Abstract
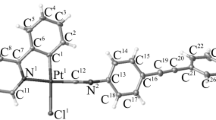
The replacement of the iodide ligands in the complex [PtI2(dpa)] (1) (dpa is 2,2′-dipyridylamine) by silver triflate in acetonitrile afforded the compound [Pt(dpa)(MeCN)2](SO3CF3)2 (2). Homoleptic complexes [Pt(dpa)2](X)2 (3·(X)2) were synthesized by the treatment of [PtI2(dpa)] (1) with 2,2′-dipyridylamine in the presence of silver salts AgX in methanol (X = NO3) or acetonitrile (X = SO3CF3). The deprotonation of the complex [3](SO3CF3)2 to give the homoleptic complex [Pt(dpa-H)2] (4) was performed by two methods, e.g., by the treatment of [3](SO3CF3)2 with 2 equiv. of NaOH in methanol or by the addition of excess Et3N to a suspension of [3](SO3CF3)2 in methanol. The structures of compounds 1–4 were established by elemental analyses, high resolution electrospray ionization mass spectrometry, IR and NMR spectroscopy; the crystal structure of complexes [2](SO3CF3)2, [3](NO3)2·H2O, [3](SO3CF3)2·2H2O, and 4 were determined by single-crystal X-ray diffraction.
Similar content being viewed by others
References
T. Kajiwara, A. Kamiyama, T. Ito, Polyhedron, 2003, 22, 1789; T. Kajiwara T. Ito, Eur. J. Inorg. Chem., 2004, 3084; T. Kajiwara, A. Kamiyama, T. Ito, Chem. Commun., 2002, 1256; L.-L. Zheng, W.-X. Zhang, L.-J. Qin, J.-D. Leng, J.-X. Lu, M.-L. Tong, Inorg. Chem., 2007, 46, 9548; A. Igashira-Kamiyama, T. Kajiwara, T. Konno, T. Ito, Inorg. Chem., 2006, 45, 6460; L.-L. Zheng, J.-D. Leng, W.-T. Liu, W.-X. Zhang, J.-X. Lu, M.-L. Tong, Eur. J. Inorg. Chem., 2008, 4616.
N. Heße, R. Fröhlich, I. Humelnicu, E.-U. Würthwein, Eur. J. Inorg. Chem., 2005, 2189.
N. Heße, R. Fröhlich, B. Wibbeling, E.-U. Würthwein, Eur. J. Org. Chem., 2006, 3923.
H. J. Breslin, M. J. Kukla, R. W. Tuman, M. C. Rebarchak, C. R. Bowden, J. Med. Chem. Commun., 1993, 36, 1597.
I. Häger, R. Fröhlich, E.-U. Würthwein, Eur. J. Inorg. Chem., 2009, 2415.
G. H. Sarova, N. A. Bokach, A. A. Fedorov, M. N. Berberan-Santos, V. Yu. Kukushkin, M. Haukka, J. J. R. Fraústo da Silva, A. J. L. Pombeiro, Dalton Trans., 2006, 3798.
P. V. Gushchin, M. R. Tyan, N. A. Bokach, M. D. Revenco, M. Haukka, M.-J. Wang, C.-H. Lai, P.-T. Chou, V. Yu. Kukushkin, Inorg. Chem., 2008, 47, 11487.
P. V. Gushchin, M. L. Kuznetsov, M. Haukka, M.-J. Wang, A. V. Gribanov, V. Yu. Kukushkin, Inorg. Chem., 2009, 48, 2583; M. N. Kopylovich, M. Haukka, A. M. Kirillov, V. Yu. Kukushkin, A. J. L. Pombeiro, Chem. Eur. J., 2007, 13, 786; M. N. Kopylovich, A. J. L. Pombeiro, A. Fischer, L. Kloo, V. Yu. Kukushkin, Inorg. Chem., 2003, 42, 7239; P. V. Gushchin, K. V. Luzyanin, M. N. Kopylovich, M. Haukka, A. J. L. Pombeiro, V. Yu. Kukushkin, Inorg. Chem., 2008, 47, 3088; A. G. Tskhovrebov, N. A. Bokach, M. Haukka, V. Yu. Kukushkin, Inorg. Chem., 2009, 48, 8678; M. N. Kopylovich, E. A. Tronova, M. Haukka, A. M. Kirillov, V. Yu. Kukushkin, J. J. R. Fraústo da Silva, A. J. L. Pombeiro, Eur. J. Inorg. Chem., 2007, 4621; M. N. Kopylovich, K. V. Luzyanin, M. Haukka, A. J. L. Pombeiro, V. Yu. Kukushkin, Dalton Trans., 2008, 5220.
