Abstract
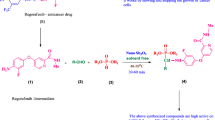
A new class of thiazolyl α-aminophosphonate derivatives was synthesized by one-pot Kabachnik–Fields reaction of ethyl 2-(3-formyl-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate with various aryl amines and diethyl phosphite under solvent-free conditions using β-cyclodextrin supported sulfonic acid (β-CD-SO3H) as an efficient, reusable and heterogeneous solid acid catalyst. The products were obtained in good to excellent yields at shorter reaction time. All the title compounds were screened for cytotoxic activity against human breast cancer (MCF-7 and MDA-MB-231), prostate cancer (DU-145) liver cancer (HepG2) and HeLa cancer cell lines using sulfarodamine-B (SRB assay). Compounds (8b, –4OMe), (8h, –4NO2) and (8j, –2I, –4CF3) showed better anticancer activity when compared with standard drug Adriamycin. Further in-silico target hunting reveals the anticancer activity of the designed compounds by inhibiting DNA topoisomerase II.
Graphical abstract








Similar content being viewed by others
References
G. Mohan, S. Santhisudha, S. Murali, N.B. Reddy, G. Sravya, Z.V. Grigory, C.S. Reddy, Res. Chem. Intermed. 44, 3475 (2018)
G. Mohan, S. Santhisudha, N.M. Reddy, S. Murali, T. Sreekanth, C.S. Reddy, Monatsh. Chem. 148, 1843 (2017)
G. Mohan, S. Santhisudha, S. Murali, T. Sreekanth, Y.S. Rao, N.B. Reddy, C.S. Reddy, J. Heterocyclic Chem. 57, 1414 (2020)
S. Murali, C. Venkataramaiah, N. Saichaithanya, G. Mohan, S.H. Yasmin, W. Rajendra, S.R. Cirandur, Med. Chem. Res. 28, 1740 (2019)
P. Sreelakshmi, M.R. Nadiveedhi, S. Santhisudha, G. Mohan, N. Saichaithanya, M. Sadik, K. Peddanna, S.R. Cirandur, Med. Chem. Res. 28, 528 (2019)
G. Mohan, S. Kumar, M. Sudileti, C. Sridevi, P. Venkatesu, C.S. Reddy, Int. J. Biol. Macromol. 165, 2010 (2020)
K.M.K. Reddy, S. Santhisudha, G. Mohan, K. Peddanna, C.A. Rao, C.S. Reddy, Phosphorus Sulfur Silicon 191, 933 (2016)
P. Dinéra, M. Amedjkouh, Org. Biomol. Chem. 4, 2091 (2006)
W.R. Gemmill, M.D. Smith, B.A. Reisner, J. Solid State Chem. 178, 2658 (2005)
L. Tusek-Bozic, Current. Med. Chem. 20, 2096 (2013)
A. Mucha, P. Kafarski, L. Berlicki, J. Med. Chem. 54, 5955 (2011)
M. Sienczyk, J. Oleksyszyn, Curr. Med. Chem. 16, 1673 (2009)
R. Damiche, S. Chafaa, J. Mol. Struct. 1130, 1009 (2017)
C. Jin-Long, W. Tang, C. Jian-Yi, C. Kai, Y. Gang, G. Yu-Cheng, S. De-Qing, J. Agri. Food Chem. 63, 7219 (2015)
Z.L. Ren, J. Zhang, H.D. Li, M.J. Chu, L.S. Zhang, X.K. Yao, Y. Xia, X.H. Lv, H.Q. Cao, Chem. Pharm. Bull. 64, 1755 (2016)
P. Kafarski, B. Lejczak, E. Slesak, J. Przetocki, Pest. Manag. Sci. 25, 137143 (1989)
L. Gu, C. Cheng, Org. Biomol. Chem. 10, 7098 (2012)
