Abstract
We report the synthesis and structural, spectroscopic and magnetic properties of new 1D coordination polymeric complex {[Cu(μ-l-Arg)2]SO4⋅1.5H2O}n (1) that contains asymmetric μ−O,O’ carboxylic bridge linking distorted square-pyramidal [Cu(μ-l-Arg)2]2+ coordination units. In 1D, the syn−anti−μ2−η1:η1 zigzag polymer conformation, the adjacent Cu(II) ions are distanced by 5.707 Å, and the subsequent Cu∙∙∙Cu proximity in 1D-coordination chain equals 6.978 Å. Detailed interpretation of IR and Raman spectra of l-arginine and 1 was performed. The principal components of the g tensor determined from EPR experiments (gx = 2.059, gy = 2.075, gz = 2.228) indicate nearly axial symmetry of Cu(II) coordination sphere and correspond to the unpaired electron occupying the dx2–y2 orbital. The single broad band at 16,200 cm–1, characteristic of d−d transition, is assigned to the dominant dublet-dublet 2B1g(dx2–y2)→ 2Eg(dyz≈dxz) transition. Magnetic susceptibility measurements have revealed ferromagnetic coupling between the Cu(II) ions within the 1D-coordination chain, while the intermolecular coupling is antiferromagnetic.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coordination polymers (CPs) of transition metal ions belong to the class of inorganic–organic hybrid materials. CPs have attracted much attention by their wide potential applications as porous magnets [1], nonlinear optics (NLO) [2], spin crossover [3] and medical [4] materials. They can be designed to have porous structure and they are then known as metal–organic frameworks (MOFs) [5]. Their structures mostly depend on the metal centres, organic ligands, counter ions and solvent molecules. In CPs, periodic architecture is accomplished by the assembly of metal entities and ligands through coordination bonds. The metal centres are connected by bridging inorganic (e.g. μ-cyano, μ-thiocyanato and μ-thiocyanamid, μ-halido, μ-oxometallates [6,7,8,9]) or organic ligands such as N-heterocycle (e.g. 1,2,4-triazole) [10] and carboxylic or dicarboxylic acids [11, 12]. Recent studies have showed that the carboxylate O-donors of amino acid are important linkers in the chemistry of homo- and heterometallic coordination polymers [13,14,15,16,17,18,19,20,21,22,23]. The carboxylate linkers represent an abundant group characterized by diversity, multifunctionality and overall stability. They display a great variety of coordination modes ranging from basic η1:η0, η1:η1, μ2−η2:η0, μ3−η3:η0, syn−syn−μ2−η1:η1, anti−anti−μ2−η1:η1, syn−anti−μ2−η1:η1 and mixed μ2−η2:η1, μ3−η2:η2, μ4−η2:η2 and μ5−η3:η3 [24] and can extend the building blocks into one, two and three dimensions [13,14,15,16,17,18,19,20,21,22,23]. Among the coordination modes, the syn−syn, anti−anti and syn−anti conformations favour the formation of polymeric chains.
Due to its properties, copper(II) is frequently chosen for the design of coordination polymers. This ion can adopt several coordination geometries and it introduces, for instance, interesting catalytic and magnetic behaviour. The copper(II)–carboxylate bridges are attracting considerable attention because their structure is directly connected to the electronic, catalytic and magnetic properties of CPs [25,26,27]. The structural, spectroscopic, magnetic and catalytic features of the copper(II) complexes with amino acids that contain carboxylate bridges have been extensively studied, as exemplified by the complexes with l-alanine [28,29,30,31], l-tyrosine [32, 33], l-glutamine [34], l-methionine [35, 36], l-leucine [36, 37], l-phenylalanine [36, 38] and l-arginine [39–41]. It was reviled that the formation of the extended 1D polymeric structures is favoured by the triatomic syn-anti conformation of Cu–O–C–O–Cu carboxylate bridge. As shown by [Cu(μ-l-Ala)2]n (l-Ala = l-alanine) and [Cu(μ-l-Arg)2](NO3)2∙3H2O [28,29,30,31, 39,40,41], this conformation of the bridge was demonstrated to favour the antiferromagnetic exchange-coupling between Cu(II) ions.
In this paper, we report the synthesis and crystal structure of copper(II) coordination polymer with l-argininato carboxylate groups as the linkers, {[Cu(l-Arg)2]SO4⋅1.5H2O}n (1). The structural features of compound 1 were compared in details with two previously reported l-argininato copper(II) polymers that also have SO42– as the counterion, i.e. {[Cu(μ-l-Arg)2(H2O)]SO4·5H2O}n (2) [42] and {[Cu(μ-l-Arg)2(H2O)]SO4}n (3) [14]. To study the electronic structure and the exchange interaction between Cu(II) ions connected by carboxylate bridges, we performed the IR/Raman, NIR−Vis−UV, X− and Q− band EPR SQUID as well as magnetic susceptibility measurements.
Experimental
Materials and methods
All substrates (CuSO4·5H2O, l-Arg, KSCN, KN3) were of reagent grade and were used as received with no further purification. All substrates were Sigma-Aldrich products.
Synthesis of {[Cu(μ-l-Arg)2]SO4⋅1.5H2O}n (1)
Aqueous solution of copper(II) sulphate (1 mmol, 249.5 mg, 10 mL) was mixed with aqueous l-arginine solution (3 mmol, 522.1 mg, 30 mL). This mixture was stirred for 15 min, and left to slowly evaporate at room temperature. After 30 days, blue crystals of 1 were formed. The crystals of compound 1 could be also obtained by alternative procedures (a) or (b) with using KSCN or KN3 salts, respectively. To the continuously stirred aqueous solution of CuSO4 (3 mmol, 748.5 mg, 10 mL), an aqueous solution of l-arginine (6 mmol, 1044.2 mg, 20 mL) was slowly added. Worth notifying is that solution of copper(II) sulphate in procedures (a) and (b) needs to be acidified before use, due to hydrolysis of copper(II) ion. Mixture was stirred for 15 min. Then:
-
1.
an aqueous solution of KSCN (3 mmol, 291.5 mg, 10 mL) or
-
2.
an aqueous solution of KN3 (3 mmol, 243.4 mg, 10 mL or 6 mmol, 486.7 mg, 20 mL)
was slowly added. If the original solution pH will reach 4.0 or lower, the amorphous seledin complex of formula [Cu(l-Arg)(NCS)2] will be formed [43]. Mixtures (a) and (b) were stirred for another 20 min and then left to slowly evaporate at room temperature. After 20 (a) and 30 days (b), blue crystals of 1 were formed. Crystals were separated from the solution by simple filtration, washed with a small amount of water and left to dry for a week. Crystals are stable in air at room temperature. At room temperature, solubility of compound 1 in water is approx. 50 mg⋅g−1. Characterization data: Yield 80% (1), Calcd for chemical formula Cu2S2N16O19C24H62 (1) (FW = 1070.09 g/mol) (%): C, 26.94; H, 5.84; N, 20.94; O, 28.41; S, 5.99; Cu, 11.88.. Found: C, 26.57; H, 5.90; N, 20.75; S, 5.80. The CHN and S analysis data were obtained using freshly prepared crystals by an Elementar Vario EL III instrument (Elementar Analysensysteme GmbH, Langenselbold, Germany).
