Abstract
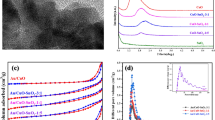
MoFe-N, MoFe/c–CeO2, MoFe/p1–CeO2, and MoFe/p2–CeO2 (where N, c, and p stand for non-supported, nanocube, and nanoparticle) oxide catalysts were designed for gas-glycerol direct catalytic conversion into allyl alcohol. The catalysts also were characterized by XRD, TEM, BET, H2-TPR, and NH3-TPD. Mo–Fe oxides were highly dispersed on the surface of c-CeO2 and p-CeO2 supports, different with the MoFe-N consist of crystalline Fe2(MoO4)3 and Fe2O3 crystalline phase. The support effect and special natural property of CeO2 significantly improve the allyl alcohol selectivity from gas-glycerol over MoFe/CeO2. The p-CeO2 with low particle sizes and crystalline degree was superior to high-crystalline nanocube c-CeO2 to promote its interaction with the MoFe oxide active components, and improve the surface acid site concentration and reducibility of MoFe/CeO2 as well as catalytic activity and stability for allyl alcohol synthesis from gas-glycerol without any extra hydrogen donors. Over the MoFe/p2–CeO2, the glycerol conversion reached 97.1%, and the selectivity of allyl alcohol, enthanal, propanoic acid, and acrylic acid were 23.3%, 8.6%, 12.6%, and 7.8%, respectively, yielding allyl alcohol of 22.6%.






Similar content being viewed by others
Change history
04 January 2019
In the original publication of the article, the chemical compounds “enthanal” and “propanal” were incorrectly published as “entanol” and “propanol”.
References
D.E. Bloom, Science 333, 562 (2011)
C.T. Wu, K.M.K. Yu, F.L. Liao, N. Young, P. Nellist, A. Dent, A. Kroner, S.C.E. Tsang, Nat. Commun. 3, 1050 (2012)
B. Obama, Science 355, 126 (2017)
C.H. Zhou, J.N. Beltramini, Y.X. Fan, G.Q. Lu, Chem. Soc. Rev. 37, 527 (2008)
F.X. Yang, M.A. Hanna, R.C. Sun, Biotechnol. Biofuels 5, 13 (2012)
P. Cintas, S. Tagliapietra, E. Calcio Gaudino, G. Palmisano, G. Cravotto, Green Chem. 16 1056 (2014)
R.A. Sheldon, Green Chem. 16, 950 (2014)
Y. Nakagawa, M. Tamura, K. Tomishige, Res. Chem. Intermed. 44, 3879 (2018)
H.T. Nguyen, G.S. Kamali Kannangara, Chem. Soc. Rev. 42, 9454 (2013)
D.L. Sun, Y. Yamada, S. Sato, W. Ueda, Appl. Catal. B: Environ. 193, 75 (2016)
NPCS, B.o. Consultants, Engineers, Industrial Alcohol Technology Handbook, Asia Pacific Business Press Inc. (2010)
K. Weissermel, H.J. Arpe, Industrial organic chemistry, 4th edition, 312 (1994)
J.G. Speight, Chemical and Process Design Handbook, McGraw Hill, (2002)
E. Arceo, P. Marsden, R.G. Bergman, J.A. Ellman, Chem. Commun. 203, 3357 (2009)
S. Tazawa, N. Ota, M. Tamura, Y. Nakagawa, K. Okumura, K. Tomishige, ACS Catal. 6, 6393 (2016)
