Abstract
In the present study, sixteen new diorganotin(IV) complexes of the general formula R2SnL1−4 where, R = methyl, ethyl, butyl phenyl and L1 = 4-chloro-2-(((2-hydroxy-5-(tert-pentyl)phenyl)imino)methyl)phenol; L2 = 3-ethoxy-2-(((2-hydroxy-5-(tert-pentyl)phenyl)imino)methyl)phenol; L3 = 4-bromo-2-(((2-hydroxy-5-(tert-pentyl)phenyl)imino)methyl)phenol; L4 = 4-nitro-2-(((2-hydroxy-5-(tert-pentyl)phenyl)imino)methyl)phenol) were synthesized and characterized by elemental analysis, molar conductance measurements, FT-IR, mass spectrometry, NMR (1H, 13C, and 119Sn), powder XRD, TGA, SEM and EDAX. Spectroscopic data elucidated that Schiff base ligands coordinate to the tin metal via ONO donor atoms in a tridentate manner. The synthesized compounds were explored for their antioxidant potential by performing DPPH assay and the data obtained demonstrated that complex 12 (IC50 = 2.57 µM) showed good antioxidant potential. The prepared compounds were also assessed for antimicrobial potential against bacterial and fungal strains and especially complex 20 being most effective against Escherichia coli (MIC = 0.0026 µmol/mL) and Candida albicans (MIC = 0.0052 µmol/mL). Further, molecular docking studies of the most potent complex 20 was performed to investigate the preferred binding modes in the active site of enzymes E. coli DNA Gyrase and C. albicans sterol 14-alpha demethylase. Additionally, in silico studies were conducted to assess the drug-like properties of the compounds (1–20), and the results indicated that the compounds have the potential to be utilized as orally active drugs.
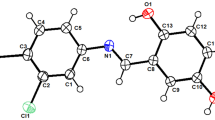
Graphical abstract
New o-aminophenol based ligands and their diorganotin(IV) complexes were synthesized, and successfully characterized. The synthesized compounds were examined for their in vitro antioxidant and antimicrobial activities. Additionally, molecular docking studies were conducted specifically on the most promising compounds identified in the antimicrobial assay.











Similar content being viewed by others
Data availability
The data analysed can be accessed from the manuscript for the current study.
References
S.H. Sumrra, F. Mushtaq, F. Ahmad, R. Hussain, W. Zafar, M. Imran, M.N. Zafar, Chem. Pap. 76, 3705 (2022)
A.J. Alanis, Arch. Med. Res. 36, 697 (2005)
M.P. Cruz, E. Santos, M.V. Cervantes, M.L. Juarez, Rev. Clin. Esp. 221, 55 (2021)
A. Fleming, Br. J. Exp. Pathol. 10, 226 (1929)
S.H. Sumrra, M. Hanif, Z.H. Chohan, J. Enzyme Inhib. Med. Chem. 30, 800 (2015)
A.U. Hassan, S.H. Sumrra, M. Imran, Z.H. Chohan, J. Mol. Struct. 1254, 132305 (2022)
S.H. Sumrra, W. Zafar, M. Imran, Z.H. Chohan, J. Coord. Chem. 75, 293 (2022)
D. Harman, Ann. N. Y. Acad. Sci. 54, 1 (1998)
S.R. Maxwell, Drugs 49, 345 (1995)
N.J. Thumar, M.P. Patel, Med. Chem. Res. 21, 1751 (2012)
Z.A. Siddiqi, M. Shahid, M. Khalid, S. Kumar, Eur. J. Med. Chem. 44, 2517 (2009)
