Abstract
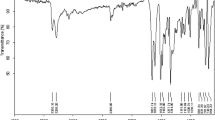
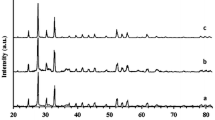
A new perlite supported Bismuth Chloride (BiCl3) was used as an efficient heterogeneous catalyst for the synthesis of heterocyclic compounds viz., quinoxalines and dihydropyrimidinones. Fourier-transform infrared spectroscopy (FT–IR), scanning electron microscopy, energy dispersive spectra, X-ray diffractometry, thermogravimetric analysis and Brunauer–Emmett–Teller surface area analytical techniques were employed to characterize the prepared catalyst. Initially, the catalytic activity of the prepared BiCl3-Perlite was tested towards synthesis of simple quinoxaline derivatives at room temperature. The effect of solvent in the preparation of quinoxaline was also examined. The formed products were confirmed by their physical (melting point) and spectral data (FT–IR, 1H and 13C-NMR). In order to implement the activity of the BiCl3-Perlite catalyst, a multicomponent reaction was adopted for synthesis of dihydropyrimidinones under solvent free conditions in a micro oven. The use of recyclable heterogeneous solid acid catalyst makes the reaction simple with minimum chemical waste, shorter reaction time, easy workup and products in good yield.







Similar content being viewed by others
References
A. Sniady, A. Durham, M.S. Morreale, K.A. Wheeler, R. Dembinski, Org. Lett. 9, 1175 (2007)
M. Terada, Synthesis (Stuttg). 12, 1929 (2010)
A. Saini, S. Kumar, J.S. Sandhu, Indian J. Chem. 45, 684 (2006)
M. Litvić, I. Večenaj, Z.M. Ladišić, M. Lovrić, V. Vinković, M. Filipan-Litvić, Tetrahedron 66, 3463 (2010)
M. Gohain, D. Prajapati, J.S. Sandhu, Synlett 2, 0235 (2004)
K. Singh, P.K. Deb, H. Singh, Tetrahedron 55, 12873 (1999)
R. Csuk, B.I. Glänzer, Chem. Rev. 91, 49 (1991)
C.O. Kappe, S.F. Falsone, Synlett 7, 718 (1998)
S.V. More, M.N.V. Sastry, C.C. Wang, C.F. Yao, Tetrahedron Lett. 46, 6345 (2005)
M. Divar, K. Zomorodian, S. Bastan, S. Yazdanpanah, S. Khabnadideh, J. Iran. Chem. Soc. 15, 1457 (2018)
L. Moradi, M. Tadayon, J. Saudi Chem. Soc. 22, 66 (2017)
Y.H. Vo, T.V. Le, H.D. Nguyen, T.A. To, H.Q. Ha, A.T. Nguyen, A.N.Q. Phan, N.T.S. Phan, J. Ind. Eng. Chem. 64, 107 (2018)
W. Xu, T. Ollevier, F. Kleitz, ACS Catal. 8, 1932 (2018)
J. Jiang, Y. Xu, C. Duanmu, X. Gu, J. Chen, Appl. Clay Sci. 95, 260 (2014)
P. Pushpaletha, M. Lalithambika, Appl. Clay Sci. 51, 424 (2011)
H. Aghahosseini, A. Ramazani, N.S. Jalayer, Z. Ranjdoost, A. Souldozi, K. Ślepokura, T. Lis, Org. Lett. 21, 22 (2019)
A. Ramazani, A. Reza Kazemizadeh, Curr. Org. Chem. 15, 3986 (2011)