Powder X-ray diffraction (PXRD) and X-ray crystallography
Powder X-ray diffraction patterns of the powdered 1 sample were checked on a PANanalytical X’Pert diffractometer equipped with a Cu–Kα radiation source (λ = 1.54182 Å). The diffraction data were recorded in the range of 5÷50° at room temperature. The simulated and experimental powder diffraction patterns of 1 are included in supporting information (Fig. S1). X-ray diffraction data for the single crystal of 1 with dimensions of 0.264×0.206×0.152 mm were collected on an Oxford Diffraction four-circle single crystal diffractometer equipped with a CCD detector using graphite-monochromatized MoKα radiation. The raw data were treated with the CrysAlis Data Reduction Program (version 1.171.38.43) [44]. The intensities of the reflection were corrected for Lorentz and polarization effects. The crystal structure was solved by direct methods [45] and refined by full-matrix least-squares method using SHELXL-2018 program [46]. Non-hydrogen atoms were refined using anisotropic displacement parameters. H atoms were visible on the Fourier difference maps, but placed by geometry and allowed to refine “riding on” the parent atom with Uiso = 1.2 Ueq(C or N). Coordinates of hydrogen atoms of the water molecules were constrained with Uiso = 1.5 Ueq(O). Visualizations of the structure were made using Diamond 3.2 k [47]. Crystal data and final refinement parameters are collected in Table 1.
Spectroscopies methods
FT-IR/FIR/Raman: The Fourier transform middle-infrared (FT-IR) and far-infrared (FT-FIR) spectra of l-arginine and complex 1 were measured on a Bruker VERTEX 70 V vacuum spectrometer equipped with a diamond ATR accessory and an air-cooled DTGS detector. The instrument was kept under vacuum during the measurements and the spectra were recorded with a resolution of 2.5 cm–1. The Raman spectra were measured on a Bruker Senterra dispersive Raman spectrometer equipped with a confocal microscope, using a 532 nm exciting laser line.
NIR–Vis–UV: The electronic diffuse-reflectance spectra of complex 1 was recorded at room temperature on a Cary 500 Scan NIR/Vis/UV spectrophotometer in the range 5000–50,000 cm–1, with a resolution of 10 cm–1. The absorbance spectrum of complex 1 dissolved in aqueous solution was measured with length of the optical path: l = 1.0 cm for CCu(II) = 1.40 × 10–2 M in 7200–23,000 cm–1 range.
X − and Q − band EPR: EPR experiments were performed using a Bruker Elexsys E500 spectrometer operating at ∼9.6 (X-band) and ∼34 GHz (Q-band) frequencies, equipped with an NMR teslameter (ER 036TM) and a frequency counter (HP5342A). Spectra were recorded for the powder samples at 77 K (X-band) and room temperature (Q-band). The samples were carefully ground before measurements to avoid incompletely averaged powder spectra. All simulations of EPR spectra were performed using EasySpin 5.2.35 [48].
DFT calculations: DFT calculations were performed using the ORCA 5 suite of programs [49, 50] with the hybrid functional B3LYP [51,52,53]. The def2-TZVP basis set for Cu and the def2-SVP basis set for the remaining atoms were employed [54]. The resolution of identity approximation was used to speed up the calculations [55], and hence, the corresponding auxiliary basis set was used [56]. The structure determined from the X-ray diffraction experiments was used in the calculations, but the non-coordinated solvent molecules and sulphate anions were removed; the positions of all hydrogen atoms were optimized. An accurate integration grid (DefGrid2) and tight SCF convergence criteria (TightSCF) were set in all calculations. Visualizations were completed with the Gabedit 2.5 software [57].
Magnetic measurements
Magnetic properties were measured by Quantum Design MPMS-XL magnetometer. The DC magnetic susceptibility in the condition of field-cooled (FC) was measured in the temperature range of 2–300 K in the presence of an applied field of 1 kOe. The field-dependent magnetization curves were obtained at 2 K in the magnetic field range of ± 7 T.
Results and discussion
Crystal structure description of 1
In order to confirm the purity of the synthesized complexes, the powder X−ray diffraction (PXRD) pattern of 1 was obtained (Fig. S1). The positions of the main peaks from the experimental data matched well with those of the simulated peaks from the crystal data, proving the single phases of the synthesized products. No major differences between experimental and simulated powder diffractogram show that examined crystal of 1 is homogeneous. The crystals of compound 1 belong to P212121 space group with unit cell edge lengths a = 6.9779 Å, b = 12.8983 Å, c = 25.477 Å (Table 1). Asymmetric unit cell consists of copper(II) coordination [Cu(l-Arg)2]2+ cation balanced by SO42– ions and three free water molecules with occupation factor of 0.5 (Fig. 1a).
Two l-Arg zwitterions chelated copper(II) ion in cis–conformation via carboxylate oxygens (O11, O21) and amino nitrogen (N11, N12) atoms, which form the O2N2 core (Fig. 1). The cis– isomerism around copper atom is also observed in other l-argininato copper(II) compounds including SO42– ion, i.e. {[Cu(μ-l-Arg)2(H2O)]SO4·5H2O}n (2) [42] and {[Cu(μ-l-Arg)2(H2O)]SO4}n (3) [14]. As shown in Table 2, the Cu−O11 (1.964 Å) and Cu−O21 (1.932 Å) bonds are slightly shorter than the Cu−N11 and Cu−N21 lengths (1.972 Å and 1.994 Å), what is also observed in compounds 2 and 3. The copper(II) sphere is completed by l-argininato carboxylate oxygen O12 atom (Cu−O12 bond length of 2.468 Å) to form the five-coordinated environment, which can be realized by square planar (SP) or trigonal bipyramidal (TB) geometry (Fig. 1b). As proposed by Addison et al. [58], the value of angular τ5 parameter allows to differentiate these two possible geometries (\(\tau_{5} = \frac{\beta - \alpha }{{60^\circ }}\); where β and α are the largest angles in the coordination sphere; τ5 = 0 stands for ideal SP geometry, and τ5 = 1 means ideal TB). In the case of compound 1, the value of geometry index based on β = 177.7° and α = 177.4° yields to τ5 = 0.005. Thus, the O11, O21, N11, N21 atoms create the basal plane, while the O12 atom occupies the axial position in square planar pyramid. The lengths of the equatorial plane bonds are in the range 1.932−1.994 Å. The carboxylate O12 atom in axial position is distanced at 2.468 Å, what is slightly shorter than that previously found for {[Cu(μ-l-Arg)2(H2O)]SO4·5H2O}n (2) [42] and {[Cu(μ-l-Arg)2(H2O)]SO4}n (3) [14] polymers (ca. 2.45 and 2.697 Å, respectively).
