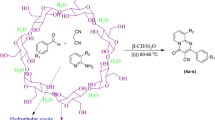
Abstract
Herein, a fast and convenient protocol for the synthesis of new isoniazid fused chromeno[4,3-b]quinolin was achieved through biomimetic catalysis by cyclodextrin in the water at 60–65 °C. The present investigation involves attractive characteristics such as the use of water as the reaction medium, one-pot conditions, short reaction periods, easy work-up/purification and reduced waste production. This method provides a green route for the synthesis of targeted scaffolds and also a wide substrate scope for several substituted aldehydes to provide good yields of the corresponding products. Furthermore, the catalyst can be easily recovered by simple filtration and reused several times without any substantial loss in activity. Our study also discloses the antimicrobial screening of new chromeno[4,3-b]quinolin-isonicotinamides against four bacterial and three fungal strains.
Graphic Abstract






Similar content being viewed by others
References
P. Tundo, P. Anastas, D.S. Black, J. Breen, T. Collins, S. Memoli, J. Miyamoto, M. Polyakoff, W. Tumas, Pure Appl. Chem. 72, 1207 (2000)
K. Alfonsi, J. Colberg, P.J. Dunn, T. Fevig, S. Jennings, T.A. Johnson, H.P. Kleine, C. Knight, M.A. Nagy, D.A. Perry, M. Stefaniak, Green Chem. 10, 31 (2008)
S. Muthusamy, C. Gangadurai, Tetrahedron Lett. 59, 1501 (2018)
V.B. Yadav, P. Rai, H. Sagir, A. Kumar, I.R. Siddiqui, New J. Chem. 42, 628 (2018)
R. Breslow, S.D. Dong, Chem. Rev. 98, 199 (1998)
A. Kumar, R.D. Shukla, Green Chem. 17, 848 (2015)
C.C. Bai, B.R. Tian, T. Zhao, Q. Huang, Z.Z. Wang, Molecules 22, 1475 (2017)
D.R. Patil, Y.B. Wagh, P.G. Ingole, K. Singh, D.S. Dalal, New J. Chem. 37, 3261 (2013)
B. Minea, N. Marangoci, D. Peptanariu, I. Rosca, V. Nastasa, A. Corciova, C. Varganici, A. Nicolescu, A. Fifere, A. Neamtu, M. Mares, M. Barboiu, M. Pinteal, New J. Chem. 40, 1765 (2016)
A. Kumar, V.D. Tripathi, P. Kumar, Green Chem. 13, 51 (2011)
F. Hapiot, S. Menuel, M. Ferreira, B. Leger, H. Bricout, S. Tilloy, E. Monflier, A.C.S. Susta, Chem. Eng. 5, 3598 (2017)
E.A. Kataev, M.R. Reddy, G.N. Reddy, V.H. Reddy, C.S. Reddy, B.V.S. Reddy, New J. Chem. 40, 1693 (2016)
D.S. Lawrence, T. Jiang, M. Levett, Chem. Rev. 95, 2229 (1995)
J. Szejtli, Cyclodextrin Technology (Kluwer Academic, Dordrecht, 1988)
J.M. Desper, J. Breslow, J. Am. Chem. Soc. 116, 12081 (1994)
J. Bjerre, T.H. Fenger, L.G. Marinescu, M. Bols, Eur. J. Org. Chem. 2007, 704 (2007)
L.G. Marinescu, E.G. Doyagueez, M. Petrillo, A. Fernandez-Mayoralas, M. Bols, Eur. J. Org. Chem. 2010, 157 (2010)
M. Zhao, H.L. Wang, L. Zhang, C.Y. Zhao, L.N. Ji, Z.W. Mao, Chem. Commun. 47, 7344 (2011)
S.P. Tang, S. Chen, G.F. Wu, H.Y. Chen, Z.W. Mao, L.N. Ji, Inorg. Chem. Commun. 14, 184 (2011)
R.S. Thombal, A.R. Jadhav, V.H. Jadhav, RSC Adv. 5, 12981 (2015)
