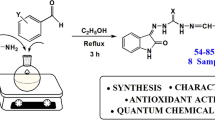
Abstract
Isatin-derived Schiff bases are the subject of many studies, finding wide application areas in polymer technology, pharmaceutical industry, and medicine. In this study, a series of new Schiff bases were prepared from monothiocarbohydrazones based on isatins with different substituents (5-F, 5-Br, 5-I, and 5-MeO). The chemical structures of the synthesized compounds were determined using 1H NMR, 13C NMR, FTIR spectroscopic techniques, and elemental analysis. Antioxidant activity determinations of 23 compounds were performed using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical quenching method. The highest percent inhibition value at 10 µM concentration was shown by compound number 22, 5-bromoisatin Schiff base containing 3-methoxy-4-hydroxy group. Compound 17, a 5-iodoisatin Schiff base containing 3-methoxy-4-hydroxy group, showed the highest antioxidant activity with an IC50 value of 9.76 ± 0.03 µM. In addition to the theoretical analysis of the compounds, both their spectroscopic and antioxidant properties were investigated. The ground-state geometries and some chemical reactivity parameters of the compounds were calculated using the B3LYP/6-311 + + G(2d,2p) approach. Besides intramolecular interactions, substituent effects, and some QTAIM parameters, the calculations were also performed to study the electronic properties of reactive N/O–H bonds and were used to interpret the experimental results. The effects of the electronic parameters and intramolecular interactions of reactive N/O–H bonds on the antioxidant properties of the compounds were investigated. Additionally, the relationships of DPPH reactions with delocalization indices of N/O-H bonds and the pattern of SET/HAT mechanisms with electronic variables were analyzed. Examination of electron and hydrogen atom transfer mechanisms has shown the dominance of electron transfer, supported by the correlation coefficients between IC50 values and SET reaction energies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schiff bases containing heterocyclic structures have a wide range of biological and pharmacological activities [1]. Isatin-derived Schiff bases have been the subject of various studies for their anticonvulsant, antibacterial, anti-HIV, and antifungal activities, thus becoming an important class of compounds in pharmaceutical chemistry [2]. Schiff bases, synthesized by the condensation reaction of different amino compounds with aldehydes or ketones, are also known as good nitrogen donor ligands. Schiff bases show a wide range of applications not only in biological activities but also in coordination chemistry, industries, food packaging, paints, and polymers [3]. The number of studies providing systematic information about antioxidant activity determinations of Schiff bases remains limited.
It has been reported that steric hydrocarbon phenols and their complex components are used as antioxidants in the polymer and food industry and as a preservative that prevents oxidation processes in pharmacy [4]. These are compounds that vary structurally from a simple phenolic molecule to complex high-molecular-weight polymers and are thought to reduce health disorders due to their antioxidant activity [5]. When antioxidant substances are added to foods, they minimize rancidity, delay the formation of toxic oxidation products, preserve food quality, and increase shelf life [6]. Current studies on derivatives of thiosemicarbazone Schiff bases and their complexes show the importance of examining the antioxidant, anticorrosive, and anticancer properties of these compounds. To understand the antioxidant activity mechanisms of these compounds, their electronic and molecular properties must be determined in detail [7,8,9].
In this study, in order to provide an important resource for current studies, new isatin-derived Schiff bases with different substituents were synthesized and their antioxidant behaviors were comparatively examined experimentally and theoretically. The antioxidant activities of the synthesized compounds were elucidated experimentally using the DPPH method and theoretically using the DFT approach. The relationship between antioxidant properties and structural and electronic properties of the compounds was also examined by Quantum Theory of Atoms in Molecules (QTAIM) analysis, single electron transfer (SET), hydrogen atom transfer (HAT), and delocalization index (DI) of active hydrogen bonds. It was proved that the QTAIM data of active hydrogen bonds play an important role in determining the antioxidant activities of the compounds.
Experimental section
Tools and chemicals
Sigma-Aldrich Co. LLC. brand 5-F, 5-MeO, 5-I and 5-Br isatin and aldehydes were purchased. FTIR analysis was performed using a Bruker Alpha Fourier brand IR spectrometer, and NMR spectra were performed with a Bruker Ultrashield Plus Biospin spectrophotometer at 400 MHz in DMSO-d6. UV absorption measurements were carried out with a SHIMADZU UVmini-1240 UV–Visible spectrophotometer. FTIR and C, H, and N elemental analysis of the synthesized compounds were performed at Kastamonu University Central Research Laboratory.
Synthesis of new isatin-schiff base derivatives
A mixture of aromatic aldehydes (5.0 mmol) and thiocarbohydrazide (5.0 mmol) in ethanol (25 mL) and two drops of hydrochloric acid was refluxed for 3 h. and mentioned in parentheses the aldehydes (A1-A23). The reaction mixture was cooled. The precipitate formed was filtered and washed with ethanol (96%) to give monocarbohydrazones (I1-I23). A mixture of monocarbohydrazones (3.0 mmol), substituted isatins (3.0 mmol), and two drops of hydrochloric acid in ethanol (15 mL) was refluxed for 5 h. The precipitate formed was filtered and washed with cold ethanol (96%) to give a product. The reaction pathway is given in Scheme 1. They were obtained with slight changes according to an earlier procedure [10].