B. Sujatha, S. Mohan, Ch. Subramanyam, K.P. Rao, Phosphorus Sulfur Silicon 192, 1110 (2017)
S. Murali, G. Mohan, S. Santhisudha, T. Sreekanth, H. Balaji, M. Balaji C. S. Reddy, Monatsh. Chem. 150, 1101 (2019)
T. Sreekanth, G. Mohan, S. Santhisudha, N.M. Reddy, S. Murali, A. Rajasekhar, C.A. Rao, C.S. Reddy, Synth. Commun. 49, 563 (2019)
Y. Xu, K. Yan, B. Song, G. Xu, S. Yang, W. Xue, D. Hu, P. Lu, G. Ouyang, L. Jin, Z. Chen, Molecules 11, 666 (2006)
S.A.R. Mulla, M.Y. Pathan, S.S. Chavan, S.P. Gample, D. Sarkar, RSC Adv. 4, 7666 (2014)
R.R. Chinthaparthi, I. Bhatnagar, C.S. Gangireddy, S.C. Syama, S.R. Cirandur, Archive. Pharm Chem. Life Sci. 346, 667 (2013)
C.B. Reddy, K.S. Kumar, M.A. Kumar, M.V.N. Reddy, B.S. Krishna, M. Naveen, M.K. Arunasree, C.S. Reddy, C.N. Raju, C.D. Reddy, Eur. J. Med. Chem. 47, 553 (2012)
S.J. Kashyap, V.J. Garg, P.K. Sharma, N. Kumar, R. Dudhe, J.K. Gupta, Med. Chem. Res. 21, 2123 (2012)
C.A. Calderón-Ospina, M.O. Nava-Mesa, CNS Neurosci. Ther. 26, 5 (2020)
G.M. Reddy, J.R. Garcia, V.H. Reddy, A.M. Andrade, A. Camilo Jr., R.A.P. Ribeiro, S.R. Lazaro, Eur. J. Med. Chem. 123, 508 (2016)
N. Siddiqui, W. Ahsan, Eur. J. Med. Chem. 45, 1536 (2010)
M.H.M. Helal, M.A. Salem, M.S.A. El-Gaby, M. Aljahdali, Eur. J. Med. Chem. 65, 517 (2013)
P. Singh, S. Gupta, S. Kumar, Med. Chem. 16, 4 (2020)
H. Yu, Z. Qin, H. Dai, X. Zhang, X. Qin, T. Wang, J. Fang, J. Agric. Food Chem. 56, 11356 (2008)
V. Jaishree, N. Ramdas, J. Sachin, B. Ramesh, J. Saudi. Chem. Soc. 16, 371 (2012)
M. Bagheri, M. Shekarchi, M. Jorjani, M.H. Ghahremani, M. Vosooghi, A. Shafiee, Archive. Pharm. Chem. Life Sci. 337, 25 (2004)
S. Anuradha, R. Patel, P. Patle, A. Parameswaran, J.A. Shard, Eur. J. Pharma. Sci. 134, 20 (2019)
R.G. Kalkhambkar, G.M. Kulkarni, H. Shivkumar, R.N. Rao, European. J. Med. Chem. 42, 1272 (2007)
T.K. Venkatachalam, E.A. Sudbeck, C. Mao, F.M. Uckun, Bioorg. Med. Chem. Lett. 11, 523 (2001)
K.V. Sashidhara, K.B. Rao, V. Kushwaha, R.K. Modukuri, R. Verma, P.K. Murthy, Eur. J. Med. Chem. 81, 473 (2014)
F. Delmas, A. Avellaneda, C.D. Giorgio, M. Robin, E.D. Clercq, P. Timon-David, J.P. Galy, Eur. J. Med. Chem. 39, 685 (2004)
P. Makam, P.K. Thakur, T. Kannan, Eur. J. Pharma Sci. 52, 138 (2014)
H. Zhao, G. Cui, J. Jin, X. Chen, B. Xu, Bioorg. Med. Chem. 24, 5911 (2016)
S. Kumar, R. Aggarwal, Mini Rev. Org. Chem. 16, 26 (2019)
T.K. Khatab, A.M. Abdelghany, E.M. Kandil, D.E. Elsefy, A. El-Mekabaty, Biointer. Res. Appl. Chem. 10, 5182 (2020)
T.K. Khatab, A.M. Abdelghany, H.A. Soliman, Silicon 10, 703 (2018)
H.A. Soliman, T.K. Khatab, Silicon 10, 229 (2018)
R.M.F. Batista, S.P.G. Costa, E.L. Malheiro, M. Belsley, M.M.M. Raposo, Tetrahedron 63, 4258 (2007)