K. Konkala, R. Chowrasia, P.S. Manjari, N.L.C. Domingues, R. Katla, RSC Adv. 6, 43339 (2016)
A. Gaspar, M.J. Matos, J. Garrido, E. Uriarte, F. Borges, Chem. Rev. 114, 4960 (2014)
A. Thakur, R. Singla, V. Jaitak, Eur. J. Med. Chem. 101, 476 (2015)
K. Nagaiah, A. Venkatesham, R. Srinivasa Rao, V. Saddanapu, J.S. Yadav, S.J. Basha, A.V.S. Sarma, B. Sridhar, A. Addlagatta, Bioorg. Med. Chem. Lett. 20, 3259 (2010)
A.K. Arya, K. Rana, M. Kumar, Lett. Drug. Des. Discov. 11, 594 (2014)
J. Magano, J.R. Dunetz, Chem. Rev. 111, 2177 (2011)
F. Jafarpour, H. Hazrati, S. Zarei, S. Izadidana, Synthesis 46, 1224 (2014)
F. Bellina, R. Rossi, Chem. Rev. 110, 1082 (2010)
M.I. Hegab, A.M. Abdel-Fattah, N.M. Yousef, H.F. Nour, A.M. Mostafa, M. Ellithey, Arch. Pharm. 340, 396 (2007)
M. Anzini, A. Cappelli, S. Vomero, G. Giorgi, T. Langer, M. Hamon, N. Merahi, B.M. Emerit, A. Cagnotto, M. Skorupska, T. Mennini, J.C. Pinto, J. Med. Chem. 38, 2692 (1995)
M.J. Coghlan, P.R. Kym, S.W. Elmore, A.X. Wang, J.R. Luly, D. Wilcox, M. Stashko, C.W. Lin, J. Miner, C. Tyree, M. Nakane, P. Jacobson, B.C. Lane, J. Med. Chem. 44, 2879 (2001)
L. Zhi, J.D. Ringgenberg, J.P. Edwards, C.M. Tegley, S.J. West, B. Pio, M. Motamedi, T.K. Jones, K.B. Marschke, D.E. Mais, W.T. Schrader, Bioorg. Med. Chem. Lett. 13(25), 2075 (2003)
F. Martins, S. Santos, C. Ventura, R. Elvas-Leitao, L. Santos, S. Vitorino, M. Reis, V. Miranda, H.F. Correia, J. Aires-de-Sousa, V. Kovalishyn, D.A.R.S. Latino, J. Ramos, M. Viveiros, Eur. J. Med. Chem. 81, 119 (2014)
L. Xia, Y.F. Xia, L.R. Huang, X. Xiao, H.Y. Lou, T.J. Liu, W.D. Pan, H. Luo, Eur. J. Med. Chem. 97, 83 (2015)
V. Judge, B. Narasimhan, M. Ahuja, D. Sriram, P. Yogeeswari, E. De Clercq, C. Pannecouque, J. Balzarini, Med. Chem. 9, 53 (2013)
M. Malhotra, S. Sharma, A. Deep, Med. Chem. Res. 21, 1237 (2012)
P. Dandawate, E. Khan, S. Padhye, H. Gaba, S. Sinha, J. Deshpande, K.V. Swamy, M. Khetmalas, A. Ahmad, F.H. Sarkar, Bioorg. Med. Chem. Lett. 22, 3104 (2012)
M.X. Wei, L. Feng, X.Q. Li, X.Z. Zhou, Z.H. Shao, Eur. J. Med. Chem. 44, 3340 (2009)
G. Nigade, P. Chavan, M. Deodhar, Med. Chem. Res. 21, 27 (2012)
M. Malhotra, V. Monga, S. Sharma, J. Jain, A. Samad, J. Stables, A. Deep, Med. Chem. Res. 21, 2145 (2012)
Y.Q. Hu, S. Zhang, F. Zhao, C. Gao, L.-S. Feng, Z.-S. Lv, Z. Xu, X. Wu, Eur. J. Med. Chem. 133, 255 (2017)
P.F.M. Oliveira, B. Guidetti, A. Chamayou, C. Andre-Barres, J. Madacki, J. Korduláková, G. Mori, B.S. Orena, L.R. Chiarelli, M.R. Pasca, C. Lherbet, C. Carayon, S. Massou, M. Baron, M. Baltas, Molecules 22, 1457 (2017)
C. Viegas-Junior, A. Danuello, V. da Silva Bolzani, E.J. Barreiro, C.A. Fraga, Curr. Med. Chem. 14(17), 1829 (2007)