Antioxidant activity analysis
The DPPH radical shows a strong absorption band at 517 nm in the visible spectrum. Thanks to the electrons becoming paired with the addition of antioxidants, this absorbance value decreases and as the antioxidant concentration increases, the colors become lighter. This reaction can be monitored with a spectrophotometer. Thus, in this study, calibration solutions of different concentrations of DPPH (5–25 × 10–5 M) were prepared, primarily prepared with ethanol. Then, the samples prepared by adding new synthesized compounds were incubated for 15 min at room temperature and in the dark. The absorbance of each sample was recorded at 517 nm against a blank consisting only of ethanol. The ethanol-DPPH solution prepared as a standard was used as a control in the absorbance measurements of the sample solutions. The results are given as % inhibition for the synthesized molecules. Radical quenching activity was calculated as percent inhibition by the following formula:
Here \({A}_{0}\) is the control absorbance and \({A}_{1}\) is the absorbance in the presence of samples [11].
Computational procedure
The DFT calculations, as described in references [12, 13], were conducted utilizing the Gaussian 09 software [14] at the B3LYP/6-311 + + G(2d,2p) level of theory. The optimized geometries of the states represent the minimum energy points on the potential energy surface. Consequently, no imaginary frequencies were detected during the IR calculations.
In consonance with the acquisition of experimental NMR data within the dimethyl sulfoxide (DMSO) solvent medium, calculations employing density functional theory (DFT)/NMR were likewise executed within the DMSO phase through the Gauge-independent atomic orbital (GIAO) method. Throughout these computational procedures, the conductor-like polarizable continuum model (CPCM) was implemented to account for solution–solvent interactions. The determination of relative chemical shift values was ensued by subtracting the absolute chemical shielding of tetramethylsilane (TMS), which was independently computed at the B3LYP/6-311 + + G(2d,2p) level of theory, yielding values of 31.8149 and 183.737 ppm for 1H and 13C NMR, respectively.
The electronic parameters of the compounds were acquired through gas-phase IR calculations. Utilizing frontier molecular orbital (FMO) energy eigenvalues derived from the IR calculations, global chemical reactivity parameters, including the HOMO-LOMO energy gap (Eg), chemical hardness (η), electronegativity (χ), electrophilic index (ω), nucleophilic index (ε), electroaccepting power (ω+), and electrodonating power (ω−) indices, were determined. Besides the single electron transfer energy (SETE), electron delocalization indices (DIs) between N/O and H atoms were also calculated. Furthermore, the electron density at both ring critical points (RCPs) and bond critical points (BCPs) was assessed using the QTAIM approach [15, 16]. To visualize intramolecular interactions, interaction region indicator (IRI) calculations were conducted through the Multiwfn software [17].
Results and discussion
Characterization of 5-isatin benzothiocarbohydrazone Schiff bases
In this study, FTIR, 1H-NMR, and 13C-NMR analysis of 23 newly synthesized compounds were performed. The molecular names of these compounds and some physicochemical properties used in their spectroscopic analysis are given in Table 1. Experimental and calculated elemental analysis results of the synthesized compounds are given in Table 2.
FTIR analysis of 5-isatin benzothiocarbohydrazone Schiff bases
In all synthesized compounds, C=O signals of the isatin ring were observed between 1705 and 1684 cm−1 and –NH stretching vibrations were observed between 3150 and 2975 cm−1. –C = S stretching vibrations were found between 1386 and 1304 cm−1. Characteristic -OH stretching vibrations were not observed in compounds 1, 7, 8, 15, and 20. In the FTIR spectra of all compounds, the aldehyde group signal of the starting material around 2750–2650 cm−1 was not seen. In molecules containing 3-OCH3-4-OH group of Schiff bases of molecules containing 5-methoxy, 5-fluorine, 5-bromine, and 5-iodine isatin groups, νC–H (Ar.) signals were seen between 2987 and 2820 cm−1. In molecules containing 5-methoxy, 5-bromine, and 5-iodine isatin group and 3,5-diOCH3-4-OH group, νC–H (Ar.) signals were seen between 3153 and 3128 cm−1. The FTIR spectrum and analysis data of compound 3 are shown in Fig. 1 and Table 3. When these analysis data are examined, the frequency values are quite compatible with the results of our previous study for 5-chloroisathin compounds and similar synthesized compounds [18,19,20]. In addition, it seems that the theoretical data are consistent with the experimental results. All FTIR spectra of other synthesized compounds are given in the supplementary material, Figs. S47–S69. Additionally, calculated data analysis for FTIR spectra is given in Table S9 in the supplemental material.
1H NMR results of 5-isatin benzothiocarbohydrazone Schiff bases
The experimental 1H-NMR spectra of the compounds were detected in DMSO-d6, and the chemical shifts are given in Table 4. As an example of 1H NMR results, the spectrum of compound 3 is given in Fig. 2.
