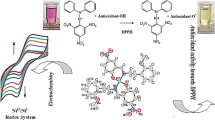
Abstract
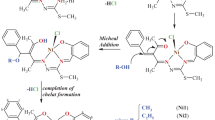
Complexes of organotin compounds R2SnCl2 with bisand trisphosphonate derivatives of 2,6-di-tert-butyl-4-methylphenol (ionol) were synthesized. X-ray diffraction studies were carried out for some of them. The redox properties of the synthesized compounds were characterized by cyclic voltammetry. Antioxidant/prooxidant activity of the complexes was studied using a new electrochemical method based on measuring the rate of hydrogen atom transfer to the stable radical 2,2´-diphenyl-1-picrylhydrazyl (DPPH). The data obtained were compared with the results of studying activity of the compounds during lipid peroxidation (LP) in biological samples. A correlation is observed between the results on antioxidant activity obtained by electrochemical DPPH test and using biological samples. Unlike the initial organotin compounds, the synthesized complexes have antioxidant activity, whereas phosphorus-containing phenols exhibit the properties of efficient antioxidants and chelating agents.
Similar content being viewed by others
References
W. Droege, Physiol. Rev., 2002, 82, 47.
E. F. Schisterman, in Methods in Molecular Biology, Vol. 196, Oxidants and Antioxidants: Ultrastructure and Molecular Biology Protocols, Ed. D. Armstrong, Humana Press Inc., Totowa, 2002, p. 343.
E. R. Milaeva, V. Yu. Tyurin, Yu. A. Gracheva, E. V. Grigor´ev, V. S. Petrosyan, Yu. T. Pimenov, N. T. Berberova, Appl. Organomet. Chem., 2002, 16, 655.
N. A. Antonova, M. N. Kolyada, V. P. Osipova, Yu. T. Pimenov, N. T. Berberova, V. Yu. Tyurin, Yu. A. Gracheva, E. R. Milaeva, Dokl. Chem. (Engl. Transl.), 2008, 419, 62 [Dokl. Akad. Nauk, 2008, 419, 342].
S. L. Davydova, Yu. T. Pimenov, E. R. Milaeva, Rtut´, olovo, svinets i ikh organicheskie proizvodnye v okruzhayushchei srede [Mercury, Tin, Lead, and Their Organic Derivatives in the Environment], AGTU, Astrakhan, 2001, 148 pp. (in Russian).
M. N. Xanthopoulou, S. K. Hadjikakou, N. Hadjiliadis, N. Kourkoumelis, E. R. Milaeva, Yu. A. Gracheva, V. Yu. Tyurin, I. Verginadis, S. Karkabounas, M. Baril, I. S. Butler, Russ. Chem. Bull. (Int. Ed.), 2007, 56, 767 [Izv. Akad. Nauk, Ser. Khim., 2007, 737].
E. B. Burlakova, in Khimicheskaya i biologicheskaya kinetika. Novye gorizonty [Chemical and Biological Kinetics. New Frontiers], Khimiya, Moscow, 2005, Vol. 2, p. 10 (in Russian).
E. T. Denisov, T. G. Denisova, Handbook of Antioxidants: Bond Dissociation Energies, Rate Constants, Activation Energies, and Enthalpies of Reactions, CRC Press, Boca Raton, 1999, p. 174.
E. N. Frankel, Autooxidation in Food and Biological Systems, Eds M. Simic, M. Karel, Plenum, New York, 1980, p. 141.
E. R. Milaeva, O. A. Gerasimova, J. Zhang, D. B. Shpakovsky, S. A. Syrbu, A. S. Semeykin, O. I. Koifman, E. G. Kireeva, E. F. Shevtsova, S. O. Bachurin, N. S. Zefirov, J. Inorg. Biochem., 2008, 102, 1348.
Z.-Q. Liu, Chem. Rev., 2010, 110, 5675.
E. Niki, Free Radical Biol. Med., 2010, 49, 503.
G. Litwinenko, K. U. Ingold, J. Org. Chem., 2003, 68, 3433.
E. Baciocchi, A. Calcagni, O. Lanzalinga, J. Org. Chem., 2008, 73, 4110.
R. Scherer, H. T. Godoy, Food Chem., 2009, 112, 654.
A. L. Dawidowicz, D. Wianowska, M. Olszowy, Food Chem., 2012, 131, 1037.
K. Mishra, H. Ojha, N. K. Chaudhury, Food Chem., 2012, 130, 1036.
O. P. Sharma, T. K. Bhat, Food Chem., 2009, 113, 1202.
S. Chevion, M. A. Roberts, M. Chevion, Free Radical Biol. Med., 2000, 28, 860.
B. H. Kipp, C. Faraj, G. Li, D. Njus, Bioelectrochem., 2004, 64, 7.
Kh. Brainina, A. Ivanova, E. Sharafutdinova, E. Lozovskaya, E. Shkarina, Talanta, 2007, 71, 13.
M. Ortiz, L. Nunez-Vergara, J. Squella, J. Electroanal. Chem., 2002, 519, 46.
R. Salazar, P. Navarrete-Encina, J. Squella, C. Barrientos, V. Pardo-Jimenes, L. Nunez-Vergara, Electrochim. Acta, 2010, 56, 841.
R. Salazar, P. Navarrete-Encina, J. Squella, L. Nunez-Vergara, J. Electroanal. Chem., 2008, 622, 29.
V. Yu. Tyurin, Wu Yaohuan, A. V. Dolganov, E. R. Milaeva, Dokl. Chem. (Engl. Transl.), 2011, 436, 31 [Dokl. Akad. Nauk, 2011, 436, 641].