A.A. Abdel Aziz, S.H. Seda, Appl. Organomet. Chem. 31, e3879 (2017)
Y. Deswal, S. Asija, A. Tufail, A. Dubey, L. Deswal, N. Kumar, N.M. Gupta, Appl. Organomet. Chem. 37, e7050 (2023)
Y. Deswal, S. Asija, A. Dubey, L. Deswal, D. Kumar, D.K. Jindal, J. Devi, J. Mol. Struct. 1253, 132266 (2022)
Y. Deswal, S. Asija, D. Kumar, D.K. Jindal, G. Chandan, V. Panwar, N. Kumar, Res. Chem. Intermed. 48, 703 (2022)
P. Agarwal, S. Asija, Y. Deswal, N. Kumar, J. Indian Chem. Soc. 99, 100556 (2022)
S. Hajra, R. Ghosh, S. Chakrabarti, A. Ghosh, S. Dutta, T.K. Dey, S. Basu, Adv. Synth. Catal. 354, 2433 (2012)
M. Iqbal, S. Ali, A. Haider, N. Khalid, Rev. Inorg. Chem. 37, 51 (2017)
K. Jamil, R. Wajid, M. Bakhtiar, M. Danish, J. Iran. Chem. Soc. 7, 495 (2010)
Q. Guo, R.F. Zhang, X.W. Hua, Q.L. Li, X.M. Du, J. Ru, C.L. Ma, New J. Chem. 46, 4314 (2022)
D. Ghazi, Z. Rasheed, E. Yousif, Development 2, 340 (2018)
D. Ghazi, E. Yousif, D.S. Ahmed, H. Thamer, R. Noaman, N.J. Hussien, A.H. Jawad, Al-Nahrain J. Sci. 22, 1 (2019)
K.K. Ho, G.J. Zhou, E.G. Xu, X. Wang, K.M. Leung, PLoS ONE 11, e0155632 (2016)
M. Hong, H. Yin, X. Zhang, C. Li, C. Yue, S. Cheng, J. Organomet. Chem. 724, 23 (2013)
E. López-Torres, F. Zani, M.A. Mendiola, J. Inorg. Biochem. 105, 600 (2011)
S. Asijaa, N. Malhotra, R. Malhotra, Phosphorus Sulf. Silicon Relat. Elem. 187, 1510 (2012)
H. Iqbal, S. Ali, S. Shahzadi, Cogent Chem. 1, 1029039 (2015)
S. Hadi, M.D. Fenska, N. Noviany, H. Satria, W. Simanjuntak, M.M. Naseer, Main Group Met. Chem. 44, 256 (2021)
W. Al Zoubi, A.A.S. Al-Hamdani, M. Kaseem, Appl. Organomet. Chem. 30, 810 (2016)
P. Debnath, K.S. Singh, T.S. Devi, S.S. Singh, R.J. Butcher, L. Sieroń, W. Maniukiewicz, Inorg. Chim. Acta 510, 119736 (2020)
A.G. Hadi, T.F. Hassen, I.J. Mahdi, Mater. Today Proc. 49, 2797 (2022)
F. Javed, M. Sirajuddin, S. Ali, N. Khalid, M.N. Tahir, N.A. Shah, M.R. Khan, Polyhedron 104, 80 (2016)
L. Hu, H. Wang, T. Xia, B. Fang, Y. Shen, Q. Zhang, Y. Tian, Inorg. Chem. 57, 6340 (2018)
H.L. Singh, J.B. Singh, S. Bhanuka, Res. Chem. Intermed. 42, 997 (2016)
M. Carcelli, G. Corazzari, S. Ianelli, G. Pelizzi, C. Solinas, Inorg. Chim. Acta 353, 310 (2003)
M.A. Girasolo, A. Attanzio, P. Sabatino, L. Tesoriere, S. Rubino, G. Stocco, Inorg. Chim. Acta 423, 168 (2014)
T.A. Antonenko, D.B. Shpakovsky, M.A. Vorobyov, Y.A. Gracheva, E.V. Kharitonashvili, L.G. Dubova, E.R. Milaeva, Appl. Organomet. Chem. 32, e4381 (2018)
Z.D. Petrović, J. Đorović, D. Simijonović, V.P. Petrović, Z. Marković, RSC Adv. 5, 24094 (2015)
A.I. Vogel, Text Book of Quantitative Chemical Analysis (Addision Wesley Longman, New York, 1999)
I. Gulcin, Arch. Toxicol. 94, 651 (2020)
I. Gülçin, H.A. Alici, M. Cesur, Chem. Pharm. Bull. 53, 281 (2005)
L. Deswal, V. Verma, D. Kumar, C.P. Kaushik, A. Kumar, Y. Deswal, S. Punia, Arch. Pharm. 353, 2000090 (2020)
L. Deswal, V. Verma, D. Kumar, A. Kumar, M. Bhatia, Y. Deswal, A. Kumar, Future Med. Chem. 13, 975 (2021)