For compound 3, the aromatic proton (H1-H2-H3) signals of isatin ring were observed between 7.09 and 6.87 ppm. The H1 proton coupled to the H2 proton and detected a doublet peak at 7.09–7.08 (d, J = 2.4 Hz) ppm. The H2 proton coupled to the H1 and H3 protons and observed a doublet of doublets peak at 6.97–6.95 (dd, J = 8.4, 2.4 Hz) ppm. The H3 proton coupled to the H2 proton and detected a doublet peak at 6.89–6.87 (d, J = 8.5 Hz) ppm. The N-H4 signal of isatin region was observed as a singlet at around 11.09 ppm. The proton signals of thiocarbohydrazone region were occurred from N-H5 and N-H6. These amino peaks were observed as a singlet at 14.66 and 12.49 ppm, respectively. The signal of imine (–CH=N, H7) was observed as a singlet at 9.68 ppm. The methoxy proton signal (5-OCH3) of isatin region was detected as a singlet in 3.79 ppm. The methoxy proton signal (H9, 3-OCH3) of aldehydic region was detected as a singlet in 3.93 ppm. The OH (H10) signal was detected as a singlet at 8.08 ppm. The aromatic protons (H8-H12) of the aldehydic region were observed at 7.70–6.83 ppm. The H8 proton coupled to the H11 proton and detected a doublet peak at 6.85–6.83 (d, J = 8.1 Hz) ppm. The H11 proton coupled to the H8 and H12 protons and observed a doublet of doublets peak at 7.16–7.14 (dd, J = 8.1. 1.5 Hz) ppm. The H12 proton coupled to the H11 proton and detected a doublet peak at 7.70–7.69 (d, J = 1.4 Hz) ppm (see Figs. S1–S23).
At 1H-NMR spectra of all compounds (1–23), the aromatic protons (H1-H3) signals of the isatin ring were observed at between 7.93 and 6.81 ppm. While the N–H4 signals of the isatin region were detected as a singlet at 11.48–11.03 ppm, the N–H5 and N–H6 signals of thiocarbohydrazone region were shown as a singlet at 14.95–12.38 ppm. The methoxy proton signals (5-OCH3) of isatin region were detected as a singlet in the range of 3.80 and 3.77 ppm. The signals of imine (–CH = N) (H7) were observed as a singlet at 11.08–8.07 ppm. The OH signal was detected as a singlet at 10.09–8.06 ppm. The aromatic protons (H8-H12) of the aldehydic region were observed at 7.91–6.32 ppm. These results are in agreement with the data that reported similar compounds [21,22,23]. DMSO-d6 and water in DMSO (HOD, H2O) signals are shown at around 2.00, 2.50 (quintet), and 3.30 (variable, based on the solvent and its concentration) ppm, respectively [24]. The theoretical 1H-NMR spectra of the compounds were detected in DMSO, and the chemical shifts are given in supplementary material, Tables S1a–S4a.
13C NMR results of 5-isatin benzothiocarbohydrazone Schiff bases
The experimental 13C-NMR spectra of the compounds were detected in DMSO-d6, and the chemical shifts are given in Table 5. As an example of 13C NMR results, the spectrum of compound 3 is given in Fig. 3.
At 13C-NMR spectra of all compounds (1–23), the –C=S (C9) peaks of the thiocarbohydrazone region were observed at 175.77–174.68 ppm. The –C=O (C7) and –C=N (C8) peaks of the isatin region were observed at 163.39–162.50 and 152.59–132.51 ppm, respectively. The characteristic –C = N (C10) peaks of imine were detected at 157.57 and 140.78 ppm. The methoxy carbon (–OCH3) resonated at 65.06–56.02 ppm. The aromatic carbon atom signals of the isatin region (C1–C6) were resonated between 160.52 and 83.93 ppm, and those from the phenyl ring (C11–C16) were detected 160.54–105.59 ppm (see Figs. S24-S26). The C1 atoms of the compounds (1–14) shifted downfield relative to the signal of benzene (128.5 ppm). These carbon atoms shifted downfield (high values of δ), which was caused by the presence of the methoxy and fluoro group. On the contrary, the C1 atoms of the compounds (15–23) shifted upfield relative to the signal of benzene (128.5 ppm). These carbon atoms shifted upfield (low values of δ), which was caused by the presence of the iodo and bromo group. Moreover, the carbon atoms (for C1–C6) were also split into doublets caused by interacting with the atomic nucleus of F for compounds 8–14. The C4 atoms of the compounds shifted downfield (140.22–136.22 ppm) relative to the signal of benzene (128.5 ppm). These carbon atoms shifted downfield (high values of δ), which was caused by the presence of the nitrogen atom. The electronic effects of groups/substituents in structures play a significant role to assignment those from the phenyl ring (C11–C16). The carbon atoms of the compounds shifted downfield or upfield relative to the signal of benzene (128.5 ppm).
