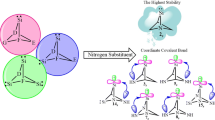
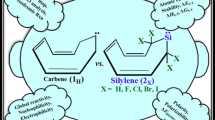
Abstract
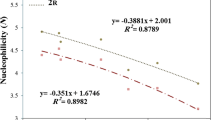
In this computational survey, substituent effects of group 17 on the stability (singlet–triplet energy gaps, ΔEs–t) and reactivity of singlet (s) and triplet (t) forms of 2-germabicyclo[1.1.1.]pentane-2-ylidenes are considered by using B3LYP/6–311 + + G**, B3LYP/aug-cc-pvtz, and B3LYP/def2-TZVP level of theories. In all germylene structures, singlets appear more stable than their corresponding triplet congeners, revealing a singlet ground state and the order of stability appears to be 1,3,4,4,5,5-hexachloro-2-germabicyclo[1.1.1.]pentane-2-ylidenes (3) > 1,3,4,4,5,5-hexabromo-2-germabicyclo[1.1.1.]pentane-2-ylidenes (4) > 1,3,4,4,5,5-hexafluoro-2-germabicyclo[1.1.1.]pentane-2-ylidenes (2) > 1,3,4,4,5,5-hexaiodo-2-germabicyclo[1.1.1.]pentane-2-ylidenes (5) > 2-germabicyclo[1.1.1.]pentane-2-ylidenes (1), at the three levels of theory. The positive and negative effects on germylene stability are LP(F, Cl, Br, and I) → LP*G̈e and σ(C-Ge) → σ*(C-F, Cl, Br, and I) interactions, respectively. The results of our calculations show that every singlet germylene with high LP(F, Cl, Br, and I) → LP*G̈e interactions has higher electrophilicity. Also, in going from the most electronegative F to the least electronegative I, the nucleophilicity index (N) for germylene increases. Finally, this survey introduces that germylene 4 s with rather high band gap (ΔEHOMO–LUMO = 97.19 kcal/mol), nucleophilicity (2.20 eV), and stability (ΔEs-t = 76.95 kcal/mol) has high proton affinity (171.55 kcal/mol) that can be applied as multidentate ligands and it is hoped that this will prompt experimental attention toward its.





Similar content being viewed by others
Data availability
“Not applicable.”
Code availability
GAMESS program package, (U)B3LYP/6–311 + + G** level of theory.
References
Tomioka H (1997) Acc Chem Res 30:315
Jutzi P, Kanne K, Krueger C (1986) Angew Chem Int Ed Engl 25(2):164–164
Barrau J, Escudie J, Satge J (1990) Chem Rev 90:283–319
Ohshita J, Iida T, Ikeda M, Uemura T, Ohta N, Kunai A, Organomet J (2004) Chem 689(9):1540–1545
Asay M, Jones C, Driess M (2011) Chem Rev 111:354–396
Yoshida M, Tamaoki N (2002) Organometallics 21:2587–2589
Nemirowski A, Schreiner PR (2007) J Org Chem 72(25):9533–9540
Neumann WP (1991) Chem Rev 91(3):311–334
Barrau J, Escudie J, Satge J (1990) Chem Rev 90(1):283–319
Lappert MF (1990) Coord Chem Rev 100:267–292
Jutzi P, Kanne K, Krueger C (1986) Angew Chem Int Ed Engl 25(2):164–164
Jutzi P, Holtmannm U, Kanne D, Kruger C, Blom R, Gleiter R, Hyla-Krypsin I (1989) Chem Ber 122(9):1629–1639
Barrau J, Rima G (1998) Coord Chem Rev 178:593–622
Vessally E (2008) Heteroat Chem 19(3):245–251
Heaven MW, Metha GF, Buntine MA (2001) J Phys Chem A 105(7):1185–1196
Biswas AK, Ganguly B (2017) Chem Eur J 23(11):2700–2705
Cernicharo J, Gottlieb CA, Guélin M, Killian TC, Paubert G, Thaddeus P, Vrtilek JM (1991) Astrophys J 368:L39–L41