V. Yu. Tyurin, N. N. Meleshonkova, A. V. Dolganov, A. P. Glukhova, E. R. Milaeva, Russ. Chem. Bull. (Int. Ed.), 2011, 60, 647 [Izv. Akad. Nauk, Ser. Khim., 2011, 633].
V. Yu. Tyurin, J. Zhang, A. P. Glukhova, E. R. Milaeva, Makrogeterotsikly [Macroheterocycles], 2011, 4, 211 (in Russian).
V. Yu. Tyurin, J. Zhang, A. A. Moiseeva, E. R. Milaeva, E. R. Belykh, E. V. Buravlev, T. K. Rocheva, I. Yu. Chukicheva, A. V. Kuchin, Dokl. Chem. (Engl. Transl.), 2013, 450, 543 [Dokl. Akad. Nauk, 2013, 450, 543].
T. Rocheva, V. Tyurin, D. Belykh, A. Moiseeva, J. Zhang, E. Buravlev, I. Chukicheva, A. Kutchin, E. Milaeva, Am. J. Anal. Chem. 2014, 5, 1028–1036, http://dx.doi.org/10.4236/ajac.2014.515109.
A. A. Prishchenko, M. V. Livantsov, O. P. Novikova, L. I. Livantsova, D. B. Shpakovskii, E. R. Milaeva, Russ. J. Gen. Chem. (Engl. Transl.), 2006, 76, 832 [Zh. Obshch. Khim., 2006, 76, 868].
A. A. Prishchenko, M. V. Livantsov, O. P. Novikova, L. I. Livantsova, D. B. Shpakovskii, E. R. Milaeva, Russ. J. Gen. Chem. (Engl. Transl.), 2006, 76, 1753 [Zh. Obshch. Khim., 2006, 76, 1834].
A. A. Prishchenko, M. V. Livantsov, O. P. Novikova, L. I. Livantsova, D. B. Shpakovskii, E. R. Milaeva, Russ. J. Gen. Chem. (Engl. Transl.), 2005, 75, 1968 [Zh. Obshch. Khim., 2005, 75, 2058].
A. A. Prishchenko, M. V. Livantsov, O. P. Novikova, L. I. Livantsova, E. R. Milaeva, Heteroat. Chem., 2008, 19, 490.
A. A. Prishchenko, M. V. Livantsov, O. P. Novikova, L. I. Livantsova, E. R. Milaeva, Heteroat. Chem., 2008, 19, 562.
A. A. Prishchenko, M. V. Livantsov, O. P. Novikova, L. I. Livantsova, E. R. Milaeva, Heteroat. Chem., 2008, 19, 733.
V. Yu. Tyurin, Yu. A. Gracheva, E. R. Milaeva, A. A. Prishchenko, M. V. Livantsov, O. P. Novikova, L. I. Livantsova, A. V. Maryashkin, M. P. Bubnov, K. A. Kozhanov, V. K. Cherkasov, Russ. Chem. Bull. (Int. Ed.), 2007, 56, 774 [Izv. Akad. Nauk, Ser. Khim., 2007, 744].
N. A. Antonova, V. P. Osipova, M. N. Kolyada, I. V. Smolyaninov, N. T. Berberova, V. Yu. Tyurin, Wu Yaohuan, E. R. Milaeva, Dokl. Chem. (Engl. Transl.), 2010, 432, 165 [Dokl. Akad. Nauk, 2010, 432, 629].
A. J. Gordon, R. A. Ford, The Chemist´s Companion, Jonh Wiley and Sons, Inc., New York, 1973, 560 pp.
X-AREA, X-RED32; Stoe and Cie GmbH, Darmstadt, Germany.
L. J. Farrugia, J. Appl. Crystallogr., 2012, 45, 849.
G. M. Sheldrick, Acta Crystallogr., Sect A: Found. Crystallogr., 2008, 64, 112.
C. F. Macrae, P. R. Edington, P. McCabe, E. Pidcock, G. P. Shields, R. Taylor, M. Towler, J. van de Streek, J. Appl. Crystallogr., 2006, 39, 453.
E. N. Stroev, V. G. Makarova, Praktikum po biologicheskoi khimii [Practical Works on Biological Chemistry], Vysshaya Shkola, 1986, 279 pp. (in Russian).
E. V. Grigoriev, N. S. Yashina, V. S. Petrosyan, L. Pellerito, A. Gianguzza, A. Pellerito, E. V. Avtomonov, J. Lorberth, A. A. Prischenko, M. V. Livantsov, J. Org. Chem., 1999, 577, 113.
V. D. Pokhodenko, A. A. Beloded, V. G. Koshechko, Okislitel´no-vosstanovitel´nye reaktsii svobodnykh radikalov [Redox Reactions of Free Radicals], Naukova Dumka, Kiev, 1977, 276 pp. (in Russian).
E. Solon, A. J. Bard, J. Am. Chem. Soc., 1964, 86, 1926.
E. Solon, A. J. Bard, J. Phys. Chem., 1964, 68, 1144.
D. A. Hall, P. J. Elvino, Electrochim. Acta, 1967, 12, 1363.
Z. Galus, Fundamentals of Electrochemical Analysis, Ellis Horwood, Chichester, 1976.
W. Brand-Williams, M. Cuvelier, C. Berset, Food Sci. Technol., 1995, 28, 25.
V. Bondet, W. Brand-Williams, C. Berset, Lebensm.-Wiss. Technol., 1997, 30, 609.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 6, pp. 1419–1429, June, 2015.
Rights and permissions
About this article
Cite this article
Tyurin, V.Y., Yaohuan, W., Prishchenko, A.A. et al. Complexes of organotin compounds with bis- and trisphosphonate derivatives of 2,6-di-tert-butylphenol having antioxidant activity. Russ Chem Bull 64, 1419–1429 (2015). https://doi.org/10.1007/s11172-015-1026-z
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-015-1026-z