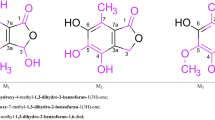
Abstract
A theoretical mechanism of nucleophilic addition of 1,3-diols to cyanoacetylene alcohols was proposed by the results of the quantum chemical study in terms of the density functional theory (DFT) by the B3LYP/6-311++G(d,p) method. In an alkaline medium, the reaction includes the nucleophilic attack of the ionized form of diol to the Cβ atom of cyanacetylene alcohol and proceeds via the formation of the intermediate vinyl carbanion that underwent intramolecular cyclization with 1,3-dioxane ring closure.
Similar content being viewed by others
References
E. A. Chirkina, K. A. Chernyshev, L. B. Krivdin, V. A. Potapov, S. V. Amosova, J. Organomet. Chem., 2014, 766, 49.
E. A. Chirkina, K. A. Chernyshev, E. Yu. Pankrat´ev, L. B. Krivdin, V. A. Potapov, S. V. Amosova, Russ. J. Org. Chem. (Engl. Transl.), 2013, 49, 508 [Zh. Org. Khim., 2013, 49, 526].
A. N. Volkov, A. N. Nikol´skaya, Russ. Chem. Rev., 1977, 46, 374.
Yu. M. Skvortsov, A. G. Mal´kina, A. N. Volkov, B. A. Trofimov, E. B. Oleinikova, I. V. Kazin, V. V. Gedymin, Bull. Acad. Sci. USSR, Div. Chem. Sci. (Engl. Transl.), 1978, 17, 872 [Izv. Akad. Nauk SSSR, Ser. Khim., 1978, 872].
B. A. Trofimov, A. G. Mal´kina, Heterocycles, 1999, 51, 2485.
A. A. Sumenov, Ocherk khimii prirodnykh soedinenii [Essay of the Chemistry of Natural Compounds], in Stat´i po khimii prirodnykh soedinenii [Articles on the Chemistry of Natural Compounds], Nauka, Novosibirsk, 2000, p. 71 (in Russian).
H. Tadashi, S. Nobuaki, Y. Hiroshi, Carbohydr. Res., 2005, 340, 2494.
I. V. Gilyautdinov, N. A. Ves´kina, S. R. Afon´kina, L. M. Khalilov, N. V. Odinokov, Russ. J. Org. Chem. (Engl. Transl.), 2006, 42, 1333 [Zh. Org. Khim., 2006, 42, 1352].
X. Li, M. Zhao, Y. R. Tang, C. Wang, Z. Zhang, S. Peng, Eur. J. Med. Chem., 2008, 43, 8.
T. Asaki, T. Aoki, T. Hamamoto, Y. Sugiyama, S. Ohmachi, K. Kuwabara, K. Murakami, M. Todo, Bioorg. Med. Chem., 2008, 16, 981.
I. L. Glazko, O. P. Gur´yanova, S. V. Levanova, S. A. Kozlova, N. S. Neiman, J. Appl. Chem. (Engl. Transl.), 2005, 78, 950 [Zh. Prikl. Khim., 2005, 78, 972].
D. N. Laikov, Yu. A. Ustynyuk, Russ. Chem. Bull. (Int. Ed.), 2005, 64, 820 [Izv. Akad. Nauk, Ser. Khim., 2005, 804].
M. Tuckerman, G. Martyna, B. J. Berne, J. Chem. Phys., 1991, 94, 6811.
C. González, H. B. Schlegel, J. Phys. Chem., 1990, 94, 5523
C. González, H. B. Schlegel, J. Chem. Phys., 1991, 95, 5853.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, D. J. Fox, Gaussian 09, Revision C.01, Gaussian, Inc., Wallingford (CT), 2009.
J. Tomasi, B. Mennucci, E. Cancès, THEOCHEM, 1999, 464, 211
J. Tomasi, B. Mennucci, R. Cammi, Chem. Rev., 2005, 105, 2999.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Academician of the Russian Academy of Sciences V. I. Minkin on the occasion of his 80th birthday.
For Part 2, see Ref. 1.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 3, pp. 0511—0517, March, 2015.
Rights and permissions
About this article
Cite this article
Chirkina, E.A., Krivdin, L.B., Mal´kina, A.G. et al. Quantum chemical study of mechanisms of organic reactions. Russ Chem Bull 64, 511–517 (2015). https://doi.org/10.1007/s11172-015-0894-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-015-0894-6