Abstract
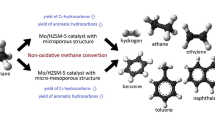
Large crystallite mesoporous MFI (ZSM-5) zeolite was synthesized by using carbon nano-powder as a secondary template. The surface properties, morphological and phase composition of the synthesized material and of the commercial ZSM-5 (Zeolyst) zeolite were studied by nitrogen porosimetry, XRD and scanning electron microscopy. The results showed that the volume of mesopores volume increases with development of a secondary mesoporosity in the structure of zeolite. The obtained zeolite supports were used to prepare molybdenum-containing catalysts for the methane aromatization by solid phase preparation technique. Based on the XPS data, molybdenum particles in these catalysts are characterized by more uniform size distribution. The formation of a secondary pore structure restrains the carbon deposit formation as well as increases the methane conversion and the yield of the aromatic compounds.
Similar content being viewed by others
References
E. V. Nikolaeva, N. A. Mamonov, L. M. Kustov, M. N. Mikhailov, Russ. Chem. Bull., 2015, 64, 269.
M. E. Davis, Nature, 2002, 417, 813.
S. Ma, X. Guo, L. Zhao, S. Scott, X. Bao, J. Energy Chem., 2013, 1, 22.
H. Tao, C. Li, J. Ren, Y. Wang, J. Solid State Chem., 2011, 184, 1820.
Y. Tao, H. Kanoh, L. Abrams, Chem. Rev., 2006, 106, 896.
A. V. Kucherov, Russ. J. Phys. Chem., 2014, 88, 386.
K. Na, M. Choi, R. Ryoo, Microporous Mesoporous Mater., 2013, 166, 3.
Y. Liu, W. Zhang, Z. Liu, S. Xu, J. Phys. Chem., 2008, 112, 15375.
L. Wang, C. Yin, Z. Shan, Colloids Surf. A: Physicochem. Eng. Aspects, 2009, 340, 126.
M. Ogura, S. Shinomiya, J. Tateno, Appl. Catal. A, 2001, 219, 33.
L. M. Kustov, Topics Catal., 1997, 4, 131.
J. H. Scoefild, J. Electr. Spectr., 1976, 9, 29.
M. Conte, B. Xu, T. E. Davies, Microporous Mesoporous Mater., 2012, 164, 207.
H. Liu, X. Bao, Y. Xu, J. Catal., 2006, 239, 441.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 11, pp. 2066—2072, November, 2017.
Rights and permissions
About this article
Cite this article
Mikhaylov, S.A., Mamonov, N.A., Kustov, L.M. et al. Selective conversion of methane to aromatic hydrocarbons on large crystallite zeolite catalysts with mesoporous structure. Russ Chem Bull 66, 2066–2072 (2017). https://doi.org/10.1007/s11172-017-1982-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-017-1982-6