Abstract
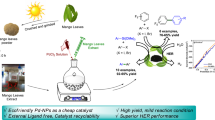
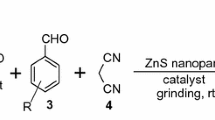
Mushroom-like mesoporous and hexagonal ZnO nanoparticles were synthesized from plant extract and chemical method respectively, by co-precipitation method in aqueous medium. Different morphological forms of ZnO NPs were characterized by XRD, TGA, FESEM, EDX, FTIR, UV–Vis and BET. Neem (Azadirachta indica) leaf extract and ultrasound irradiation have a crucial role in the formation of different morphologies of ZnO NPs. ZnO NPs synthesized from plant extract and hydrotrope show a synergistic effect that leads to efficient synthesis of benzylidenemalonitrile and tetraketone derivatives at room temperature in water. Simple preparation of the catalyst, excellent yields, reusability of catalyst with consistent activity and ease of product isolation are the most significant advantages of this green protocol.
Graphic abstract















Similar content being viewed by others
References
R.A. Sheldon, Green Chem. 72, 67 (2005)
F. Mohammadi, M. Yousefi, R. Ghahremanzadeh, Adv. J. Chem. Sect. A 2, 266 (2019)
H. R. Madan, S. C. Sharma, Udayabhanu, D. Suresh, Y. S. Vidya, H. Nagabhushana, H. Rajanaik, K. S. Anantharaju, S. C. Prashantha, P. Sadananda Maiya, Spectrochimica Acta Part A, 1386 (2015)
A. Kumar, D. Saxena, M.K. Gupta, Green Chem. 10, 2699 (2013)
P.C. Nagajyothi, T.N. Minh An, T.V.M. Sreekanth, J.I. Lee, D.J. Lee, K.D. Lee, Mater. Lett. 108, 160 (2013)
H. Gao, R. Tayebee, M.F. Abdizadeh, E. Mansouri, M. Latifinia, Z. Pourmojahed, RSC Adv. 10, 3005 (2020)
A. Maleki, P. Ravaghi, M. Aghaei, Res. Chem. Intermed. (2017)
S. Kamble, A. Kumbhar, G. Rashinkar, M. Barge, R. Salunkhe, Ultrasound Sonochem. 19, 812 (2012)
A. Maleki, Ultrason. Sonochem. 28, 115 (2017)
T. Bhuyan, K. Mishra, M. Khanuja, R. Prasad, A. Varma, Mater. Sci. Semicond. Process. 32, 55 (2015)
J.S. Rathore, M. Upadhyay, Res. J. Pharm. Sci. 2, 15 (2013)
D. Nath, P. Banerjee, Environ. Toxicol. Pharmacol 33, 997 (2013)
A. Bayrami, S. Alioghli, S.R. Pouran, A.H. Yangjeh, A. Khataee, S. Ramesh, Ultrason. Sonochem. 55, 57 (2019)
F. Shirini, N. Daneshvar, RSC Adv. 6, 110190 (2016)
K.M. Parida, D. Rath, J. Mol. Catal. A: Chem. 310, 93 (2009)
B. Karmakar, B. Chowdhury, J. Banerji, Catal. Commun. 11, 601 (2010)
U.P.N. Tran, K.K.A. Le, N.T.S. Phan, ACS Catal. 1, 120 (2011)
R.A. Agarwal, S. Mukherjee, Polyhedron 105, 228 (2016)
A. Karmakar, A. Paul, K.T. Mahmudov, M.F.C. Guedes Da Silva, A.J.L. Pombeiro, New J. Chem. 40, 1535 (2016)
A. Taher, D.-J.J. Lee, B.-K.K. Lee, I.-M.M. Lee, Synlett 27, 1433 (2016)
A. Zanon, S. Chaemchuen, F. Verpoort, Catal. Lett. 147, 2410 (2017)
H. Goksu, E. Gu, H. Goksu, E. Gultekin, Chem. Select 2, 458 (2017)
S. Hasanzadeh Banakar, M.G. Dekamin, A. Yaghoubi, New J. Chem. 42, 14246 (2018)
D.H. Jung, Y.R. Lee, S.H. Kim, W.S. Lyoo, Bull. Korean Chem. Soc. 30, 1989 (2009)
Y. Ren, B. Yang, X. Liao, RSC Adv. 6, 22034 (2016)