When we examine the 13C NMR spectrum of compound 3, there are 18 different resonated carbon atoms consistent with the target structure (Fig. 3). In compound 3, the –C=S (C9) peaks of the thiocarbohydrazone region were observed at 175.20 ppm. The –C=O (C7) and –C=N (C8) peaks of the isatin region were observed at 163.24 and 138.23 ppm, respectively. The characteristic –C=N (C10) peaks of imine were detected at 145.13 ppm. The methoxy carbons (–OCH3) of isatin and phenyl ring resonated at 56.17 and 56.10 ppm, respectively. The C1 atom was resonated at 155.79 ppm. This carbon atom shifted downfield (high values of δ). Due to in the presence of the methoxy group, the carbons (C1–C6) of isatin region were detected at 155.79, 112.42, 121.31, 136.27, 118.18, and 105.84 ppm, respectively. The aromatic C signals of the phenyl ring (C11–C16) were observed at 150.06 and 109.40 ppm. The C13 (148.80 ppm) and C14 (150.06 ppm) carbon atoms shifted downfield (high values of δ) caused by the presence of –OCH3 and –OH groups, respectively. These results are in agreement with the data that reported similar compounds [11, 21,22,23]. The theoretical 13C-NMR spectra of the compounds were detected in DMSO, and the chemical shifts are given in supplementary material, Tables S5a–S8a.
Examination of antioxidant activities
The compounds synthesized in this project were compared against gallic acid as a standard antioxidant using the DPPH radical scavenging method. The antioxidant activities of the Schiff base compounds we obtained using aldehyde derivatives are given in Fig. 4, with concentration changes versus calculated inhibition percentages. All compounds showed a steady increase in direct proportion to the increase in concentration.
Among the synthesized 23 compounds, compound 22 showed the highest antioxidant activity at 10 µM concentration. Then, compounds 11, 14, 18, 19, and 17 followed this order. Compounds 1 and 8 exhibited the lowest antioxidant activity. Gallic acid, used as standard, showed the highest activity at all concentrations.
In order to regularly compare the antioxidant activities of all compounds, IC50 values were calculated. The amount of antioxidant required to reduce the initial DPPH concentration by 50% expresses its antiradical activity and is called IC50 (mg/mL) [25]. IC50 value is a widely used parameter to measure antioxidant activity. In this study, the R2 values and IC50 values of the linear regression equations of gallic acid using the DPPH method are summarized in Table 6.
The IC50 value calculated from the inhibition values of different concentrations was found to be 6.48 ± 0.01 µM for gallic acid. Considering the IC50 values, it can be said that the lower the value, the greater the antioxidant activity. This means that substances that can scavenge the same amount of free radicals at the lowest concentration show stronger antioxidant activity. Accordingly, among the IC50 values, compound 17 was the compound closest to gallic acid and with the highest antioxidant activity, with 9.76 ± 0.03 µM. Compound 8 showed the lowest antioxidant activity with an IC50 value of 282.18 ± 0.25 µM.
Graphs were presented separately in order to examine in more detail the changes in the antioxidant properties of the synthesized compounds according to the groups to which they are systematically affiliated. Accordingly, methoxy, fluorine, bromine, and iodine groups attached to the isatin group could be compared among them. It was found that the aldehyde structures of the synthesized Schiff bases containing 3-OC2H5–4-OH, 3,5-diOCH3–4-OH, and 4-OH groups showed a higher antioxidant effect in the halogen-bonded isatin groups than in the methoxy isatin structure. Similarly, Kıran et al., in their study with new bis-isatin carbohydrazone derivatives, reported that the halogen groups attached to the aromatic ring were responsible for the antioxidant activity [23]. Many previous studies have reported that halogen-containing systems significantly increase the antioxidant effect [26].
Molecules containing 3-OCH3–4-OH groups were the structures showing the highest antioxidant activity in all compounds with both halide and methoxy groups attached. Nagaraja Naik and colleagues reported that aniline structures attached to isatin significantly increased antioxidant effectiveness and compounds containing the electron-donating methoxy substituent showed higher antioxidant activity compared to butylated hydroxyanisole (BHA) [27]. The IC50 value (34.61 µM) we found for the compound containing the 3-OH–4-OCH3 group, which we synthesized previously with 5 chlorides of isatin, showed that the antioxidant power of molecules containing halogens attached to isatin is higher than the compounds containing methoxy groups attached to isatin. [23, 26].