Redondo P, Redondo JR, Largo A (2000) J Mol Struct Theochem 505(1–3):221–232
Thaddeus P, Vrtilek JM, Gottlieb CA (1985) Astrophys J 299:L63–L66
Reisenauer HP, Maier G, Riemann A, Hoffmann RW (1984) Angew Chem Int Ed Engl 23(8):641–641
Barthelat JC, Roch BS, Trinquier G, Satge J (1980) J Am Chem Soc 102(12):4080–4085
Hadlington TJ, Driess M, Jones C (2018) Chem Soc Rev 47:4176–4197
Kirilchuk AA, Rozhenko AB, Leszczynski J (2017) Comput Theor Chem 1103:83–91
Mizuhata Y, Sasamori T, Tokitoh N (2009) Chem Rev 109:3479–3511
Holthausen MC, Koch W, Apeloig Y (1999) J Am Chem Soc 121:2623–2624
Jiang P, Gaspar PP (2001) J Am Chem Soc 123:8622–8623
Schreiner PR, Reisenauer HP, Allen WD, Sattelmeyer KW (2004) Org Lett 6:1163–1166
Abedini N, Kassaee MZ (2020) J Mol Model 26(11):1–11
Abedini N, Kassaee MZ, (2021) J Phys Org Chem 34(8).
Hoffmann R, Schleyer PvR, Schaefer HF (2008) Angew Chem Int Ed 47(38):7164–7167
Schmidt W, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) J Comput Chem 14:1347–1363
Bao W, Li Y, Lu X (2013) Struct Chem 24:1615–1619
Aysin RR, Bukalov SS, Leites LA, Zabula AV (2017) Dalton Trans 46:8774–8781
Schlegel HB, Frisch MJ (1995) Int J Quantum Chem 54:83
Rostami Z, Asnaashariisfahan M, Ahmadi S, Hosseinian A, Ebadi A (2021) J Mol Struct 1238:130427. https://doi.org/10.1016/j.molstruc.2021.130427
Aysin RR, Bukalov SS, Leites LA, Lalov AV, Tsys KV, Piskunov AV (2019) Organometallics 38(16):3174–3180
Schleyer PvR, Maerker C, Dransfeld A, Jiao H, Rv NJ, Hommes E (1996) J Am Chem Soc 118:6317–6318
Domingo LR, Chamorro E, Perez P (2008) J Org Chem 73:4615–4624
Parr RG, Szentpaly L, Liu S (1999) J Am Chem Soc 121:1922–1924
Parr RG, Pearson RG (1983) J Am Chem Soc 105:7512–7516
Abedini N, Kassaee MZ (2021) Struct Chem 32(3):1105–1112
Scrocco E, Tomasi J (1973) New Concepts II 42:95–170
Abedini N, Kassaee MZ, Cummings PT (2020) Theor Chem Acc 139(8):1–11
Abedini N, Kassaee MZ, Cummings PT (2021) Silicon 13(10):3377–3383
Abedini N, Kassaee MZ (2020) Comput Theor Chem 1190:112998
Abedini N, Kassaee MZ (2021) J Mol Model 27(5):1–13
Abedini N, Kassaee MZ (2022) Silicon 14:2089–2095
Acknowledgements
The support from Tarbiat Modares University (TMU) is gratefully acknowledged. Special thanks are due to Mrs. Shokufeh Mojtahedi for his continued encouragement and moral support.
Funding
This study is supported by the Tarbiat Modares University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
“Not applicable.”
Consent to participate
We have consent to participate.
Consent for publication
We consent for publication.
Competing interests
“The authors declare no competing interests.”
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abedini, N., Kassaee, M.Z. Estimating structure, stability, and electronic properties on halogenated derivatives of 2-germabicyclo[1.1.1.]pentane-2-ylidenes at density functional theory. J Mol Model 28, 207 (2022). https://doi.org/10.1007/s00894-022-05202-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05202-y