In crystal structures of compounds 1−3, the sulphate ions are uncoordinated to the metal centres. For 1, the SO42– ion is deformed from ideal tetrahedral sp3 geometry, as indicated by the respective values of O−S−O angles ranging from 106.96° to 112.95° (Table 2). The magnitude of this deformation can be described using the angular τ4 geometry parameter proposed by Yang et al. [59]. (\(\tau_{4} = \frac{{360^\circ - \left( {\alpha + \beta } \right)}}{141^\circ }\); α and β are the largest angles). τ4 = 0 corresponds to ideal square planar geometry and τ4 = 1 stands for ideal tetrahedral geometry. The value of τ4 = 0.993 obtained for SO42– ion in 1 indicates slight angular deformation from tetrahedral shape (α = 112.95° (O1–S–O4); β = 111.31° (O1–S–O2)). Apart from the angular distortions, there are also different S–O bond lengths, ranging from 1.469 Å (Table 2).
Compound 1 exhibits 1D polymer structure similar to the 2 and 3 compounds (Fig. 1b). The copper centres are linked by carboxylate oxygen atoms and form syn−anti−μ2−η1:η1 Cu–O–C–O–Cu zigzag bridges in which the difference in Cu−O basal plane (1.965 Å) and axial (2.468 Å) bond lengths generated the bridge asymmetry similar to structures 2 and 3. Along Cu–O–C–O–Cu bridge in compound 1, the adjacent Cu(II) ions are distanced by 5.707 Å, what is slightly shorter than 5.908 Å found for structure 2 but longer than 5.310 Å observed in crystals of {[Cu(glygly)(H2O)][Cu(glygly)(H2O)2]}n 5nH2O (glygly = glycylglycine) [60]. The further inspection of compound 1 indicates that subsequent Cu∙∙∙Cu proximity in 1D–coordination polymeric chain equals 6.978 Å (Fig. 1b).
The oxygen sulphate atoms are engaged in Namino–H∙∙∙O1/O4 and O2/O3∙∙∙H–Nguanidine hydrogen bonds systems which linked polymeric 1D chains and further generate 2D layers (Fig. 2, Table 3). It is suspected that a deformation of the ideal tetrahedral geometry of the sulphate anion is due to the existence of hydrogen bonds involving oxygen atoms of sulphate group (Fig. 2, Table 3). The largest difference is observed for the S–O3 bond length (1.511 Å). This effect is mainly caused by two relatively strong hydrogen bonds N12–H12A∙∙∙O3 and N14–H14D∙∙∙O3vi involving nitrogen atoms of one guanidine group. Moderately elongated S–O4 bond is connected with N24–H24D∙∙∙O4 hydrogen bond created also by guanidine nitrogen atom and N11–H11A∙∙∙O4ii hydrogen bond with amino nitrogen atom as donor. Bond angle in the second of them is noticeably smaller and bond length is significantly larger. Additionally, S–O2 bond is involved in one moderately strong N22–H22A∙∙∙O2 hydrogen bond which also results in slight elongation of this bond.
Spectroscopic studies (IR/Raman, NIR–Vis–UV, EPR)
IR and Raman spectroscopy
Figures 3 and 4 illustrate the experimental infrared and Raman spectra of 1, in the ranges 3750–600 cm–1 and 600–50 cm–1, respectively. The analogous spectra of pure l-arginine are shown in Figs. S2 and S3 (Supp. material). Table 4 lists the characteristic bands in the IR and Raman spectra of l-arginine and complex 1. All the bands observed in the vibrational spectra of these molecules are shown in Table S1 (Supp. material). Band assignments have been made by the comparison with the results reported in Refs [14, 61,62,63,64,65,66,67,68,69].
As is seen in Table 3, the lattice aqua molecules, the l-arginine ligand and the SO42– group in complex 1 are involved in an extensive hydrogen bonding network in the crystal. This is confirmed by the complicated pattern and broadening of the IR bands, in the range 3500–2700 cm–1 (Fig. 3). In the Raman spectrum of 1, the medium intensity bands at 3368 and 3281 cm–1 arise from the ν(NH2) stretching vibrations in the guanidinium moiety. However, the ν(NH2) modes of the coordinating amino group may also contribute to these Raman bands. The broad infrared bands with maxima at about 3342 and 3148 cm–1 are probably due to the overlapping ν(H2O) vibrations of the lattice aqua molecules and ν(N–H) modes. The characteristic, intense Raman bands at 2952, 2916 and 2868 cm–1 correspond to the stretching vibrations of the CH2 and C–H groups. The scissoring δsc(CH2) vibrations are observed at 1467 and 1450 cm–1 in the Raman spectrum. The other vibrations of the CH2 groups (wagging, rocking and twisting) are strongly coupled [61, 62]; therefore, they are described as CH2 deformation modes (Tables 4 and S1).
It is known that l-arginine in the solid state exists as zwitterion [63] with the negative charge on the carboxylate COO− group and the positive charge on the protonated guanidinium moiety, as in the crystals of 1. The characteristic strong bands at 1674 and 1676 cm–1 in the IR spectra of l-arginine and 1, respectively, arise from the guanidinium ν(CN) stretching vibrations coupled with the δsc(NH2)guan. A similar assignment of this band in the IR spectrum of l-arginine was reported by Krishnan et al. [64]. The very strong band at 1552 cm–1 in the IR spectrum of l-arginine should be assigned to the δsc(NH2)am vibrations, since this band is absent in the spectra of complex 1 (this work) and 3 [14]. The corresponding vibration of the coordinating NH2 group in 1 is shifted to a higher frequency (1632 cm–1) upon binding with the copper(II) ion (Table 4).
In the studied IR spectrum of l-arginine, the νas(COO) vibration appears as the very strong band at 1614 cm–1, whereas the νs(COO−) mode contributes to the strong band at 1419 cm–1 (Table 4). The ν(C=O) and ν(C–O) stretching vibrations in 1 contribute to the strong and medium intense IR bands at 1591 and 1391 cm–1, respectively. The corresponding vibrations, in complex 3, Alikhani et al. assigned to the IR bands at 1594 and 1377 cm–1 [14]. Moreover, for the cis-[Cu(glycine)2]·H2O complex [65], the carboxylate ν(C=O) and ν(C–O) bands were reported at 1595 and 1389 cm–1, which supports our assignment. It should be noted that in metal ion complexes of amino acids, the non-coordinating C=O groups are hydrogen bonded to the neighbouring complex molecules or water of crystallization, or weakly bonded to the metal ion of the other complex unit. All these interactions affect the ν(C=O) stretching vibration [66].
As revealed by the DFT studies and the calculated potential energy distribution (PED) for l-arginine [61], the symmetric stretching vibration in the guanidinium group, νs(CN)3, can be attributed to the medium intensity Raman band at 920 cm–1. Thus, the corresponding vibration in complex 1 is assigned to the similar Raman band at 935 cm–1. As follows from our X–ray study of 1, the three CN bond lengths in the guanidinium unit (e.g. 1.33(1), 1.30(1) and 1.338(9) Å for C26–N22, C26–N23 and C26–N24, respectively) are very close to each other. This is consistent with the delocalization of the positive charge, i.e. a resonance structure between the three CN bonds in the guanidinium moiety of 1. The other marker bands for the C–NH2 groups are observed in the Raman spectra at 1100 and 1103 cm–1 for free l-arginine and 1, respectively. According to the calculated PED [61], these bands correspond to the bending vibration, δ(C–NH2)guan (Table 4).