N. A. Bokach, T. V. Kuznetsova, S. A. Simanova, M. Haukka, A. J. L. Pombeiro, V. Yu. Kukushkin, Inorg. Chem., 2005, 44, 5152.
M. J. Rauterkus, S. Fakih, C. Mock, I. Puscasu, B. Krebs, Inorg. Chim. Acta, 2003, 350, 355.
C. Tu, X. Wu, Q. Liu, X. Wang, Q. Xu, Z. Guo, Inorg. Chim. Acta, 2004 357, 95.
I. Puscasu, C. Mock, M. Rauterkus, A. Roendigs, G. Tallen, Z. Anorg. Allg. Chemie, 2001, 627, 1292.
R. Romeo, N. Nastasi, L. M. Scolaro, M. R. Plutino, A. Albinati, A. Macchioni, Inorg. Chem., 1998, 37, 5460.
F. Zhang, M. C. Jennings, R. J. Puddephatt, Chem. Commun., 2007, 1496.
F. B. Zhang, E. M. Prokopchuk, M. E. Broczkowski, M. C. Jennings, R. J. Puddephatt, Organometallics, 2006, 25, 1583.
E. Guney, V. T. Yilmaz, O. Buyukgungor, Inorg. Chim. Acta, 2010, 363, 2416.
D. Fraccarollo, R. Bertani, M. Mozzon, U. Belluco, R. Michelin, Inorg. Chim. Acta, 1992, 201, 15.
V. Yu. Kukushkin, T. B. Pakhomova, Yu. N. Kukushkin, R. Herrmann, G. Wagner, A. J. L. Pombeiro, Inorg. Chem., 1998, 37, 6511.
A. G. Orpen, L. Brammer, F. H. Allen, O. Kennard, D. G. Watson, R. Taylor, J. Chem. Soc., Dalton. Trans., 1989, S1.
F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen, R. Taylor, J. Chem. Soc., Perkin Trans. 2, 1987, S1.
V. Yu. Kukushkin, A. J. L. Pombeiro, Inorg. Chem. Acta, 2005, 358, 1; V. Yu. Kukushkin, A. J. L. Pombeiro, Chem. Rev., 2002, 102, 1771.
E. A. Klimova, Osnovnye mikrometody analisa organicheskich soedinenii [Basic microanalytical methods in organic chemistry], Khimiya, Moscow, 1967 (in Russian).
A. J. M Duisenberg, L. M. J. Kroon-Batenburg, A. M. M. Schreurs, J. Appl. Crystallogr., 2003, 36, 220.
Z. Otwinowski, W. Minor, Processing of X-ray Diffraction Data Collected in Oscillation Mode, Academic Press, New York, 1997, pp. 307–326.
Bruker AXS, APEX2, Software Suite for Crystallographic Programs, Bruker AXS, Inc., Madison, WI, USA, 2009.
G. M. Sheldrick, Acta Crystallogr., Sect. A, A64, 2008, 112.
L. J. Farrugia, J. Appl. Crystallogr., 1999, 32, 837.
G. M. Sheldrick, SADABS. Bruker AXS scaling and absorption correction, Bruker AXS, Inc., Madison, Wisconsin, USA, 2008.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 4, pp. 0825–0832, April, 2012.
Rights and permissions
About this article
Cite this article
Wang, Q., Gushchin, P.V., Bokach, N.A. et al. Platinum complexes bearing 2,2′-dipyridylamine ligand. Russ Chem Bull 61, 828–835 (2012). https://doi.org/10.1007/s11172-012-0115-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-012-0115-5