Abstract
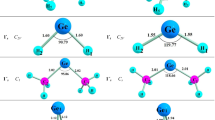
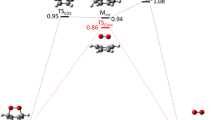
The formation and decomposition pathways of germiranes (germacyclopropanes), i.e., products of reactions of the GeH2 and GeMe2 germylenes with ethylene, tetramethylethylene, buta-1,2,3-triene, and tetramethylbuta-1,2,3-triene, were studied using the density functional approach (PBE/TZ2P approximation). The thermodynamic stabilities of the structures under consideration were evaluated by calculating the Gibbs free energies under normal conditions (ΔG°298). Addition of germylenes to the C=C bond can proceed as a single-step process without a barrier or involve the formation of a π-complex (the barrier to this process is lower than the sum of the energies of isolated reactants). Stability of the germiranes formed is determined by their stability to retrodecomposition into the initial germylene and olefin and to the three-membered ring opening followed by simultaneous 1,2-migration of the substituent at the Ge atom and formation of the secondary germylene. Alkyl substituents can efficiently block the opening of the three-membered ring and transformation of the cyclic structure into the secondary germylene, simultaneously decreasing the germirane stability to retrodecomposition. Decomposition into germylene and olefin under normal conditions is thermally favorable for hexamethylgermirane (ΔG°298 = −5.7 kcal mol−1), being thermally forbidden for the other germiranes studied in this work (Δ G°298 > 0). The activation energy (E a) for the germirane ring opening depends on the substituents at the germanium atom, namely, E a ≤ 10 kcal mol−1 for unsubstituted germiranes and E a > 30 kcal mol−1 for methyl-substituted germiranes. Taking the experimentally isolated germirane as an example, it was shown how the introduction of substituents and modification of the carbon skeleton make it possible to stabilize the germacyclopropane system.
Similar content being viewed by others
References
P. P. Gaspar and R. West, in The Chemistry of Organic Silicon Compound, Eds Z. Rappoport and Y. Apeloig, Wiley, 1998, 2, p. 2463.
W. P. Neumann, Chem. Rev., 1991, 91, 311.
W. Ando, H. Ohgaki, and Y. Kabe, Angew. Chem., Int. Ed. Engl., 1994, 33, 659.
Y. Kabe, H. Ohgaki, T. Yamagaki, H. Nakanishi, and W. Ando, J. Organomet. Chem., 2001, 636, 82.
R. Becerra, S. E. Boganov, M. P. Egorov, O. M. Nefedov, and R. Walsh, Chem. Phys. Lett., 1996, 260, 433.
R. Becerra, S. E. Boganov, M. P. Egorov, V. I. Faustov, V. M. Promyslov, O. M. Nefedov, and R. Walsh, Phys. Chem. Chem. Phys., 2002, 4, 5079.
R. Becerra, S. E. Boganov, M. P. Egorov, V. Ya. Lee, O. M. Nefedov, and R. Walsh, Chem. Phys. Lett., 1996, 250, 111.
M.-D. Su and S.-Y. Chu, J. Am. Chem. Soc., 1999, 121, 11478.
S. Sakai, Int. J. Quant. Chem., 1998, 70, 291.
J. P. Perdew, K. Burke, and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865.
D. N. Laikov, Ph.D. (Phys.-Math.) Thesis, M. V. Lomonosov Moscow State University, Moscow, 2000 (in Russian).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, Jr., R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, A. G. Baboul, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, J. L. Andres, C. Gonzalez, M. Head-Gordon, E. S. Replogle, and J. A. Pople, GAUSSIAN 98, Revision A.7, Gaussian, Inc., Pittsburgh (PA), 1998.
D. N. Laikov, Chem. Phys. Lett., 1997, 281, 151.
R. Becerra, S. E. Boganov, M. P. Egorov, V. Ya. Lee, O. M. Nefedov, and R. Walsh, Chem. Phys. Lett., 1996, 250, 111.
K. M. Baines and W. G. Stibbs, Coord. Chem. Rev., 1995, 145, 157.
S. E. Boganov, M. P. Egorov, V. I. Faustov, I. V. Krylova, O. M. Nefedov, R. Becerra, and R. Walsh, Izv. Akad. Nauk, Ser. Khim., 2005, 477 [Russ. Chem. Bull., Int. Ed., 2005, 44, 483].
R. D. Bach and O. Dmitrenko, J. Org. Chem., 2002, 67, 2588; Y. Naruse, J. Ma, and S. Inagaki, Tetrahedron Lett., 2001, 42, 6553; B. M. Gimarc and M. Zhao, Coord. Chem. Rev., 1997, 158, 385.
Author information
Authors and Affiliations
Additional information
Dedicated to Academician A. L. Buchachenko on the occasion of his 70th birthday.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 9, pp. 1943–1951, September, 2005.
Rights and permissions
About this article
Cite this article
Birukov, A.A., Faustov, V.I., Egorov, M.P. et al. Isomerization and decomposition of germiranes: a density functional study. Russ Chem Bull 54, 2003–2012 (2005). https://doi.org/10.1007/s11172-006-0072-y
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11172-006-0072-y