The inhibition percentages we observed in our study varied depending on the position of the phenolic structures attached to the ring and the number of hydroxy groups they contained. This change was previously reported to be affected by the presence of electron-withdrawing groups on the ring. [28,29,30]. In this study, compounds 14, 2, 9, 21, and 16, which have 2,4-diOH and 4-OH positions attached to the ring, enabled us to compare the antioxidant activity of compounds against the bonding type and amount of phenolic structures. In particular, it was observed that the 2,4-diOL and 3-OC2H5–4-OH linked aldehyde groups in the 5-fluoroisatin compound exhibited higher antioxidant activity than the others. Likewise, percentage inhibition increases varied in direct proportion to the abundance of phenolic structures. This result showed that antioxidant activity depends on the position of phenolic structures relative to the meta- or meta-promoters [11]. In our study with 5-chloroisathin, the Schiff base molecule containing a 3-OCH3-4-OH group in the ring showed the highest antioxidant activity compared to other molecules (2,4 di OH; 2-OH; 2-OH; 3-OH–4-OCH3) [21]. Likewise, in this study, for all 5-methoxyisatin, 5-fluoroisatin, 5-bromoisatin, and 5-iodoisatin structures, methoxy groups exhibited the highest antioxidant effect when added to the structure in the meta position. The data we obtained from these studies showed that the position of -OMe groups significantly affects antioxidant activity [20]. In this study, compounds 1, 6, 8, and 15 showed the lowest antioxidant effect. The common feature of these compounds is that all isatin molecules (5-methoxyisatin, 5-fluoroisatin, 5-bromoisatin, 5-iodoisatin) are molecules that do not have a structure attached to the aldehyde group.
DFT analysis
The 23 compounds were divided into two different groups for detailed examination, and both inter- and intra-group analysis was performed. The first group includes compounds with the same substituent (methoxy-substituted compounds 1–7, fluorine-substituted compounds 8–14, iodine-substituted compounds 15–19, and bromine-substituted compounds 20–23). In the second group, compounds 1, 8, 15, 20; 2, 9, 16, 21; 3, 10, 17, 22; 4, 18, 23; 5, 19, 11; 6, 12; 14, 13 were examined in order to analyze the effects of compounds with different substituents (methoxy, fluorine, iodine, and bromine) at the same position on their antioxidant properties.
HOMO and LUMO energies, EHOMO and ELUMO, are indicators of a molecule’s tendency to donate and accept electrons, respectively. (Calculated electronic parameters are given in Supplementary Table S10.) Additionally, chemical reactivity is directly related to the interaction between the HOMO-LUMOs of the reacting compounds (Fig. 5; the HOMO and LUMO surfaces of all the compounds are given in Supplementary Figs. S70–S73). As the HOMO–LUMO energy gap, Eg, decreases, the reactivity of the molecule increases, i.e., the lower energy gap suggests higher reaction expectations since the energy required to remove an electron from the last occupied orbital is lower. Conversely, a larger HOMO–LUMO gap means higher kinetic stability and lower chemical reactivity, such that it is energetically unfavorable to form an activated complex of any potential by adding electrons to a higher LUMO and removing electrons from a lower HOMO. Furthermore, the analysis of FMO energies obtained from DFT calculations performed by considering the bare/non-environment-interactive states of molecules and other electronic parameters derived from these energy eigenvalues is difficult due to the fact that these parameters are directly affected by the conformational freedom of compounds, as well as by dynamic variables such as intramolecular/intermolecular interactions, solvent effects, and temperature. In other words, due to the dynamic nature of reactions, which include both environmental interactions and internal properties of compounds, care must be taken when trying to elucidate a reaction mechanism through these parameters. Based on this point, some approaches/predictions regarding the antioxidant properties of compounds can be made based on calculated electronic parameters.
Calculations reveal that there is a correlation between the Eg and IC50 values of the compounds. (see Table S11 for data in Supplementary file). Pearson correlation coefficients between the Eg and IC50 values of compounds 1–7, 8–14, 15–19, and 20–23 were calculated as 0.649, 0.762, 0.871, and 0.890, respectively. (Note: While the Eg values of the compounds were generally calculated close to each other, a decrease in correlation was seen due to the presence of large IC50 values in each group, i.e., large IC50 values deviating from the mean within the group reduced the correlation.) Although the data reveal a partial correlation between Eg and IC50 values, they are insufficient to explain the source of the presence of considerably larger IC50 values within each group relative to other compounds of the group. (All calculated electronic parameters are given in Supplementary Table S10.) Another interesting high correlation was observed between the HOMO energy eigenvalues of the compounds and their 1/IC50 values. The Pearson correlation coefficients between the HOMO energies and 1/IC50 values of compounds 1–7, 8–14, 15–19, and 20–23 have been calculated as 0.556, 0.858, 0.954, and 0.976, respectively, indicating that the data demonstrate an inverse relationship between the HOMO energy of the compounds and their IC50 values (see Table S12 for data in Supplementary file). Lower correlations are especially noticeable for methoxy group compounds 1–7.
