Abstract
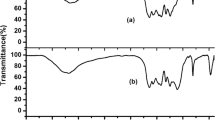
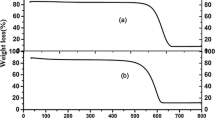
NiO@g-C3N4 as an efficient catalyst for the synthesis of spirooxindole derivatives was prepared by impregnation of g-C3N4 with NiO nanoparticles and characterized by various techniques including thermogravimetric analysis, transmission electron microscopy, X-ray diffraction and Fourier transform infrared spectroscopy. The one-pot synthesis of spirooxindole derivatives using 1 mmol isatin, 1 mmol dimedone (or 4-hydroxycoumarin or ethyl acetoacetate) and 1 mmol malononitrile was carried out in the presence of 50 mg NiO@g-C3N4 in EtOH media at reflux conditions. The results showed that both series of reactions had short reaction times (less than 7 min) and high reaction efficiency (greater than 87%). Some advantages can be cited for this method, including short reaction time, excellent yields, easy workup and a reusable and inexpensive nanocatalyst.








Similar content being viewed by others
References
I.O. Ghinea, R.M. Dinica, New synthetic pathways, new applications, in Scope of Selective Heterocycles from Organic and Pharmaceutical Perspective, ed. by R. Varala (InTech, Rijeka, 2017), p. 115
I.V. Borovlev, O.P. Demidov, G.A. Amangasieva, E.K. Avakyan, N.A. Kurnosova, J. Heterocycl. Chem. 406, 54 (2016)
N. Azizi, E. Gholibeghlo, Z. Manocheri, Sci. Iran. 19, 574 (2012)
S. Budde, J.K. Ega, B. Gavaji, R. Vadde, Int. J. Res. Appl. 2, 365 (2015)
G. Mohammadi Ziarani, R. Moradi, A. Badiei, N. Lashgari, B. Moradi, A. Abolhasani Soorki, J. Taibah Univ. Sci. 9, 555 (2015)
K. Rad-Moghadama, S. Gholizadeh, IJC 4, 41 (2014)
F.X. Felpin, O. Ibarguren, L. Nassar-Hardy, E. Fouquet, J. Org. Chem. 74, 1349 (2009)
J. Kothandapani, A. Ganesan, G.K. Mani, A.J. Kulandaisamy, J.B. Balaguru Rayappan, S.S. Ganesan, Tetrahedron Lett. 57, 3472 (2016)
A. Allahresani, Iran. J. Catal. 7, 293 (2017)
J.A. Makawana, C.B. Sangani, Y.F. Yao, Y.T. Duan, P.C. Lv, H.L. Zhu, Mini Rev. Med. Chem. 16, 1303 (2016)
M.A. Nasseri, F. Kamali, B. Zakerinasab, RSC Adv. 5, 26517 (2015)
J.A. Tanna, R.G. Chaudhary, N.V. Gandhare, A.R. Rai, H.D. Juneja, IJSER 6, 93 (2015)
R.J. Kalbasi, N. Mosaddegh, Bull. Korean Chem. Soc. 32, 2584 (2011)
L.L. Chng, N. Erathodiyil, J.Y. Ying, Acc. Chem. Res. 46, 1825 (2013)
H. Sachdeva, D. Dwivedi, R.R. Bhattacharjee, S. Khaturia, R. Saroj, J. Chem. 2013, 1 (2013)
X. Miao, X. Shen, J. Wu, Z. Ji, J. Wang, L. Kong, M. Liu, C. Song, Appl. Catal. A Gen. 539, 104 (2017)
H. Liu, Z. Xu, Z. Zhang, D. Ao, Appl. Catal. A Gen. 518, 150 (2016)
L. Ge, C. Han, J. Liu, Y. Li, Appl. Catal. A Gen. 409–410, 215 (2011)
Q. Hao, X. Niu, C. Nie, S. Hao, W. Zou, J. Gea, D. Chen, W. Yao, Phys. Chem. Chem. Phys. 18, 31410 (2016)
B. Lin, C. Xue, X. Yan, G. Yang, G. Yang, Appl. Surf. Sci. 357, 346 (2015)
L.C. Chen, X.T. Zeng, P. Si, Y.M. Chen, Y.W. Chi, D.H. Kim, G. Chen, Anal. Chem. 86, 4188 (2014)
A. Thomas, A. Fischer, F. Goettmann, M. Antonietti, J.-O. Müller, R. Schlogl, J.M. Carlsson, J. Mater. Chem. 18, 4893 (2008)
X. Wang, K. Maeda, A. Thomas, K. Takanabe, Nat. Mater. 8, 76 (2009)
B. Long, J. Lin, X. Wang, J. Mater. Chem. A 2, 2942 (2014)
A. Allahresani, B. Taheri, M.A. Nasseri, Res. Chem. Intermed. 44, 6979 (2018)
A. Allahresani, B. Taheri, M.A. Nasseri, Res. Chem. Intermed. 44, 1173 (2018)
A. Allahresani, M.A. Nasseri, A. Nakhaei, Res. Chem. Intermed. 43, 6367 (2017)
S. Riyaz, A. Indrasena, A. Naidu, P.K. Dubey, Indian J. Chem. B 53, 1442 (2014)
R.Y. Guo, Z.M. An, L.P. Mo, S.-T. Yang, H.-X. Liu, S.-X. Wang, Z.-H. Zhang, Tetrahedron 69, 9931 (2013)
C. Wu, R. Shen, J. Chen, C. Hu, Bull. Korean Chem. Soc. 34, 2431 (2013)
S.P. Satasia, P.N. Kalaria, J.R. Avalani, D.K. Raval, Tetrahedron 70, 5763 (2014)
P. Rai, M. Srivastava, J. Singh, J. Singh, New J. Chem. 38, 3181 (2014)
A. Hasaninejad, N. Golzar, M. Beyrati, A. Zare, M.M. Doroodmand, J. Mol. Catal. A Chem. 372, 137 (2013)
L.-M. Wang, N. Jiao, J. Qiu, J.-J. Yu, J.-Q. Liu, F.-L. Guo, Y. Liu, Tetrahedron 66, 339 (2010)
M.N. Elinson, R.F. Nasybullin, F.V. Ryzhkov, T.A. Zaimovskaya, G.I. Nikishi, Monatsh. Chem. 146, 631 (2015)
S.J. Chai, Y.F. Lai, J.C. Xu, H. Zheng, Q. Zhu, P.F. Zhang, Advanc. Synth. Catal. 353, 371 (2011)
L. Zhao, B. Zhou, Y. Li, Heteroatom Chem. 22, 673 (2011)
Acknowledgements
The authors are grateful to the University of Birjand and Payam Noor University of Mashhad for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amini Moqadam, Z., Allahresani, A. & Hassani, H. An efficiently and quickly synthesized NiO@g-C3N4 nanocomposite-catalyzed green synthesis of spirooxindole derivatives. Res Chem Intermed 46, 299–311 (2020). https://doi.org/10.1007/s11164-019-03951-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-03951-9