The comparison of the IR and Raman spectra of 1 with the other compounds containing the sulphate group [14, 66,67,68] confirms the presence of the SO42– ion in the studied complex. For an isolated SO42– ion (Td symmetry), there are four normal modes: ν3(F2) = 1150 cm–1 (triply degenerate νas(S–O)); ν1(A1) = 983 cm–1 (νs(S–O)); ν4(F2) = 611 cm–1 (triply degenerate δas(OSO)) and ν2(E) = 450 cm–1 (doubly degenerate δs(OSO)). All the four vibrations are Raman active, whereas only ν3 and ν4 are infrared active [66, 67]. In the crystal of 1, the Td symmetry of SO42– ion is lowered because of intermolecular hydrogen bonds (Table 3). This causes a splitting of the degenerate vibrations and the Raman-active modes become also infrared active. In the IR spectrum of 1, the very strong band at 1063 cm–1 with a shoulder at 1080 cm–1 and the medium band at 1157 cm–1 unambiguously originate from the split of ν3 mode. Very similar assignments of degenerate ν3 vibrations were reported for bis(melaminium) sulphate dihydrate, where the corresponding ν3 bands appeared at 1058, 1090 and 1146 cm–1, respectively [67]. The symmetric S–O stretching vibration ν1 should be assigned to the very strong Raman band at 970 cm–1, because this mode was reported in the range 970–995 cm–1 for several other compounds with the sulphate group [14, 66,67,68]. However, it should be noted that in l-arginine, the coupled ν(C–C) and ν(C–N) vibrations were observed at very similar frequencies: 981(R) cm–1 [61], 985(R) cm–1 [62], 973(IR)/982(R) cm–1 [69]. Thus, it can be concluded that the ν1 mode coincides with the ligand band and the latter is observed as a shoulder at 982 (IR) and 985 cm–1 (Raman). A similar effect has been found for the ν4 mode, which usually generates strong and split bands, in the range 570–645 cm–1 in the IR spectra of metal ions sulphate complexes [14, 66,67,]. The very strong and broad band with a maximum at 602 cm–1 in the IR spectrum of 1 may arise from the two overlapping modes: l-arginine skeletal vibration and the ν4 mode (Table 4). A shoulder at 592 cm–1 can also be attributed to the ν4. Furthermore, the ν2 vibration appears at 454 cm–1 in the Raman spectrum of 1, and this assignment is supported by the literature data [66,67,, 68].
In the low-frequency region (below 600 cm–1), the metal–ligand vibrations are expected to occur. According to the symmetry consideration, the cis–isomer exhibits more bands in the infrared and Raman spectra than does the trans-isomer; for example, the cis–isomer has two ν(Cu–O) stretching vibrations, which are both IR- and Raman-active, while the trans-isomer shows only one IR-active (νas) and one Raman-active (νs) stretching vibration (mutual exclusion rule).
The far-infrared and Raman spectra of 1 show that the studied complex has the cis–conformation. The IR band of medium intensity at 444 cm–1 and the corresponding Raman band (a shoulder) at 444 cm–1 are attributed to the νas(Cu–N) stretching vibration. The νs(Cu–N) mode contributes to the IR/Raman shoulders at 430 cm–1. It should be noticed that the ν(Cu–N) stretching vibration was assigned at 440 cm–1 for [Cu(N2H4)2]Cl2, 445 cm–1 for (glycylglycino)Cu(II) trihydrate and 440 cm–1 for bis(aminomethanesulfonato)Cu(II) compounds [65]. This indicates a similar strength of the Cu–N bonds, in all these complexes. In the infrared spectrum of cis–[Cu(glycino)2]·H2O the two ν(Cu–O) vibrations were observed at 335 and 285 cm–1, while in the IR spectrum of trans-[Cu(glycino)2]·2H2O only one νas(Cu–O) band appeared at 337 cm–1 [65, 66,67,]. The νas(Cu–O) and νs(Cu–O) vibrations in the title complex 1 are observed at 340(IR)/343(R) and 264(IR)/273(R) cm–1, respectively (Table 4, Fig. 4). According to Condrate and Nakamoto [65], a large frequency separation between the antisymmetric and symmetric ν(Cu–O) stretching vibrations (approx. 70 cm–1) may be ascribed to a large repulsive force between two neighbouring oxygens of the two ligands. Conversely, the charge on nitrogen atoms is less than that on oxygen atoms, hence the ν(M–N) frequencies do not split appreciably.
In the low-frequency region, the crystals containing lattice water molecules exhibit librational modes. These modes generate very broad bands in the range from 600 to 300 cm–1 [66,67,]. Thus, in the IR spectrum of 1, the very broad band with a maximum at 500 cm–1 should be assigned to librational modes of water of crystallization in 1.
Solid-state and absorbance electronic spectra NIR–Vis–UV
The diffuse-reflectance solid-state electronic spectrum exhibits broad band at 16,200 cm–1 (Fig. 5) characteristic for d−d transition for copper(II) (d9 configuration). The similar broad band centred at 16,160 cm−1 (619 nm) was found for cis−complex {[Cu(μ-l-Arg)2(H2O)]SO4·5H2O}n (2) [42]. The l-argininato N11, N21, O11, O21 and O12 coordinated atoms form CuN2O3 chromophore of distorted square-pyramidal coordination environment resulting in a tetragonal crystal field of the C4v symmetry. In this crystal filed, the degenerate spin-allowed 2Eg(Oh) and 2T2g(Oh) states are split into a pair of components 2B1g(dx2–y2) + 2A1g(dz2) and 2B2g(dxy) + 2Eg(dyz≈dxz), respectively, and the 2B1g level is the ground state [70,71,72]. Unfortunately, the splitting of the main band is not observed probably due to the very slightly square planar pyramid distortion towards the trigonal bipyramid (τ5 = 0.005, see Crystal structure description of 1). The close energy values of 2B1g(dx2–y2) → 2A1g(dz2), 2B2g(dxy), 2Eg(dyz≈dxz) transitions result one unresolved band assigned to the dominant dublet-dublet 2B1g(dx2–y2) → 2Eg(dyz≈dxz) transition.
Similarly to the solid-state spectrum, the absorbance spectrum of compound 1 dissolved in water shows broad and unsplitted absorption band with a maximum at 16,160 cm–1 (Fig. S4). The value of molar absorption coefficient (ε), being ca. 60 dm3 mol–1 cm–1, indicates the d-d nature of this transition.
EPR spectroscopy
EPR spectroscopy has been showed as an efficient tool for the determination of a molecular structure and electronic state of Cu(II) systems [73,74,75,76,77]. The 3d9 Cu(II) ion in an octahedral ligand field is a subject to the Jahn–Teller distortion, which decreases the molecular symmetry by lifting the eg and t2g orbital degeneracy. If the resulting system becomes an elongated octahedral, or square-pyramidal or square planar molecular structure, then the unpaired electron occupies the 3dx2−y2 orbital and the relationship between the principal values of the g tensor is gz ≫ gx = gy > 2.0023. The 3dz2 orbital becomes the ground state if the molecular structure is a compressed octahedral or trigonal bipyramidal and the correlation is gx = gy > gz ≈ 2.0023. For intermediate situations, the EPR spectrum is characterized by gz > gy > gx values and reveals the ground state with the unpaired electron occupying a molecular orbital being the linear combination of 3dx2−y2 and 3dz2. The parameter G = (gy − gx)/(gz − gx) can determine whether the unpaired electron occupies the dz2 (G > 1) or dx2−y2 (G < 1) orbital in the ground state [78].