It is difficult to definitively state that the reactions of compounds with DPPH occur via electron or hydrogen atom transfer, generally speaking, as often these reactions occur concurrently. The correlation coefficients between IC50 values and possible SET reaction energies were calculated as 0.516, 0.715, 0.914, and 0.924 for compounds 1–7, 8–14, 15–19, and 20–23, respectively (see Table S13 for data in Supplementary file), which parallel the correlation obtained between HOMO energy and 1/IC50 values. On the other hand, since compounds have more than one reactive H atom (there are many N‒H and O‒H bonds), it is difficult to make predictions to determine which active hydrogens a possible HAT reaction takes place. At this point, electron delocalization on N‒H and O‒H bonds can be used as an indicator for the reactivity of the bonds, and one of the most appropriate approximations can be to examine the effects of hydroxyl and N‒H groups separately and make a general analysis on the relationship between them. For this purpose, the correlation between the average electron delocalization of N‒H bonds and IC50 values, due to the presence of more than one N‒H in the compounds, was discussed, while the effect of the O‒H bond within the group was taken into account. For the possible HAT reaction, the correlation coefficients between IC50 values and the average electron delocalization index of N–H bonds were calculated as − 0.840, − 0.692, − 0.851, and − 0.873 for compounds 1–7, 8–14, 15–19, and 20–23, respectively (see Table S14 for data in Supplementary file). The positive and negative correlations calculated for the SET and HAT mechanisms, respectively, strengthen the idea that the reactions with DPPH occur more predominantly through the SET mechanism. In addition, considering groups containing methoxy, fluorine, iodine, and bromine among themselves will produce stronger interpretations in determining the antioxidant properties of the compounds. For example, considering that the inhibition effects are partially linear with the concentrations of the compounds on average, the correlations of the inhibition values of compounds 1–7 with the SETE and DI data at 1.25 µM concentrations were calculated as − 0.473 and 0.785, respectively, while it was calculated as − 0.251 and 0.422 for 8–14, − 0.951 and 0.992 for 15–19, and − 0.739 and 0.730 for 20–23 (see Tables S15 and S16 for data in Supplementary file). These data predict that the DPPH reactions predominantly occur via the HAT mechanism for compounds 1–7, especially, while for compounds 8–14 containing the fluorine group, they partially occur via the HAT mechanism, but for compounds 15–23 containing iodine and bromine, both mechanisms are predicted to have similar effectiveness on average. These data support the assumption that the DPPH reactions of compounds 1–7 predominantly occur via the HAT mechanism, while for compounds 8–14 with the fluorine group, it occurs partially via the HAT mechanism. However, for compounds 15–23 containing iodine and bromine, it strengthens the assumption that both mechanisms are closely dominant. (DIs of active hydrogens and SETE values of the compounds are given in Supplementary Table S17.)
Another investigation was performed on compounds 1–3, 8–10, 15–17, and 20–22 containing p-OH and p-OH/m-MeO substituents to observe the effect of halogen and methoxy substituents on the antioxidant properties of the compounds. Inhibition data reveal that compounds 3, 10, 17, and 22, containing p-OH and m-MeO, show high antioxidant inhibition effects compared to other compounds in their group, while the lowest inhibition effect is exhibited by compounds 1, 8, 15, and 20, which do not contain any substituents on the phenyl ring. Additionally, it was observed that electron-donating hydroxyl and methoxy substituents increased the HOMO energies of the compounds in these groups, while also decreasing their Eg values. Similar effects are not limited to this; it was calculated that hydroxyl and methoxy substituents decrease the electronegativity value of the compounds while increasing the polarizability and nucleophilic index values. All these effects occurred similarly for the compounds in this group and were experimentally observed to be proportional to the increase in inhibition (Supplementary Figs. S74 and S75).
In order to analyze the effects of methoxy, fluorine, iodine, and bromine substituents on the electronic and antioxidant properties of the compounds, the compounds were grouped as 1, 8, 15, 20; 2, 9, 16, 21; 3, 10, 17, 22. For compounds 1, 8, 15, and 20, methoxy and bromine were generally found to produce lower inhibitory effects than fluorine and iodine, such that methoxy’s contribution to the antioxidant inhibition effect of the compounds was weakest. On the other hand, at low concentrations, it was observed that iodine atom caused a more effective inhibition effect than fluorine. Compounds 1, 8, 15, and 20 are not hydroxyl-substituted and are not favorable for the HAT reaction because, although they have active hydrogens via N‒H, we can say that these compounds are likely to be subject to steric effect. In this context, a SET-type mechanism is expected in the DPPH reactions of these compounds, such that the nucleophilic indices in the halogen group were generally found to be proportional to the inhibition effects of fluorine-, iodine-, and bromine-substituted compounds 8, 15, and 20, respectively. In this context, a SET-type mechanism is expected in the DPPH reactions of these compounds, such that the nucleophilic indices (6.155, 6.262, 6.266; 6.397, 6.503, 6.512) of two groups of halogens-substituted compounds 9, 16, 21 and 10, 17, 22 were found to be generally proportional to their inhibition effects (11.520, 11.713, 12.778; 11.713, 16.941, 13.553 at 1.25 µM concentration). Except for the strong inhibitory effect exhibited by fluorine-containing compound 9 at high concentrations, methoxy, bromine, and iodine (iodine being the most effective and methoxy the weakest) increased the inhibition effects of the p-OH-substituted compounds 2, 9, 16, and 21 at concentrations above 2.50 µM. A partially similar situation was observed for p-OH- and m-MeO-substituted compounds 3, 10, 17, and 22. At concentrations above 2.50 µM, fluorine, methoxy, bromine, and iodine structures were observed to increase the inhibition, respectively. (Iodine is the most effective.) Although the steric effect of adjacent methoxy reduces the contribution of O‒H to the HAT mechanism in the DPPH reaction of the compounds, it is predicted that the probability of collision increases at high concentrations above 5 µM and therefore the contribution of SET reactions becomes stronger (Supplementary Fig. S76). Furthermore, it can be said that the exchange of p-OH and m-MeO or halogen groups in the compounds of the three groups considered (1, 8, 15, 20; 2, 9, 16, 21; 3, 10, 17, 22) resulted in parallel changes in electronic data, with one of the most important reasons being the neglect of intermolecular interactions in the calculations.
