports.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
11243_2015_9966_MOESM1_ESM.doc
CCDC 1063688 and 1063689 contain the supplementary crystallographic data for [ZnLNO3]NO3 and [CuLNO3]NO3 compounds, respectively. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk
Supplementary material 1 (DOC 1348 kb)
Rights and permissions
About this article
Cite this article
Keypour, H., Shooshtari, A., Rezaeivala, M. et al. Synthesis and characterization of transition metal complexes of a hexadentate N4O2 donor Schiff base ligand: X-ray crystal structures of the copper(II) and zinc(II) complexes and their antibacterial properties. Transition Met Chem 40, 715–722 (2015). https://doi.org/10.1007/s11243-015-9966-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-015-9966-6