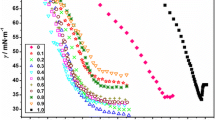
Abstract
The variation of the microscopic properties (surface potential, micropolarity, etc.) of the interface of cetyltrimethylammonium bromide micelles upon the addition of a background electrolyte or the nonionic surfactant Triton X-100 decreases the rates of ion-molecular reactions, namely, alkaline hydrolysis of carboxylic acid esters and tetracoordinate phosphorus acid esters, and results in the shift of acid-base equilibria.
Similar content being viewed by others
References
I. V. Berezin, K. Martinek, and A. K. Yatsimirskii, Usp. Khim., 1973, 42, 1729 [Russ. Chem. Rev., 1973, 42, 1343 (Engl. Transl.)].
C. A. Bunton and G. Savelli, Adv. Phys. Chem., 1986, 22, 213.
L. S. Romsted, Surfactants in Solution, Ed. K. L. Mittal, Plenum Press, New York-London, 1984, 4, 1015.
E. J. Fendler and J. H. Fendler, Adv. Phys. Org. Chem., 1970, 8, 271.
K. Holmberg, Current Opinion in Colloid Interface Sci., 2003, 8, 187.
K. Shinoda, T. Nakagawa, B. Tamamushi, and T. Isemura, Colloid Surfactants. Some Physicochemical Properties, Academic Press, New York-London, 1963.
D. B. Kudryavtsev, L. Ya. Zakharova, and L. A. Kudryavtseva, Zh. Fiz. Khim., 2003, 77, 443 [Russ. J. Phys. Chem., 2003, 77 (Engl. Transl.)].
L. Ya. Zakharova, D. B. Kudryavtsev, F. G. Valeeva, and L. A. Kudryavtseva, Zh. Obshch. Khim., 2002, 72, 1296 [Russ. J. Gen. Chem., 2002, 72 (Engl. Transl.)].
R. A. Hobson, F. Grieser, and T. W. Healy, J. Phys. Chem., 1994, 98, 274.
N. A. Smirnova, Usp. Khim., 2005, 74, 138 [Russ. Chem. Rev., 2005, 74 (Engl. Transl.)].
L. Ya. Zakharova, F. G. Valeeva, A. V. Zakharov, A. B. Mirgorodskaya, L. A. Kudryavtseva, and A. I. Konovalov, Kinet. Katal., 2007, 48, 221 [Kinet. Catal., 2007, 48 (Engl. Transl.)].
US Pat. N 2922810 (1960); Chem. Abstrs, 1960, 54, 9848.
D. N. Rubingh, Solution Chemistry of Surfactants, Ed. K. L. Mittal, Plenum Press, New York, 1979, Vol. 1, p. 337.
L. Ya. Zakharova, F. G. Valeeva, A. R. Ibragimova, L. A. Kudryavtseva, N. N. Valeev, T. L. Didenko, V. I. Kovalenko, and A. I. Konovalov, Izv. Akad. Nauk, Ser. Khim., 2002, 2019 [Russ. Chem. Bull., Int. Ed., 2002, 51, 2176].
L. Zakharova, F. Valeeva, A. Zakharov, A. Ibragimova, L. Kudryavtseva, and H. Harlampidi, J. Colloid Interface Sci., 2003, 263, 597.
N. O. Mchedlov-Petrosyan, Differentsirovanie sily organicheskikh kislot v istinnykh i organizovannykh rastvorakh [Differentiation of the Strength of Organic Acids in True and Organized Solutions], Izd-vo Khar’kov Nats. Univ., Kharkov, 2004, 326 (in Russian).
A. B. Mirgorodskaya, L. A. Kudryavtseva, L. Ya. Zakharova, and V. E. Bel’skii, Izv. Akad. Nauk, Ser. Khim., 1998, 1333 [Russ. Chem. Bull., 1998, 47, 1296 (Engl. Transl.)].
K. M. Solntsev, S. A. Al-Ainain, Y. V. Il’ichev, and M. G. Kuzmin, J. Phys. Chem., A, 2004, 108, 8212.
L. Ya. Zakharova, S. B. Fedorov, L. A. Kudryavtseva, V. E. Bel’skii, and B. E. Ivanov, Izv. Akad. Nauk SSSR, Ser. Khim., 1993, 1396 [Russ. Chem. Bull., 1993, 42, 1329 (Engl. Transl.)].
L. Ya. Zakharova, D. B. Kudryavtsev, L. A. Kudryavtseva, A. I. Konovalov, Y. F. Zuev, N. N. Vylegzhanina, N. L. Zakhartchenko, and Z. Sh. Idiyatullin, Mendeleev Commun., 1999, 245.
A. I. Serdyuk and R. V. Kucher, Mitsellyarnye perekhody v rastvorakh poverkhnostno-aktivnykh veshchestv [Micellar Transitions in Surfactant Solutions], Naukova Dumka, Kiev, 1987, 208 (in Russian).
V. R. Aswal and P. S. Goyal, Phys. Rev. E, 2000, 61, 2947.
L. J. Magid, Z. Han, G. G. Warr, M. A. Kassidi, P. B. Bulter, and W. A. Hamilton, J. Phys. Chem. B, 1997, 101, 7919.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 10, pp. 1933–1940, October, 2007.
Rights and permissions
About this article
Cite this article
Mirgorodskaya, A.B., Zakharova, L.Y., Valeeva, F.G. et al. Control of the catalytic effect of cetyltrimethylammonium bromide micelles by the addition of background electrolyte and nonionic surfactant. Russ Chem Bull 56, 2000–2007 (2007). https://doi.org/10.1007/s11172-007-0312-9
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11172-007-0312-9