IRI calculations were performed for an analysis of intramolecular interactions (Figure IRI for compound 17, see Supplementary Figs. S77 and S78 for all). Calculations revealed that the isatin structure performs intramolecular interactions with the ‒OH and MeO substituents of the benzene ring via the oxygen atom. A linear decrease in the RCP values of the isatin (benzene) ring of compounds 1, 8, 15, and 20 was observed with the electronegativity of the halogen and methoxy substituents, with the presence of iodine calculated as 0.023150 e/bohr3, while for bromine, fluorine, and MeO, it was obtained as 0.023124, 0.022949, and 0.022779 e/bohr3, respectively. Except for iodine-substituted compound 15, a linear proportion is observed between electronegativity and inhibition values. Although some partial correlations can be established between electronic data and antioxidant inhibition values, the uncertainty surrounding the interactions of compounds with the solvent, DPPH, and among themselves, as well as the manner in which they affect their conformations and consequently their charge distributions, makes it quite challenging to make generalized predictions about the antioxidant properties of the compounds. However, it can be said that iodine and bromine atoms, while difficult to elucidate their mechanisms of action on inhibition, create strong inhibitory effects (Fig. 6).
It was observed that compounds 4, 18, and 23 containing MeO, I, and Br, respectively, iodine atom had a higher inhibitory effect compared to others at all concentrations. The only electronic parameter compatible with the inhibition data of these compounds is the polarizability of the compounds, which alone is not sufficient to comment on the general inhibition effects of the compounds. Although the effect of the presence of the iodine atom on the inhibition was partially noticeable for compounds 5, 11, and 19, it was seen from the inhibition data of compounds 6 and 12 that the effect of the fluorine atom on the inhibition was greater than MeO, so that, in these compounds, the presence of the fluorine atom instead of MeO reduced the Eg value and nucleophilic index (Eg and nucleophilic values for compounds 6 and 12 are 3.154 eV and 3.069 eV; 3.588 eV and 3.498 eV, respectively) while increasing the electronegativity and electroaccepting power values. (Electronegativity and electroaccepting power values for compounds 6 and 12 are 4.330 eV, 4.462 eV; 1.202 eV, 1.396 eV, respectively.) Furthermore, looking at compounds 13 and 14, it appears that the hydroxyl substituent has a direct contribution to the inhibition effects of the compounds.
Conclusions
In this study, 23 Schiff bases were synthesized using different substituents (5-F, 5-Br, 5-I and 5-MeO). After the molecules were characterized and confirmed by NMR and spectroscopic methods, the antioxidant properties of the compounds were examined by experimental and theoretical studies. Experimental studies showed that the differentiation of substitution groups attached to isatin groups is effective in changing antioxidant activities, but the main effect occurs with the change of aldehyde groups attached to thiocarbohydrazide. Accordingly, 2,4 diOH, 3-OCH3–4-OH, 3-OC2H5–4-OH and 3,5-diOCH3–4-OH groups enabled the compounds to show higher antioxidant activity.
Calculations reveal specific correlations between the energy levels, reactivity, and antioxidant activities of the compounds. An increase in the reactivity of the molecule has been detected as the HOMO–LUMO energy gap decreases. Additionally, an inverse relationship was detected between HOMO energy and IC50 values. Analysis of the reaction mechanisms of the compounds with DPPH shows that the single electron transfer (SET) mechanism is dominant. In addition, the effects of methoxy-, fluorine-, iodine-, and bromine-containing groups on inhibition were investigated and it was concluded that different reaction mechanisms play a role in halogen groups with lower electronegativity compared to others. Studies on the change of antioxidant effects according to p-OH and p-OH/m-MeO substituents show that compounds containing p-OH/m-MeO generally exhibit higher inhibition effects. The study also revealed that due to single-state calculations, the electronic properties showed similar changes with the change of p-OH/m-MeO and halogen groups. Our study, which aims to elucidate the complex relationships between the electronic and antioxidant properties of compounds, underlines the sensitivity of antioxidant activity to molecular structural changes and reveals important clues for the molecular design of antioxidants. Overall, the study provides insight into the complex interplay between molecular structure, electronic properties, and antioxidant behavior; however, difficulties remain in accurately predicting antioxidant properties due to the dynamic nature of the reactions and the influence of various factors such as solvent effects, conformational changes, and intra- and intermolecular interactions.
