Abstract
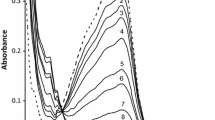
Oxidation kinetics of complexes [CrIII(OR)(HOX)(H2O)3]+ and [CrIII(OR)(HM)(H2O)3]+ (OR is orotic acid, HOX is oxalic acid, HM is malonic acid) by periodate in aqueous acidic medium was studied using spectrophotometric measurements in the pH range 3.34–4.63 at different temperatures. The reaction is first order in concentrations of both the IO4 − ion and complex. For both complexes, the oxidation rate increases with the pH increase. The experimental rate law is consistent with a mechanism in which the deprotonated ([CrIII(OR)(OX)(H2O)2]) and protonated ([CrIII(OR)(HM)(H2O)3]+) species are significantly more reactive. Apparently, electron transfer proceeds through an inner sphere mechanism via coordination of the IO4 − ion to chromium(iii). Thermodynamic activation parameters were calculated using the transition state theory equations.
Similar content being viewed by others
References
P. Ruasmadiedo, J. C. Badagancedo, E. G. Fernandez, D. G. Dellano, C. G. Reyes, J. Food Prot., 1996, 59, 502.
K. Matsumoto, Inorg. Chim. Acta, 1988, 151, 9.
G. Maistralis, A. Koutsodimou, N. Katsaros, Transition Met. Chem., 2000, 25, 166.
A. Levina, R. Codd, C. T. Dillon, P. A. Lay, Prog. Inorg. Chem., 2003, 51, 145.
D. J. B. Galliford, J. M. Ottaway, Analyst, 1972, 91, 412.
F. R. El-Eziri, Y. Sulfab, Inorg. Chim. Acta, 1977, 25, 15.
I. H. Ali, Y. Sulfab, Int. J. Chem. Kinet., 2011, 43, 563.
H. A. Ewais, S. M. Hassun, Oxid. Commun., 2012, Book 2, 340.
E. S. H. Khaled, Inorg. React. Mech., 2007, 6, 247.
A. M. Abdel-Hady, Transition Met. Chem., 2000, 25, 437.
H. A. Ewais, S. A. Ahmed, A. A. Abdel-Khalek, Inorg. React. Mech., 2004, 5, 125.
A. A. Abdel-Khalek, M. M. Elsemongy, Transition Met. Chem., 1989, 14, 206.
H. A. Ewais, S. A. Elroby, M. A. Habib, Transition Met. Chem., 2010, 35, 37.
A. A. Abdel-Khalek, I. M. I. Ismail, M. A. Nagdy, H. A. Ewais, Oxid. Commun., 2012, Book 2, 327.
H. A. Ewais, M. A. Nagdy, A. A. Abdel-Khalek, Transition Met. Chem., 2012, 37, 525.
F. S. Head, H. A. Standing, J. Chem. Soc., 1953, 75, 5670.
H. A. Ewais, F. D. Dahaman, A. A. Abdel-Khalek, Chem. Cent. J., 2009, 3, 3.
H. A. Ewais, J. Coord. Chem., 2009, 62, 940.
I. Hadinec, L. Jenšovsky, A. Linek, V. Syneek, Naturwissenschaften, 1960, 47, 377.
P. Ray, Inorg. Synth., 1957, 5, 201.
C. E. Crouthamel, A. M. Hayes, D. S. Martin, J. Am. Chem. Soc., 1951, 73, 82.
A. Y. Kassim, Y. Sulfab, Inorg. Chim. Acta, 1977, 22, 169.
M. Rizzotto, A. Levina, M. Santoro, S. Garcia, M. I. Frascaroli, S. Signorella, L. F. Sala, P. A. Lay, J. Chem. Soc., Dalton Trans., 2002, 3206.
A. Levina, P. A. Lay, Coord. Chem. Rev., 2005, 249, 281.
T. W. Kallen, M. J. Root, K. A. Schroeder, Inorg. Chem., 1979, 18, 3318.
M. J. Weaver, E. L. Yee, Inorg. Chem., 1980, 19, 1936.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 3, pp. 0591–0598, March, 2014.
Rights and permissions
About this article
Cite this article
Ismail, I.M.I., Ewais, H.A. & Elroby, S.A.K. Kinetics and mechanism of periodate oxidation of ternary orotatochromium(iii) complexes bearing oxalate or malonate co-ligands. Russ Chem Bull 63, 591–598 (2014). https://doi.org/10.1007/s11172-014-0478-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-014-0478-x