J. Devi, S. Kumari, S. Asija, R. Malhotra, Phosphorus Sulf. Silicon Relat. Elem. 187, 1409 (2012)
L. Deswal, V. Verma, J.S. Kirar, D. Kumar, Y. Deswal, A. Kumar, M. Bhatia, Res. Chem. Intermed. 49, 1059 (2022)
F. Jesus, H. Passos, A.M. Ferreira, K. Kuroda, J.L. Pereira, F.J. Gonçalves, S.P. Ventura, Green Chem. 23, 3683 (2021)
J.M. Yang, C.C. Chen, Proteins Struct. Funct. Genet. 55, 288 (2004)
D. Systèmes, Dassault Systèmes (Biovia, San Diego, 2016)
E.F. Pettersen, T.D. Goddard, C.C. Huang, E.C. Meng, G.S. Couch, T.I. Croll, T.E. Ferrin, Protein Sci. 30, 70 (2021)
S. Saroya, S. Asija, Y. Deswal, N. Kumar, A. Kumar, Res. Chem. Intermed. 48, 2949 (2022)
H. Ullah, V. Previtali, H.B. Mihigo, B. Twamley, M.K. Rauf, F. Javed, I. Rozas, Eur. J. Med. Chem. 181, 111544 (2019)
S. Sharma, S. Gupta, A.K. Narula, NISCAIR-CSIR, India (1994)
M.M. Slaihim, F.S.R. Al-Suede, M. Khairuddean, M.B.K. Ahamed, A.M.S.A. Majid, J. Mol. Struct. 1196, 78 (2019)
N. Kumar, S. Asija, Y. Deswal, S. Saroya, A. Kumar, J. Devi, Phosphorus Sulf. Silicon Relat. Elem. 197, 952 (2022)
M. Sirajuddin, S. Ali, A. Haider, N.A. Shah, A. Shah, M.R. Khan, Polyhedron 40, 19 (2012)
N. Kumar, S. Asija, Y. Deswal, S. Saroya, A. Kumar, Res. Chem. Intermed. 48, 5133 (2022)
T.P. Lockhart, W.F. Manders, Inorg. Chem. 25, 892 (1986)
B. Nisha, M. Nidhi, A. Sonika, J. Chem. Pharm. Res. 6, 194 (2014)
J. Ordonez-Hernandez, R. Arcos-Ramos, H. Garcıa-Ortega, E. Munguıa-Viveros, M. Romero-Avila, M. Flores-Alamo, I. Gracia-Mora, F. Sanchez-Bartez, R. Santillan, N. Farfan, J. Mol. Struct. 1180, 462 (2019)
Y.X. Tan, Z.J. Zhang, Y. Liu, J.X. Yu, X.M. Zhu, D.Z. Kuang, W.J. Jiang, J. Mol. Struct. 1149, 874 (2017)
M. Sirajuddin, S. Ali, V. McKee, A. Matin, J. Mol. Struct. 1206, 127683 (2020)
S. Saroya, S. Asija, Y. Deswal, N. Kumar, D. Kumar, D.K. Jindal, S. Kumar, Res. Chem. Intermed. 48, 4671 (2022)
P. Khatkar, S. Asija, Phosphorus Sulf. Silicon Relat. Elem. 192, 446 (2017)
B.K. Singh, A. Prakash, D. Adhikari, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 74, 657 (2009)
P. Bera, P. Brandão, G. Mondal, H. Jana, A. Jana, A. Santra, P. Bera, Polyhedron 134, 230 (2017)
C. Sánchez, Synth. Syst. Biotechnol. 2, 13 (2017)
S. Saroya, S. Asija, N. Kumar, Y. Deswal, J. Indian Chem. Soc. 99, 100379 (2022)
V.Y. Tyurin, W. Yaohuan, A.A. Prishchenko, D.B. Shpakovsky, Y.A. Gracheva, T.A. Antonenko, E.R. Milaeva, Russ. Chem. Bull. 64, 1419 (2015)
G. Mustafa, M. Zia-ur-Rehman, S.H. Sumrra, M. Ashfaq, W. Zafar, M. Ashfaq, J. Mol. Struct. 1262, 133044 (2022)
R.S. Srivastava, Inorg. Chim. Acta 56, L65–L67 (1981)
F. Javed, M. Sirajuddin, S. Ali, N. Khalid, M.N. Tahir, N.A. Shah, Z. Rasheed, M.R. Khan, Polyhedron 104, 80 (2016)
B. Coyle, K. Kavanagh, M. McCann, M. Devereux, M. Geraghty, Biometals 16, 321 (2003)
L. Deswal, V. Verma, D. Kumar, Y. Deswal, A. Kumar, R. Kumar, M. Bhatia, Chem. Pap. 76, 7607 (2022)