The EPR spectrum recorded for 1 at 9.6 GHz comprised a single, but noticeably anisotropic line (Fig. 6). Similarly, the EPR spectrum recorded for the crystalline sample of 2 was broad and unresolved [42]. The spectrum of 1 was simulated using gx = 2.070, gy = 2.075 and gz = 2.200, but accuracy of these three values is somewhat limited because the spectrum remained unresolved due to the g tensor at 9.6 GHz. However, a satisfactory resolution of the g tensor into its three components gx = 2.059, gy = 2.075 and gz = 2.227 (gavg = 2.120) was obtained at 34 GHz frequency. These values show that the unpaired electron occupies a molecular orbital with the dominant contribution from the 3dx2−y2 atomic orbital of Cu(II) (G = 0.21). This finding stays in line with the conclusion we drew from the analysis of absorbance electronic spectra. Moreover, the determined values of the principal g tensor components are in accordance with values expected for CuN2O2 chromophore [73, 78,79,80] and similar to these observed for other Cu(II) complexes containing l-arginine as a ligand [39, 81].
The DFT calculations corroborated the conclusion that in 1 the unpaired electron occupies the molecular orbital formed from 3dx2−y2. Figure 7 shows the spin density and singly occupied molecular orbital (SOMO) isosurfaces, which demonstrate that the dx2−y2 is engaged in strong antibonding interactions with the ligands’ lone pairs. This antibonding interaction causes a significant transfer of spin density onto the ligands. According to Löwdin populations analysis, only 57.5% of the spin population is located on the Cu(II) ions in 1.
Results of the DFT calculations with the hybrid functional B3LYP: a molecular model of 1 used in the calculations along with the spin density isosurfaces contoured at 0.0025 a.u.; b splitting of the quasi-restricted orbitals and their isosurfaces contoured at 0.05 a.u., orbital symbols for idealized symmetry (C4v) are used
The EPR spectra for compound 1 recorded at 9.6 and 34 GHz frequencies remained unresolved due to the hyperfine interaction of the unpaired electron with 63,65Cu (I = 3/2), indicating that the spin exchange between the adjacent Cu(II) is fast enough to average the hyperfine interaction (short spin–spin relaxation time) [82]. This stays in line with short 5.707 Å Cu⋯Cu distance between the adjacent Cu(II) ions in the Cu–O–C–O–Cu bridge. This distance is considerably shorter than the Cu⋯Cu gaps of 8.4, 9.6, 8.1 and 8.6 Å observed for [Cu(his)4](NO3)2, [Cu(1–Bz–2–CH2OHIm)4](NO3)2, [Cu(4–Me–5–CHOIm)4](H2O)2(NO3)2 and [Cu(Hdien)2(H2O)2](pnb)4, respectively, for which hyperfine interaction due to 63,65Cu was resolved for the polycrystalline samples (his = histaminol, 1–Bz–2–CH2OHIm = 1–benzyl–2–hydroxymethylimidazole, 4–Me–5–CHOIm = 4–methyl–5–imidazole–carboxaldehyde, Hdien = monoprotonated diethylenetriamine and pnb = p–nitrobenzoate) [73, 83, 84].
Magnetic measurements
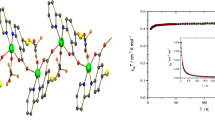
The temperature dependence of χT product (χ- molar magnetic susceptibility per CuII ion) measured for compound 1 in the temperature range 2–300 K and in the field of 1 kOe is given in Fig. 8. The χT values show an almost perfectly linear decreasing trend within the full temperature range with the most apparent deviations located in the low-temperature regime. The value of χT at 2 K amounts to 0.420 cm3 K mol–1 and it decreases steadily to reach the value of 0.162 cm3 K mol–1. This behaviour of the raw χT data implies a substantial diamagnetic contribution intrinsic to the sample. The magnetization at 2 K increases smoothly with the applied field to reach saturation at the maximal field of 70 kOe with the value of 0.99 NAμB. This value of the spectroscopic factor points to a moderate magnetic anisotropy of the Cu(II) ion.
a The molar susceptibility of the studied compound represented in terms of χT (left axis) and χ–1 (right axis). The solid lines show the best-fit curves. Green symbols show the χT values corrected for the diamagnetic contribution. Cyan symbols show the χ–1 values calculated upon subtraction of the diamagnetic corrections. b Magnetization of the studied compound detected at 2 K. Solid lines show the best-fit curves disregarding (green) and accounting for (red) intramolecular interactions between the Cu(II) ions along the chains units obtained with the anisotropic model for magnetization
We fitted to the experimental χT values the following function
where NA is the Avogadro number, μB is the Bohr magneton, kB is the Boltzmann constant, g is the average spectroscopic factor of the Cu(II) ion, SCu = ½ is its spin value, and χ0 is the diamagnetic correction. The best fit yielded g = 2.112(2), χ0 = − 0.000878(3) cm3 mol–1. It is important to notice that the g parameter from the χT fitting stays in line with gavg = 2.120 determined from EPR experiment at 34 GHz. The diamagnetic contribution of the sample can be estimated using the Pascal constants [85] to amount to − 0.000281 cm3 mol−1, which indicates that the diamagnetic contribution of the sample holder accounts for more than 65% of the total correction. The red solid line in Fig. 8a shows the corresponding best-fit curve. Green symbols show the χT values corrected for the diamagnetic contribution. Cyan symbols show the χ−1 values calculated upon subtraction of the diamagnetic corrections. We fitted these points within the interval [Tmin, 300 K], where Tmin varies from 2 K up to 100 K, to the function
where C is the Curie constant and θ is the Weiss temperature. The best fit was selected as the first local minimum of the estimated variance corresponding to Tmin = 2 K (see Fig. S5 in Supporting Data). It yielded C = 0.4212(5) cm3 K mol−1 and θ = − 1.0(2) K. The magenta solid line in Fig. 8a shows the best-fit curve. The relation between the spectroscopic factor and the Curie constant, i.e. \(C = N_{{\text{A}}} \mu_{{\text{B}}}^{2} g^{2} S_{{{\text{Cu}}}} (S_{{{\text{Cu}}}} + 1)/3k_{{\text{B}}}\), implies the value of the spectroscopic factor g = 2.119(1), which is consistent with previous fit result. The negative and small value of the Weiss temperature indicates a weak and antiferromagnetic intermolecular coupling between the −Cu−O−C−O−Cu− chains. Two possible sources of this interaction can be given, one is the H−bond-mediated coupling and the other is the dipole−dipole through space interaction.
