Abstract
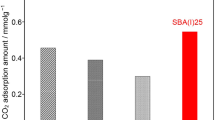
Adsorption properties of amine-functionalized mesoporous silica NH2-SBA-15, zeolite-like imidazole framework ZIF-8, and amine-functionalized metal-organic polymer NH2-MIL-53 have been investigated. Non-modified mesoporous adsorbent SBA-15 has a higher sorption capacity for CO2 than microporous ZIF-8, although microporous sample is characterized by a larger surface area and the values of total pore volume are close. When amine groups are present on the surface of the adsorbents, the chemical adsorption contributes more then the physical one. The adsorption capacity increases with increasing concentration of the functional groups which, in its turn, correlates with adsorbent surface area. Among the studied samples, the best adsorption properties demonstrate amine-functionalized adsorbents, aminefunctionalized mesoporous silica NH2-SBA-15, and amine-functionalized metal-organic polymer NH2-MIL-53.
Similar content being viewed by others
References
N. Du, H. B. Park, M. M. Dal-Cin, M. D. Guiver, Energy Environ. Sci., 2012, 5, 7306–7322.
E. S. Rubin, Elements, 2008, 4, 311–317.
E. S. Rubin, C. Chen, A. B. Rao, Energy Policy, 2007, 35, 4444–4454.
X. Luo, M. Wang, J. Chen, Fuel, 2015, 151, 110–117.
Z. H. Lee, K. T. Lee, S. Bhatia, A. R. Mohamed, Renewable and Sustainable Energy Reviews, 2012, 16, 2599–2609.
G. Calleja, J. Pau, J. A. Calles, J. Chem. Eng. Data, 1998, 43, 994–1000.
J. S. Lee, J. H. Kim, J. T. Kim, J. K. Suh, J. M. Lee, C. H. Lee, Chem. Eng. Data, 2002, 47, 1237–1242.
A. Sayari, Y. Belmabkhout, J. Am. Chem. Soc., 2010, 132, 6312–6314.
J. J. Lee, C.-H. Chen, D. Shimon, S. E. Hayes, C. Sievers, C. W. Jones, J. Phys. Chem. C, 2017, 121, 23480–23487.
A. Samanta, A. Zhao, G. K. H. Shimizu, P. Sarkar, P. Gupta, Ind. Eng. Chem. Res., 2012, 51, 1438–1463.
V. Zelenak, M. Badanicova, D. Halamova, J. Cejka, A. Zukal, N. Murafa, G. Goerigk, J. Chem. Eng., 2008, 144, 336–347.
N. Rao, M. Wang, Z. Shang, Y. Hou, G. Fan, J. Li, Energy Fuels, 2018, 32, 670–677.
B. Arstad, H. Fjellvag, K. O. Kongshaug, O. Swang, R. Blom, Adsorption, 2008, 14, 755–762.
H. R. Abid, Z. H. Rada, X. Duan, H. Sun, S. Wang, Energy Fuels, 2018, 32, 4502–4510; DOI: 10.1021/acs.energyfuels. 7b03240.
L. S. Lai, Y. F. Yeong, N. C. Ani, K. K. Lau, A. M. Shariff, Particulate Sci. Technol., 2014, 32, 520–528.
J.-R. Li, Y. Ma, M. C. McCarthy, J. Sculley, J. Yu, H.-K. Jeong, P. B. Balbuena, H.-C. Zhou, Coord. Chem. Rev., 2011, 255, 1791–1800.
D. Zhao, Q. Huo, J. Feng, B. F. Chmelka, G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024–3036.
W. Klinthong, K. Chao, C. Tan, Ind. Eng. Chem. Res., 2013, 52, 9834–9842.
N. A. H. M. Nordin, A. F. Ismail, A. Mustafa, P. S. Goh, D. Rana, T. Matsuura, RSC Adv., 2014, 4, 33292–33300.
S. Couck, J. F. M. Denayer, G. V. Baron, T. Remy, J. Gascon, F. Kapteijn, J. Am. Chem. Soc., 2009, 131, 6326–6327.
X. Liu, Y. Li, Y. Ban, Chem. Commun., 2013, 49, 9140–9142.
X. Cheng, A. Zhang, K. Hou, Dalton Trans., 2013, 42, 13698–13705.
N. Hiyoshi, K. Yogo, T. Yashima, Micropor. Mesopor. Mater., 2005, 84, 357–365.
A. C. C. Chang, S. S. C. Chuang, M. Gray, Energy Fuels, 2003, 17, 468–473.
R. Banerjee, H. Furukawa, D. Britt, C. Kobler, M. O’Keeffe, O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 3875–3877.
Y. Belmabkhout, G. D. Weireld, A. Sayari, Langmuir, 2009, 25, 13275–13278.
J. Liu, P. K. Thallapally, B. P. McGrail, Chem. Soc. Rev., 2012, 41, 2308–2322.
A. Zhao, A. Samanta, P. Sarkar, R. Gupta, Ind. Eng. Chem. Res., 2013, 52, 6480–6491.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 9, pp. 1595–1600, September, 2018.
Rights and permissions
About this article
Cite this article
Mamonov, N.A., Mikhailov, S.A., Dzhungurova, G.E. et al. Investigation of СО2 adsorption on amine-functionalized silicas and metal-organic polymers. Russ Chem Bull 67, 1595–1600 (2018). https://doi.org/10.1007/s11172-018-2263-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-018-2263-8