J.O. Adeyemi, D.C. Onwudiwe, A.C. Ekennia, R.C. Uwaoma, E.C. Hosten, Inorg. Chim. Acta 477, 148 (2018)
C.Y. Lee, C.N. Nanah, R.A. Held, A.R. Clark, U.G. Huynh, M.C. Maraskine, A. Sharma, Biochimie 111, 125 (2015)
S.H. Sumrra, A. Suleman, Z.H. Chohan, M.N. Zafar, M.A. Raza, T. Iqbal, Russ. J. Gen. Chem. 87, 1281 (2017)
S.H. Sumrra, W. Zafar, H. Javed, M. Zafar, M.Z. Hussain, M. Imran, M.A. Nadeem, Biometals 34, 1329 (2021)
M.I. Setyawati, C.Y. Tay, D.T. Leong, Biomaterials 34, 10133 (2013)
C. Ning, X. Wang, L. Li, Y. Zhu, M. Li, P. Yu, L. Zhou, Z. Zhou, J. Chen, G. Tan, Y. Zhang, Y. Wang, C. Mao, Chem. Res. Toxicol. 28, 1815 (2015)
P.B. Jadhav, A.R. Yadav, M.G. Gore, Int. J. Pharm. Biol. Sci. 6, 142 (2015)
R. Kadu, H. Roy, V.K. Singh, Appl. Organomet. Chem. 29, 746 (2015)
Funding
The research received financial support from the Council of Scientific and Industrial Research (CSIR), New Delhi, in the form of a JRF-SRF fellowship with reference number 09/752(0120)/2021-EMR-I.
Author information
Authors and Affiliations
Contributions
PB: Writing-original draft, Conceptualization, Methodology, Data curation, Investigation. SA: Supervision, Formal analysis, Validation. YD: Writing-original draft, Writing-review and editing, Software, Data curation, Validation. NK: Formal analysis. AK: Software. JD: Investigation.
Corresponding author
Ethics declarations
Conflict of interest
The authors affirm that they have no known financial or personal conflicts that could have influenced the research findings and the content presented in this paper.
Ethical approval
The authors can guarantee their adherence to Springer's ethical standards. The manuscript being submitted to Research on Chemical Intermediates represents original work that has not been published before in any format. The authors also confirm that the manuscript is not currently being considered for publication elsewhere and that its publication has been approved by all authors, as well as the responsible authorities where the research was conducted, either tacitly or explicitly. The authors affirm that they have no disclosed financial interests or personal relationships that could potentially influence the findings and conclusions presented in this paper. Each author was aware that the submission also signifies that if accepted, the manuscript will not be published elsewhere in the same format, whether in English or any other language, without obtaining written consent from the copyright holder.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barwa, P., Asija, S., Deswal, Y. et al. New pentacoordinated diorganotin(IV) complexes of o-aminophenol based Schiff base ligands: Synthesis, characterization, antioxidant, antimicrobial and molecular docking studies. Res Chem Intermed 49, 3411–3440 (2023). https://doi.org/10.1007/s11164-023-05053-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-023-05053-z