A. Reza Kazemizadeh, A. Ramazani, Curr. Org. Chem. 16, 418 (2012)
A. Ramazani, F. Moradnia, H. Aghahosseini, I. Abdolmaleki, Curr. Org. Chem. 21, 1612 (2017)
H. Aghahosseini, A. Ramazani, F. Gouranlou, S. Woo Joo, Curr. Org. Synth. 14, 810 (2017)
S.W. Joo, K. Ślepokura, H. Ahankar, A. Ramazani, T. Lis, Green Chem. 18, 3582 (2016)
A. Ramazani, M. Rouhani, S.W. Joo, Ultrason. Sonochem. 28, 393 (2016)
I. Yavari, H. Djahaniani, M.T. Maghsoodlou, N. Hazeri, J. Chem. Res. (S) 6, 382 (1999)
I. Yavari, A. Ramazani, Synth. Commun. 27, 1385 (1997)
A. Ramazani, P. Asiabi, H. Aghahosseini, F. Gouranlou, Curr. Org. Chem. 21, 908 (2017)
S. Gupta, M. Lakshman, J. Med. Chem. Sci. 2, 51 (2019)
H. Aghahosseini, A. Ramazani, K. Ślepokura, T. Lis, J. Colloid Interface Sci. 511, 222 (2018)
M. Rouhani, A. Ramazani, S.W. Joo, Ultrason. Sonochem. 21, 262 (2014)
M. Rouhani, A. Ramazani, S.W. Joo, Ultrason. Sonochem. 22, 391 (2015)
A. Ramazani, M. Khoobi, A. Torkaman, F. Zeinali Nasrabadi, H. Forootanfar, M. Shakibaie, M. Jafari, A. Ameri, S. Emami, M. A. Faramarzi, A. Foroumadi, and A. Shafiee, Eur. J. Med. Chem. 78, 151 (2014)
R. Motamedi, F. Ebrahimi, G.R. Bardajee, Asian J. Green Chem. 3, 22 (2019)
Z. Arzehgar, S. Sajjadifar, M.H. Fekri, Chem. Methodol. J. 3, 251 (2019)
S. Sajjadifar, K. Pal, H. Jabbari, O. Pouralimardan, F. Divsar, S. Mohammadi-Aghdam, I. Amini, H. Hamidi, Chem. Methodol. 3, 226 (2019)
M. Azizmohammadi, M. Khoobi, A. Ramazani, S. Emami, A. Zarrin, O. Firuzi, R. Miri, A. Shafiee, Eur. J. Med. Chem. 59, 15 (2013)
S.T. Fardood, A. Ramazani, S. Moradi, J. Sol-Gel. Sci. Technol. 82, 432 (2017)
Z. Arzehgar, S. Sajjadifar, H. Arandiyan, Asian J. Green Chem. 3, 43 (2019)
A. Y.-Z. Yavari, Issa, Ramzani, Ali, Synth. Commun. An Int. J. Rapid Commun. Synth. Org. Chem. 26, 4495 (1996)
M. Arifuzzaman, H.S. Kim, Constr. Build. Mater. 141, 201 (2017)
T. Y. Erdem, T.K, Meral, C, Tokyay, M, Erdogan, Gazi Univ. J. Sci. 23, 305 (2010)
S.N. Hosseini, S.M. Borghei, M. Vossoughi, N. Taghavinia, Appl. Catal. B Environ. 74, 53 (2007)
O. Sengul, S. Azizi, F. Karaosmanoglu, M.A. Tasdemir, Energy Build. 43, 671 (2011)
S. Yilmazer, M.B. Ozdeniz, Build. Environ. 40, 311 (2005)
E. Mirhadi, A. Ramazani, M. Rouhani, S.W. Joo, Chemija 24, 320 (2013)
A. Ramazani, M. Rouhani, E. Mirhadi, M. Sheikhi, K. Ślepokura, T. Lis, Nanochem. Res. 1, 87 (2016)
G. Sabitha, E. Venkata Reddy, J. S. Yadav, K. V. S. Rama Krishna, and A. Ravi Sankar, Tetrahedron Lett. 43, 4029 (2002)
H. Li, H. Yao Zeng, and H. Wu Shao, Tetrahedron Lett. 50, 6858 (2009)