The Brillouin function for spin SCu = ½ was adjusted to the experimental magnetization data at 2 K to reproduce the saturation value. This yielded the value of the spectroscopic factor of g = 2.022(2). The green solid curve in Fig. 8b shows the result of the adjustment. It can be seen that the experimental points lie above the adjusted curve, which implies the presence of the ferromagnetic coupling between the Cu(II) ions within the chain units. To estimate the value of this coupling, we employed the molecular field approach and fitting to the experimental points the iterative solution of the following equation
where λ is the molecular field constant and the magnetization M was calculated using the spin ½ Brillouin function. The iterations were stopped when the difference in the magnetization values for two consecutive steps was less than 10–3 NAμB. The value of the spectroscopic factor was fixed during the procedure at the value g = 2.022 given by the previous adjustment. Hence, the only free parameter of the fit was the molecular field constant for which we obtained the value λ = 0.89(2) cm–3 mol. Using the relation between λ and the intramolecular coupling constant J, \(\lambda = \frac{zJ}{{N_{{\text{A}}} \mu_{{\text{B}}}^{2} g^{2} }}\), where z = 2 denotes the number of the nearest neighbour Cu(II) ions coupled along the chain unit, we arrive at the following estimate of the coupling constant J = 0.68(2) K (0.47(1) cm–1). The red solid curve in Fig. 9 shows the best-fit curve. Taking into account that the fit was obtained within the framework of the rough molecular field approach the agreement with the experimental points is perfect.
Encouraged by the success of the last approach, we ventured to recalculate it with an anisotropic model for magnetization. We assumed a diagonal spectroscopic tensor \(\hat{g} = {\text{diag}}(g_{xx} ,g_{yy} ,g_{zz} )\). Then, the magnetization value depends on the orientation of the applied field \(\vec{H} = H[\sin \theta \cos \varphi ,\sin \theta \sin \varphi ,\cos \theta ]\), where \((\theta ,\varphi )\) is the couple of spherical angles, and it is given by the following formula
where \(\beta = (k_{{\text{B}}} T)^{ - 1}\). The powder sample signal is obtained by the appropriate averaging over all possible orientation of the external magnetic field, i.e.
The fitting was repeated by solving the same Eq. (3) with M given by Eq. (5). The function to be minimized was the dimensionless agreement quotient \(Q_{M} = \sum {[M_{\exp } - M_{{{\text{calc}}}} ]^{2} /\sum {M_{\exp }^{2} } }\). The best fit yielded gxx = gyy = gzz = 2.0(1), λ = 0.89(1) cm–3 mol. The value of QM corresponding to the fit was 1.6 × 10–5. The red solid line in Fig. 8b shows the best-fit curve. The green solid line shows the signal for isolated Cu(II) ions (with the intramolecular interactions being switched off, i.e. λ = 0). The value of the intramolecular coupling constant implied by the fit is J = 0.68(9) K (0.47(6) cm–1) and is perfectly consistent with the value obtained from the isotropic fit. The average value of the spectroscopic factor \(\overline{g} = \sqrt {(g_{{{\text{xx}}}}^{2} + g_{{{\text{yy}}}}^{2} + g_{{{\text{zz}}}}^{2} )/3} = 2.0(1)\) is consistent with the adjusted value of 2.022(2). Let us note that the uncertainty of the spectroscopic factor amounting to 0.1 makes it comparable with its counterparts obtained from the susceptibility analysis.
Finally, we ventured to perform the fitting of the complete magnetization data. Because that the feedback loop calculations together with the averaging over field directions turn out quite lengthy for the anisotropic model, we adopted the isotropic model, where the magnetization is given by the Brillouin function. For variable values of the molecular field, constant λ and the averaged g-factor Eq. (3) was solved iteratively and the dimensionless agreement quotient
was calculated. These calculations were implemented within the built-in optimization procedure of the Mathematica8.0 package. The best fit yielded λ = 0.56(3) cm–3 mol, g = 2.084(2) and QM = 1.7 × 10–3. The value of the intramolecular coupling constant implied by the fit is J = 0.45(3) K (0.31(2) cm–1) and is lower than the values obtained in the previous fit. Figure 9 shows the complete magnetization data (symbols) together with the best-fit curves (black solid lines). Although there are apparent discrepancies between the experimental and calculated magnetization values for high fields and low temperatures, the overall agreement is satisfactory. The strength of the intramolecular coupling is slightly diminished as compared to the values obtained from the single magnetization data at 2 K, while the value of the average g-factor is enhanced making it more consistent with the predictions of the susceptibility fits.
Conclusions
In this paper, we present three alternative methods of synthesis of 1D copper(II) coordination polymer based on μ−O,O’ l-argininato carboxylic bridge. The added SCN– and N3− ions to the aqueous CuSO42– and l-arginine mixture have not affected the crystal structure of {[Cu(l-Arg)2]SO4⋅1.5H2O}n (1) although both the SCN– and N3– ions show the better coordination properties than SO42–. In the crystal structure of 1, the copper atom has almost ideal square planar geometry with cis–equatorial coordinated two l-Arg zwitterions, while the apical site is occupied by the oxygen atom of neighbouring amino acid ligand. The copper centres are linked in 1D polymer chain through asymmetric carboxylate zigzag bridges in which the Cu(II) ions are distanced by 5.707 Å.
In the vibrational spectra of 1, the bands characteristic for the SO42– group occur at 1063vs (IR)/1062m (R), 1157m (IR), 970m (IR)/970vs (R), 602vs (IR) and 454m (R) cm–1. The νas(Cu−N) stretching vibration is observed at 444 cm–1 (IR/R), while the νs(Cu−N) stretching mode contributes to the shoulders at 430 cm–1 (IR/R). The ν(Cu−O) stretching vibrations are assigned at 340(IR)/343(R) and 264(IR)/273(R) cm–1 for νas and νs, respectively. The frequencies of Cu−ligand stretching vibrations indicate that the studied complex has the cis–conformation.
In electronic spectra, the characteristic broad band of d−d type found at 16,200 cm–1 confirms the square-pyramidal coordination environment of the C4v symmetry around copper(II) ions. This band is mostly generated by dublet-dublet 2B1g(dx2−y2) → 2Eg(dyz≈dxz) transition. The values of the g tensor gx = 2.059, gy = 2.075 and gz = 2.227 (gavg = 2.120) showed that the unpaired electron occupies a molecular orbital with the dominant contribution from the 3dx2−y2 atomic orbital of Cu(II) (G = 0.21). The DFT calculations demonstrate that the dx2−y2 is engaged in strong antibonding interactions with the ligands’ lone pairs and only 57.5% of the spin population is located on the Cu(II) ions in 1.
The susceptibility fits give the value of g by only about 1% larger than the magnetization fits. They both point to a moderate anisotropy of the spectroscopic factor. The value of g may be concluded to be contained in the interval [2.08, 2.12], the upper bound of which is perfectly consistent with the EPR prediction of \(\overline{g} = \sqrt {(g_{{{\text{xx}}}}^{2} + g_{{{\text{yy}}}}^{2} + g_{{{\text{zz}}}}^{2} )/3} = 2.122\) with gxx = 2.059, gyy = 2.075 and gzz = 2.227. The coupling between the Cu(II) ions within the chain units mediated by the carboxylate bridges is rather weak and of the ferromagnetic character. The intermolecular coupling is antiferromagnetic and comparable with the intramolecular one giving rise to the Weiss theta being small and negative. Among the previous studies, there are reports of both the weakly ferromagnetic [86,87,88,89] and weakly antiferromagnetic [29, 31, 36, 39, 90] couplings through the syn-anti carboxylate bridges.