P.V.G. Reddy, Y.W. Lin, H.T. Chang, Arkivoc 16, 113 (2007)
D. Xie, A. Zhang, D. Liu, L. Yin, J. Wan, S. Zeng, D. Hu, Phosphorus Sulfur Silicon 192, 1061 (2017)
D. Ravikumar, S. Mohan, Ch. Subramanyam, K.P. Rao, Phosphorus Sulfur Silicon 193, 400 (2018)
F. Tamaddon, S.E. Tadayonfar, J. Mol. Liq. 283, 51 (2019)
N. Grimblat, A.M. Sarotti, T.S. Kaufmanaand, S.O. Simonetti, Org. Biomol. Chem. 14, 10496 (2016)
S. Lianyong, Z. Haibo, H. Tao, C. Lingwu, Chin. J. Pharma. 47, 22 (2016)
V. Vichai, K. Kirtikara, Nature Proto. 1, 1112 (2006)
S. Munusamy, V.N. Badavath, S. Maji, M. Sekar, M.N. Shabbir, New J. Chem. 44, 17231 (2019)
C. Nath, V.N. Badavath, A. Thakur, G. Ucar, O. Acevedo, M.U.M. Siddique, V. Jayaprakash, Med. Chem. Commun. 9, 1164 (2018)
B.V. Nayak, S. Ciftci-Yabanoglu, S. Bhakat, A.K. Timiri, B.N. Sinha, G. Ucar, M.E.S. Soliman, V. Jayaprakash, Bioorg. Chem. 58, 72 (2015)
V.N. Badavath, C. Nath, N.M. Ganta, G. Ucar, B.N. Sinha, V. Jayaprakash, Chin. Chem. Lett. 28, 1528 (2017)
V.N. Badavath, I. Baysal, G. Ucar, B.N. Sinha, V. Jayaprakash, A.C.S. Med, Chem. Lett. 7, 56 (2016)
V.N. Badavath, G. Ucar, B.N. Sinha, S.K. Mondal, V. Jayaprakash, Chem. Select. 1, 5879 (2016)
V.B. Nayak, S. Ciftci-Yabanoglu, S.S. Jadav, M. Jagrat, B.N. Sinha, G. Ucar, V. Jayaprakash, Eur. J. Med. Chem. 69, 762 (2013)
H. Wei, A.J. Ruthenburg, S.K. Bechis, G.L. Verdine, J. Biol. Chem. 280, 37041 (2005)
M. Mantipally, M.R. Gangireddy, R. Gundla, V.N. Badavath, S.R. Mandha, V.C. Maddipati, Bioorg. Med. Chem. Lett. 29, 2248 (2019)
G.M. Reddy, M. Mantipally, R. Gundla, B.V. Nayak, K. Paidikondala, A. Yamala, Chem.Select. 4, 13622 (2019)
B.V. Kumbhar, D. Panda, A. Kunwar, PLoS ONE 13, e0194934 (2018)
Acknowledgments
The author G. Mohan thanks to Prof. C. Devendranath Reddy, Department of Chemistry, S.V. University, Tirupati for his helpful discussions and acknowledges DST-PURSE 2nd Phase Programme facilitated in S.V. University, Tirupati funded by DST, New Delhi, India for providing instrumentation facility and funding (File No: 17118-UGC-III(3)/DST-PURSE 2nd Phase/2017, Dt: 23-08-2018). One of the authors S.H. Yasmin acknowledges DST, New Delhi, India for the financial support under DST-INSPIRE Fellowship Programme (File No. DST/INSPIRE Fellowship/2017/IF170317, Dated: 24/05/2017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gundluru, M., Badavath, V.N., Shaik, H.Y. et al. Design, synthesis, cytotoxic evaluation and molecular docking studies of novel thiazolyl α-aminophosphonates. Res Chem Intermed 47, 1139–1160 (2021). https://doi.org/10.1007/s11164-020-04321-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04321-6