T. Matsumura, M. Nakada, Tetrahedron Lett. 55, 1829 (2014)
B.D. Jadhav, S.K. Pardeshi, Tetrahedron Lett. 55, 4948 (2014)
T. Ollevier, G. Lavie-Compin, Tetrahedron Lett. 43, 7891 (2002)
K. Ravi, B. Krishnakumar, M. Swaminathan, Res. Chem. Intermed. 41, 5353 (2015)
K. Ravi, B. Krishnakumar, M. Swaminathan, I.S.R.N. Org, Chem. 2012, 1 (2012)
R. Peraman, R. Kuppusamy, S.K. Killi, Y.P. Reddy, Int. J. Med. Chem. 2016, 1 (2016)
R. Teja, S. Kapu, S. Kadiyala, V. Dhanapal, A.N. Raman, J. SAUDI Chem. Soc. 20, S387 (2013)
M. Hajri, M. Esteve, O. Khoumeri, R. Abderrahim, T. Terme, M. Montana, P. Vanelle, Eur. J. Med. Chem. 124, 959 (2016)
L. Achutha, R. Parameshwar, B. M. Reddy, and V. H. Babu, 2013, 3 (2013)
M. Quiliano, A. Pabón, G. Ramirez-calderon, C. Barea, E. Deharo, S. Galiano, I. Aldana, Bioorg. Med. Chem. Lett. 27, 1820 (2017)
L. M. Ramos, B. C. Guido, C. C. Nobrega, J. R. Corrþa, R. G. Silva, H. C. B. De Oliveira, A. F. Gomes, F. C. Gozzo, and B. A. D. Neto, Chem.—A Eur. J. 19, 4156 (2013)
S. A. T. Elham Rezaee, M. Hedayati, L. H. Rad, Soraya Shahhosseini, M. Faizi, Medchemcomm 7, 2128 (2016)
J. Kim, T. Ok, C. Park, W. So, M. Jo, Y. Kim, M. Seo, D. Lee, S. Jo, Y. Ko, I. Choi, Y. Park, J. Yoon, M. Kyeong, J. Ahn, J. Kim, S. Han, T. Kim, J. Cechetto, J. Nam, M. Liuzzi, P. Sommer, Z. No, Bioorg. Med. Chem. Lett. 22, 2522 (2012)
R. Chikhale, S. Thorat, A. Pant, A. Jadhav, K. Chary, R. Bansode, G. Bhargavi, N. Karodia, M.V. Rajasekharan, A. Paradkar, P. Khedekar, Bioorg. Med. Chem. 23, 6687 (2015)
S.D. Guggilapu, S.K. Prajapti, A. Nagarsenkar, G. Lalita, G. Modi, N. Vegi, B.N. Babu, N. J. Chem. 40, 838 (2015)
S.M. Abo-Naf, R.L. Elwan, G.M. Elkomy, J. Non. Cryst. Solids 358, 964 (2012)
E. Kolvari, N. Koukabi, M.M. Hosseini, J. Mol. Catal. A: Chem. 397, 68 (2015)
B. Krishnakumar, R. Velmurugan, S. Jothivel, M. Swaminathan, Catal. Commun. 11, 997 (2010)
B. Krishnakumar, M. Swaminathan, J. Mol. Catal. A: Chem. 350, 16 (2011)
Acknowledgements
The authors are thankful to the management of the GRG Trust for providing financial support and necessary facilities. One of the authors, Balu Krishnakumar, is also thankful to FCT, for a post-doc grant (SFRH/BPD/86971/2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Brindha, K., Amutha, P., Krishnakumar, B. et al. BiCl3-modified perlite as an effective catalyst for selective organic transformations: a green protocol. Res Chem Intermed 45, 4367–4381 (2019). https://doi.org/10.1007/s11164-019-03836-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-03836-x