M. Rastroshan, S. Sayyahi, V. Zare-Shahabadi, R. Badri, J. Iranian Chem Res. 5, 265 (2012)
A. Ilangovan, S. Malayappasamy, S. Muralidharan, S. Maruthamuthu, Chem. Cent. J. 5, 81 (2011)
N. Azizi, S. Dezfooli, M.M. Hashemi, C. R. Chim. 16, 997 (2013)
J. Khurana, K. Vij, J. Chem. Sci. 124, 907 (2012)
S. Das, S. Paul, J. Chem. Inf. Model 57, 1461 (2017)
S.D. Sharma, P. Gogoy, D. Konwar, Green Chem. 9, 153 (2007)
M.R. Parra, F.Z. Haque, J. Mater. Res. Technol. 3(4), 363 (2014)
MdR Shakil, A.G. Meguerdichian, H. Tasnim, A. Shirazi-Amin, M.S. Seraji, S. Suib, Inorg. Chem. (2019)
M.H. Kahsay, A. Tadesse, D. Rama Devi, N. Belachew, K. Basavaiah, RSC Adv. 9, 36967 (2019)
B. Shinde, S. Kamble, P. Gaikwad, V. Ghanwat, S. Tanpure, P. Pagare, B. Karale, A. Burungale, Res. Chem. Intermed. 44, 3097 (2018)
V.N. Pham, Do-Gun Kim, Seok-Oh Ko, Science of Total. Environment 631, 609 (2018)
P.P. Ghosh, A.R. Das, J. Org. Chem. 12, 6170 (2013)
B. Shinde, S.B. Kamble, D.M. Pore, P. Gosavi, A. Gaikwad, H.S. Jadhav, B. Karale, A.S. Burungale, Chemistry Select 3, 13197 (2018)
N. Lolak, E. Kuyuldar, H. Burhan, H. Goksu, S. Akocak, F. Sen, ACS Omega 4, 6848 (2019)
R. Maleki, E. Kolvari, M. Salehi, M. Koukabi, Appl. Organomet. Chem. 31, 3795 (2017)
F. Nemati, M.M. Heravi, R. Saeedi Rad, Chin. J. Catal. 33, 1825 (2012)
S.M. Islam, A.S. Roy, R.C. Dey, S. Paul, J. Mol. Catal. A Chem. 394, 66 (2014)
M. Gilanizadeh, B. Zeynizadeha, New J. Chem. 42, 8553 (2018)
M.B. Deshmukh, S.S. Patil, S.D. Jadhav, P.B. Pawar, Synth. Commun. 42, 1177 (2012)
C.I. Ezugwu, B. Mousavi, M.A. Asraf, Z. Luo, F. Verpoort, J. Catal. 344, 445 (2016)
B. Viswanadham, P. Jhansi, K.V.R.R. Chary, H.B. Friedrich, S. Singh. Catal. Lett. 146, 364 (2016)
Yu Jian-Jun, L.-M. Wang, J.-Q. Liu, F.-L. Guo, Y. Liu, Ning Jiao. Green Chem. 12, 216 (2010)
J.K. Rajput, G. Kaur, Catal. Sci. Technol. 4, 142 (2014)
M. Tajbakhsh, M. Heidary, R. Hosseinzadeh, Res. Chem. Intermed. 42, 1425 (2016)
A. Kumbhar, S. Kamble, M. Barge, G. Rashinkar, R. Salunkhe, Tetrahedron Lett. 53, 2756 (2012)
Acknowledgements
The authors gratefully acknowledge the financial support from the Department of Science Technology–Science and Engineering Research Board (DST-SERB) and University Grants Commission (UGC) as major research project and Y.C.I.S. Satara (Autonomous) as seed money. The authors are also thankful to the (DST-FIST) Analytical Instrumentation Lab, Jaysingpur College, Jaysingpur, for providing BET and UVDRS analysis. One of the authors S.A. thanks Krishna Mahavidyalaya, Rethare, for the partial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Attar, S.R., Shinde, B. & Kamble, S.B. Enhanced catalytic activity of bio-fabricated ZnO NPs prepared by ultrasound-assisted route for the synthesis of tetraketone and benzylidenemalonitrile in hydrotropic aqueous medium. Res Chem Intermed 46, 4723–4748 (2020). https://doi.org/10.1007/s11164-020-04233-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04233-5