G.M. Lari, Z.P. Chen, C. Mondelli, J. Pérez-Ramírez, ChemCatChem. 9, 2195 (2017)
Y. Liu, H. Tüysüz, C.J. Jia, M. Schwickardi, R. Rinaldi, A.H. Lu, W. Schmidt, F. Schüth, Chem. Commun. 46, 1238 (2010)
T. Yoshikawa, T. Tago, A. Nakamura, A. Konaka, M. Mukaida, T. Masuda, Res. Chem. Intermed. 37, 1247 (2011)
L. Harvey, G. Sánchez, E.M. Kennedy, M. Stockenhuber, Asia-Pac. J. Chem. Eng. 10, 598 (2015)
G. Sánchez, B.Z. Dlugogorski, E.M. Kennedy, M. Stockenhuber, Appl. Catal. A: Gen. 509, 130 (2016)
A. Konak, T. Tag, T. Yoshikaw, A. Nakamur, T. Masud, Appl. Catal. B: Environ. 146, 267 (2014)
G. Sánchez, J. Friggieri, C. Keast, M. Drewery, B.Z. Dlugogorski, E. Kennedy, M. Stockenhuber, Appl. Catal. B: Environ. 152–153, 117 (2014)
H. Lan, X. Xiao, S.L. Yuan, B. Zhang, G.L. Zhou, Y. Jiang, Acta Phys. Chim. Sin. 33, 2301 (2017)
H. Lan, X. Xiao, S.L. Yuan, B. Zhang, G.L. Zhou, Y. Jiang, Catal. Lett. 147, 2187 (2017)
M.H. Haider, N.F. Dummer, D.W. Knight, R.L. Jenkins, M. Howard, J. Moulijn, S.H. Taylor, G.J. Hutchings, Nature Chem. 7, 1028 (2015)
H.X. Mai, L.D. Sun, Y.W. Zhang, R. Si, W. Feng, H.P. Zhang, H.C. Liu, C.H. Yan, Nanorods, and Nanocubes. J. Phys. Chem. B 109, 24380 (2005)
Y.M. Liu, L.F. Luo, Y.X. Gao, W.X. Huang, Appl. Catal. B Environ. 197, 214 (2016)
S.J. Chang, M. Li, Q. Hua, L.J. Zhang, Y.S. Ma, B.J. Ye, W.X. Huang, J. Catal. 293, 195 (2012)
Y.J. Lee, G.H. He, A.J. Akey, R. Si, I.P. Herman, J. Am. Chem. Soc. 133, 12952 (2011)
G.L. Zhou, B.G. Gui, H.M. Xie, F. Yang, Y. Chen, S.M. Chen, X.X. Zheng, J. Ind. Eng. Chem. 20, 160 (2014)
G.L. Zhou, H. Lan, T.T. Gao, H.M. Xie, Chem. Eng. J. 246, 53 (2014)
H. Lan, G.L. Zhou, C.J. Luo, Y.R. Yu, G.Z. Zhang, Int. J. Chem. React. Eng. 14, 757 (2016)
R.M.M. Abbaslou, A. Tavassoli, J. Soltan, A.K. Dalai, Appl. Catal. A: Gen. 367, 47 (2009)
Z.B. Lei, S.Y. Bai, L.Q. Dang, H.A. Xia, S.B. Liu, Micropor. Mesopor. Mater. 123, 306 (2009)
Y.S. Li, Y. Chen, L. Li, J.L. Gu, W.R. Zhao, L. Li, J.L. Shi, Appl. Catal. A: Gen 366, 57 (2009)
S.D. Qina, C.H. Zhang, J. Xu, B.S. Wu, H.W. Xiang, Y.W. Li, J. Mol. Catal. A: Chem. 304, 128 (2009)
Author information
Authors and Affiliations
Corresponding author
Additional information
The original version of this article is revised: The chemical compounds “entanol” and “propanol” were updated as “enthanal” and “propanal”.
Rights and permissions
About this article
Cite this article
Lan, H., Zeng, J., Zhang, B. et al. CeO2 promoting allyl alcohol synthesis from glycerol direct conversion over MoFe/CeO2 oxide catalysts: morphology and particle sizes dependent. Res Chem Intermed 45, 1565–1580 (2019). https://doi.org/10.1007/s11164-018-3694-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-018-3694-4