The successful synthesis of the presented in this paper copper(II) l-argininato coordination polymer has primarily enriched carboxylate-bridged 1D coordination polymers not only structurally, but also spectroscopically and magnetically.
CCDC 2,213,322 contains the supplementary crystallographic data for the complex {[Cu(l-Arg)2]SO4⋅1.5H2O}n (1). These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+ 44) 1223–336-033; or email: deposit@ccdc.cam.ac.uk.
References
D. Maspoch, D. Ruiz-Molina, J. Veciana, J. Mater. Chem. 14, 2713 (2004)
L.R. Mingabudinova, V.V. Vinogradov, V.A. Milichko, E. Hey-Hawkins, A.V. Vinogradov, Chem. Soc. Rev. 45, 5408 (2016)
Z. Yan, M. Li, H.-L. Gao, X.-C. Huang, D. Li, Chem. Comm. 48, 3960 (2012)
J. S. Barbosa, F. Figueira, S. S. Braga, F.A. Almeida Paz, Metal-Organic Frameworks for Biomedical Applications, ed. By M. Mozafari (Elsevier Inc., 2020) p. 69–92
B. Li, Q.-Q. Yan, Z.-Q. Xu, Y.-B. Xu, G.-P. Yong, Cryst. Eng. Comm. 22, 506 (2020)
H.-Z. Kou, B.C. Zhou, S. Gao, D.-Z. Liao, R.-J. Wang, Inorg. Chem. 42, 5604 (2003)
B. Machura, A. Świtlicka, I. Nawrot, J. Mroziński, R. Kruszynski, Polyhedron 30, 832 (2011)
S.V. Voitekhovich, M.M. Degtyarik, A.S. Lyakhov, L.S. Ivashkevich, J. Klose, B. Kersting, O.A. Ivashkevich, Z. Anorg, Allg. Chem. 646, 1331 (2020)
N. Gerasimchuk, Polymers 3, 1475 (2011)
A.D. Naik, M.M. Dîrtu, A.P. Railliet, J. Marchand-Brynaert, Y. Garcia, Polymers 3, 1750 (2011)
J. Zhao, X.-L. Wang, X. Shi, Q.-H. Yang, C. Li, Inorg. Chem. 50, 3198 (2011)
W. Yang, X. Lin, A.J. Blake, C. Wilson, P. Hubberstey, N.R. Champness, M. Schröder, Inorg. Chem. 48, 11067 (2009)
M.L. Hu, A. Morsali, L. Aboutorabi, Coord. Chem. Rev. 255, 2821 (2011)
M. Alikhani, M. Hakimi, K. Moeini, V. Eigner, M. Dusek, J. Inorg. Organomet. Polym. Mater. 30, 2907 (2020)
S. Konar, P.S. Mukherjee, M.G.B. Drew, J. Ribas, N.R. Chaudhuri, Inorg. Chem. 42, 2545 (2003)
W. Gao, F. Liu, B.Y. Zhang, X.M. Zhang, J.P. Liu, E.Q. Gao, Q.Y. Gao, Dalton Trans. 46, 13878 (2017)
J.H. Qing, H.R. Wang, Q. Pan, S.Q. Zang, H. Hou, Y. Fan, Dalton Trans. 44, 17639 (2015)
W. Li, H.P. Jia, Z.F. Ju, J. Zhang, Dalton Trans. 39, 5350 (2008)
P. Lama, A. Aijaz, E.C. Sanudo, P.K. Bharadwaj, Cryst. Growth Des. 10, 283 (2010)
H. Arora, R. Mukherjee, New. J. Chem. 11, 2357 (2010)
K.Y. Choi, Y.M. Jeon, H. Ryu, J.J. Oh, H.H. Lim, M.W. Kim, Polyhedron 23(6), 903 (2004)
L.F. Chen, J. Zhang, L.J. Song, Z.F. Ju, Inorg. Chem. Commun. 8, 555 (2005)
K. Yoneda, M. Ohba, T. Shiga, H. Oshio, S. Kitagawa, Chem. Lett. 36, 1184 (2007)
Y.Z. Zheng, Z. Zheng, X.M. Chen, Coord. Chem. Rev. 258–259, 1 (2014)
S. Massignami, R. Scatena, A. Lanza, M. Monari, F. Condello, F. Nestola, C. Pettinari, F. Zorzi, L. Pandolfo, Inorganica Chim. Acta 455, 618 (2017)
Ch. Hawes, R. Babarao, M.R. Hill, K.F. White, B.F. Abrahams, P.E. Kruger, Chem. Commun. 94, 11558 (2012)
R. Cao, Q. Shi, D. Sun, M. Hong, W. Bi, Y. Zhao, Inorg. Chem. 41, 6161 (2002)
A. Dijkstra, Acta Crystallogr. 20, 588 (1966)
R. Bikas, B. Soltani, H. Sheykhi, M. Korabik, M. Hossaini-Sadr, J. Mol. Struct. 1168, 195 (2018)
M. A. Hitchman, L. Kwan, J. Chem. Soc., Dalton trans. 2, 457 (1987)
R. Calvo, M.C.G. Passeggi, M.A. Novak, O.G. Symko, S.B. Oseroff, O.R. Nascimento, M.C. Terrile, Phys. Rev. B43, 1074 (1991)
J. Weng, M. Hong, Q. Shi, R. Cao, A.S.C. Chan, Eur. J. Inorg. Chem. 10, 2553 (2002)
A. Wojciechowska, M. Daszkiewicz, A. Bieńko, Polyhedron 28, 1481 (2009)
J.M. Schveigkardt, A.C. Rizzi, O.E. Piro, E.E. Castellano, R.C. de Santana, R. Calvo, C.D. Brondino, Eur. J. Inorg. Chem. 11, 2913 (2002)
C.-C. Ou, D.A. Powers, J.A. Thich, T.R. Felthouse, D.N. Hendrickson, J.A. Potenza, H.J. Schugar, Inorg. Chem. 17, 34 (1978)
P.R. Levstein, R. Calvo, Inorg. Chem. 29, 1581–1582 (1990)
T.G. Fawcett, M. Ushay, J.P. Rose, R.A. Lalancette, J.A. Patenza, H.J. Schugar, Inorg. Chem. 18, 327 (1979)
D. Van der Helm, M.B. Lawson, E.L. Enwall, Acta Crystallogr. B27, 2411 (1971)
M.F. Gerard, C. Aiassa, N.M.C. Casado, R.C. Santana, M. Perec, R.E. Rapp, R. Calvo, J. Phys. Chem. Solids 68, 1533 (2007)
R. Hu, Q. Yu, F. Liang, L. Ma, X. Chen, M. Zhang, H. Liang, K. Yu, J. Coord. Chem. 61, 1265 (2008)
H. Masuda, A. Odani, T. Yamazaki, T. Yahima, O. Yamauchi, Inorg. Chem. 32, 1111 (1993)
N. Ohata, H. Masuda, O. Yamauchi, Inorg. Chim. Acta 300–302, 749 (2000)
A. Wojciechowska, J. Janczak, T. Rojek, A. Gorzsas, M. Malik-Gajewska, M. Duczmal, J. Coord. Chem. 72, 1358 (2019)
CrysAlis CCD and CrysAlis Red 1.171.38.43, Rigaku Oxford Diffraction, 2015. PROGRAM
G.M. Sheldrick, Acta Crystallogr. A64, 112 (2008)
G.M. Sheldrick, Acta Crystallogr. A71, 3 (2015)
K. Brandenburg, H. Putz, Diamond: crystal and Molecular Structure Visualization, Cryst. Impact Bonn, Germany. (2008) http://www.crystalimpact.com/diamond.