References
M. Rudrapal, B. De, Int. Res. J. Pure Appl. Chem. 3(3), 232 (2013)
A. Dzeikala, A. Sykula, J. Pharm. Pharmacol. 6, 989 (2018)
M.S. More, P.G. Joshi, Y.K. Mishra, P.K. Khanna, Mater Today Chem. 14, 100195 (2019)
T.H. Donelly, J. Chem. Edu. 73(2), 158 (1996)
F. Shahidi, P. Ambigaipalan, J. Funct. Foods 18, 820 (2015)
S.J. Jadhav, S.S. Nimbalkar, A.D. Kulkarni, D.L. Madhavi, Lipid oxidation in biological and food systems, in Food Antioxidants. (Dekker, New York, 1996), pp.5–63
A.I. Elshamy, T. Yoneyama, N. Van Trang, N.T. Son, Y. Okamoto, S. Ban, M. Noji, A. Umeyama, J. Mol. Struct. 1200, 127061 (2020)
N.T. Son, D.T.M. Thanh, N. Van Trang, J. Mol. Struct. 1193, 76 (2019)
T.S. Ahamed, V.K. Rajan, K. Sabira, K. Muraleedharan, Comput. Biol. Chem. 80, 66 (2019)
K. Gangarapu, S. Manda, A. Jallapally, S. Thota, S.S. Karki, J. Balzarini, E.D. Clercq, H. Tokuda, Med. Chem. Res. 23(2), 1046 (2014)
H. Muğlu, M.S. Çavuş, T. Bakır, H. Yakan, J. Mol. Struct. 1196, 819 (2019)
W. Kohn, L.J. Sham, Phys. Rev. 140, A1133 (1965)
P. Hohenberg, W. Kohn, Phys. Rev. 136, B864 (1964)
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G.A. Petersson, H. Nakatsuji, GAUSSIAN09 (Gaussian Inc., Wallingford, 2009)
R.F.W. Bader, Chem. Rev. 91, 893 (1991)
R.F.W. Bader, Acc. Chem. Res. 18, 9 (1985)
T. Lu, F. Chen, J. Comput. Chem. 33, 580 (2012)
A. Jarrahpour, D. Khalili, E. De Clercq, C. Salmi, J.M. Brunel, Molecules 12(8), 1720 (2007)
O. Bekircan, H. Bektas, Molecules 13(9), 2126 (2008)
H. Yakan, T. Bakır, M.S. Çavuş, H. Muğlu, Res. Chem. Intermed. 46, 5417 (2020)
H. Muğlu, H. Yakan, T. Bakır, Turk. J. Chem. 44, 237 (2020)
S.Y. Abbas, A.A. Farag, Y.A. Ammar, A.A. Atrees, A.F. Mohamed, A.A. El-Henawy, Monatsh. Chem. 144, 1725 (2013)
G. Kiran, M. Sarangapani, T. Gouthami, A.R. Narsimha Reddy, Toxicol. Environ. Chem. 95(3), 367 (2013)
W. Kemp, Organic Spectroscopy (Macmillan International Higher Education, New York, 2017)
E.N. Frankel, A.S. Meyer, J. Sci. Food Agric. 80(13), 1925 (2000)
G. Sammaiah, G. Brahmeshwari, M. Sarangapani, J. Adv. Pharm. Sci. 1, 47 (2011)
N. Naik, H. Vijay Kumar, P.B. Vidyashree, J. Pharm. Res. 4, 2686 (2011)
K.M. Khan, M. Khan, N. Ambreen, F. Rahim, B. Muhammad, S. Ali, S.M. Haider, S. Perveenb, M.I. Choudharya, J. Pharm. Res. 4(10), 3402 (2011)
A. Kıvrak, C. Yılmaz, M. Konus, H. Koca, S. Aydemir, J.A. Oagaz, Turk. J. Chem. 42, 306 (2018)
P. Pakravan, S. Kashanian, M.M. Khodaei, F.J. Harding, Pharmacol. Rep. 65, 313 (2013)
Acknowledgements
This work was supported by Kastamonu University, Research Fund, KÜBAP Project No: KÜ-BAP01/2019-18. Dr. Bakır would like to thank Kastamonu University, Research Fund, for these support. Also, the numerical calculations reported in this paper were partially performed at TUBITAK ULAKBIM, High Performance and Grid Computing Center (TRUBA resources).
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Temel Kan Bakır contributed to spectroscopic characterization, antioxidant assay, and writing—review. Halit Muğlu was involved in synthesis and spectroscopic characterization. M. Serdar Çavuş contributed to theoretical calculations and their visualizations and writing—review. Hasan Yakan was involved in spectroscopic characterization, visualization, and writing—review.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bakır, T.K., Çavuş, M.S., Muğlu, H. et al. Synthesis, structure elucidation, antioxidant properties, and theoretical calculations of new Schiff bases–isatin derivatives. Res Chem Intermed (2024). https://doi.org/10.1007/s11164-024-05318-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11164-024-05318-1