J.R. Harrison, S. Brand, V. Smith, D.A. Robinson, S. Thompson, A. Smith, K. Davies, N. Mok, L.S. Torrie, I. Collie, I. Hallyburton, S. Norval, F.R.C. Simeons, L. Stojanovski, J.A. Frearson, R. Brenk, P.G. Wyatt, I.H. Gilbert, K.D. Read, J. Med. Chem. 61, 8374 (2018)
A. Yahya-Meymandi, H. Nikookar, S. Moghimi, M. Mahdavi, L. Firoozpour, A. Asadipour, P.R. Ranjbar, A. Foroumadi, J. Iran, Chem. Soc. 14, 771 (2017)
S. Kumari, J.M. Khurana, J. Chem. Sci. 129(8), 1225 (2017)
K.V. Sashidhara, G.R. Palnati, L.R. Singh, A. Upadhyay, S. Rao Avula, A. Kumara, R. Kant, Green Chem. 17, 3766 (2015)
K.C. Majumdar, S. Ponra, A. Taher, Synthesis 2011, 463 (2011)
S. Ramesh, R. Nagarajan, Tetrahedron Lett. 52, 4857 (2011)
S. Ramesh, V. Gaddam, R. Nagarajan, Synlett 2010, 757 (2010)
R. Bera, G. Dhananjaya, S.N. Singh, B. Ramu, S.U. Kiran, P.R. Kumar, K. Mukkanti, M. Pal, Tetrahedron 64, 582 (2008)
M.M. Tomashevskaya, O.A. Tomashenko, A.A. Tomashevskii, V.V. Sokolov, A.A. Potekhin, Russ. J. Org. Chem. 43, 77 (2007)
K. Aradi, P. Bombicz, Z. Novák, J. Org. Chem. 81, 920 (2016)
X. Yu, J. Wang, Z. Xu, Y. Yamamoto, M. Bao, Org. Lett. 18, 2491 (2016)
M.R. Bhosle, D.B. Wahul, G.M. Bondle, A. Sarkate, S.V. Tiwari, Synth. Commun. 48(16), 2046 (2018)
M.R. Bhosle, L.D. Khillare, J.R. Mali, A.P. Sarkate, D.K. Lokwani, S.V. Tiwaree, New J. Chem. 42, 18621 (2018)
C. Jadhav, L.D. Khillare, M.R. Bhosle, Synth. Commun. 48(3), 233 (2018)
M.R. Bhosle, P. Andil, D. Wahul, G.M. Bondle, A. Sarkate, S.V. Tiwari, J. Iran. Chem. Soc. 16, 1553 (2019)
M.R. Bhosle, D. Nipte, J. Gaikwad, M.A. Shaikh, G.M. Bondle, J.N. Sangshetti, Res. Chem. Intermed. 44, 7047 (2018)
M. Kour, M. Bhardwaj, H. Sharma, S. Paul, J.H. Clark, New J. Chem. 41, 5521 (2017)
S.-F. Gan, J.-P. Wan, Y.-J. Pan, C.-R. Sun, Synlett 6, 973 (2010)
C.J. Li, T.H. Chan, Organic Reactions in Aqueous Media (Wiley, New York, 1997)
P.A. Grieco, Organic Synthesis in Water (Blackie, London, 1998)
D. Kong, Q. Wang, Z. Zhu, X. Wang, Z. Shi, Q. Lin, M. Wuc, Tetrahedron Lett. 58, 2644 (2017)
F. Hapiot, A. Ponchel, S. Tilloy, E. Monflier, C. R. Chimie 14, 149 (2011)
J.-A. Shin, Y.-G. Lim, K.-H. Lee, J. Org. Chem. 77, 4117 (2012)
Acknowledgements
The authors are thankful to Professor Ramrao A. Mane for his invaluable discussions and guidance. Authors acknowledge to CDRI, Lucknow, for providing spectral facilities. The authors are also thankful to the Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad and Central Drug Research Institute (CDRI), Lucknow, for providing necessary facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bhosle, M.R., Joshi, S.A., Bondle, G.M. et al. Supramolecular biomimetic catalysis by β-cyclodextrin for the synthesis of new antimicrobial chromeno[4,3-b]quinolin-isonicotinamides in water. Res Chem Intermed 46, 737–753 (2020). https://doi.org/10.1007/s11164-019-03987-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-03987-x