S. Stoll, A. Schweiger, J. Magn. Reson. 178, 42 (2006)
F. Neese, Wiley Interdiscip. Rev. Comput. Mol. Sci. 8, e1327 (2018)
F. Neese, F. Wennmohs, U. Becker, C. Riplinger, J. Chem. Phys. 152, 224108 (2020)
A.D. Becke, Phys. Rev. A38, 3098 (1988)
A.D. Becke, J. Chem. Phys. 98, 1372 (1993)
C. Lee, W. Yang, R.G. Parr, Phys. Rev. B37, 785 (1988)
F. Weigend, R. Ahlrichs, Phys. Chem. Chem. Phys. 7, 3297 (2005)
F. Neese, J. Comput. Chem. 24(14), 1740 (2003)
F. Weigend, Phys. Chem. Chem. Phys. 8, 1057 (2006)
A.-R. Allouche, J. Comput. Chem. 32, 174 (2011)
A.W. Addison, T.N. Rao, J. Reedijk, J. van Rijn, G.C. Verschoor, J. Chem. Soc. Dalton Trans. 7, 1349 (1984)
L. Yang, D.R. Powell, R.P. Houser, Dalton Trans. 9, 955 (2007)
W.L. Liu, Y. Zou, Ch.L. Ni, Z.P. Ni, Y.Z. Li, Q.J. Meng, J. Coord. Chem. 57, 657 (2004)
B. Hernández, F. Pflüger, N. Derbel, J. De Coninck, M. Ghomi, J. Phys. Chem. B114, 1077 (2010)
G. Zhu, X. Zhu, Q. Fan, X. Wan, Spectrochim. Acta 78, 1187 (2011)
E. Courvoisier, P.A. Williams, G.K. Lim, C.E. Hughes, K.D.M. Harris, Chem. Commun. 48(22), 2761 (2012)
R.S. Krishnan, V.N. Sankaranarayanan, K. Krishnan, J. Indian Inst. Sci. 55, 66 (1973)
R.A. Condrate, K. Nakamoto, J. Chem. Phys. 42, 2590 (1965)
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, part B (New York Inc, J. Wiley & Sons, 2009)
M.K. Marchewka, J. Chem. Res. 8, 518 (2003)
E.A. Secco, Can. J. Chem. 66, 329 (1988)
A. Wojciechowska, A. Kochel, W. Zierkiewicz, J. Coord. Chem. 69, 886 (2016)
T. Murakami, T. Takei, Y. Ishikawa, Polyhedron 16, 89 (1997)
S. Saha, N. Biswas, A. Sasmal, C.J. Gomez Garcia, E. Garriba, A. Bauza, A. Frontera, G. Pilet, G.M. Rosair, S. Mitra, C. R. Choudhury, Dalton Trans. 47, 16102 (2018)
D.E. Billing, B.J. Hathaway, P. Nicholls, J. Chem. Soc. A, 316 (1969)
P. Maślewski, D. Wyrzykowski, M. Witwicki, A. Dołęga, Eur. J. Inorg. Chem. 12, 1399 (2018)
E.I. Solomon, Inorg. Chem. 45, 8012 (2006)
D.L. Reger, A.E. Pascui, M.D. Smith, J. Jezierska, A. Ozarowski, Inorg. Chem. 54, 1487 (2015)
S. Kumar, R.P. Sharma, P. Venugopalan, V.S. Gondil, S. Chhibber, T. Aree, M. Witwicki, V. Ferretti, Inorg. Chim. Acta 469, 288 (2018)
J. Gregoliński, K. Ślepokura, J. Kłak, M. Witwicki, Dalton Trans. 51, 9735 (2022)
B.J. Hathaway, D.E. Billing, Coord. Chem. Rev. 5, 143 (1970)
A. Bykowska, R. Starosta, J. Jezierska, M. Jeżowska-Bojczuk, RSC Adv. 5, 80804 (2015)
J. Peisach, W.E. Blumberg, Arch. Biochem. 165, 691 (1974)
R.C. Santana, R.O. Cunha, J.F. Carvalho, I. Vencato, R. Calvo, J. Inorg. Biochem. 99, 415 (2005)
M.M. Roessler, E. Salvadori, Chem. Soc. Rev. 47, 2534 (2018)
S. Kumar, R.P. Sharma, P. Venugopalan, M. Witwicki, V. Ferretti, J. Mol. Struct. 1123, 124 (2016)
B. Barszcz, T. Głowiak, J. Jezierska, Polyhedron 18, 3713 (1999)
G.A. Bain, J.F. Berry, J. Chem. Edu. 85, 532 (2008)
E. Colacio, J.-M. Dominguez-Vera, J.-P. Costes, R. Kivekäs, J.-P. Laurent, J. Ruiz, M. Sundberg, Inorg. Chem. 31, 774 (1992)
E. Colacio, M. Ghazi, R. Kivekäs, J.M. Moreno, Inorg. Chem. 39, 2882 (2000)
C. Ruiz-Pérez, J. Sanchiz, M.H. Molina, F. Lloret, M. Julve, Inorg. Chem. 39, 1363 (2000)
F. Su, L. Lu, S. Feng, M. Zhu, Z. Gao, Y. Dong, Dalton Trans. 44, 7213 (2015)
S. Shit, M. Nandy, C. Rizzoli, C. Desplanches, S. Mitra, J. Chem. Sci. 128, 913 (2016)
Funding
The work was financed by a statutory activity subsidy from the Polish Ministry of Science and Education for the Faculty of Chemistry of Wrocław University of Science and Technology. We also gratefully acknowledge the instrumental grant 6221/IA/119/2012 from Polish Ministry of Science and Higher Education, which supported our Integrated Laboratory of Research and Engineering of Advanced Materials where FT−IR and Raman measurements were performed. DFT computations were performed using computers of the Wroclaw Center for Networking and Supercomputing (Grant No. 47).
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. KM—synthesis, IR and electronic measurements, editing. JJ crystal structure, investigation. KH—IR/Raman spectroscopy, visualization, writing. MW—EPR spectroscopy and DFT calculations, visualization, writing. MF—magnetism, visualization, writing. RP—magnetism, visualization, writing. AW project administration, methodology, synthesis, electronic spectroscopy, investigation, visualization, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
Not applicable.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Musioł, K., Janczak, J., Helios, K. et al. Copper(II) coordination polymer based on l-arginine as a supramolecular hybrid inorganic–organic material: synthesis, structural, spectroscopic and magnetic properties. Res Chem Intermed 49, 3563–3587 (2023). https://doi.org/10.1007/s11164-023-04957-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-023-04957-0