Abstract
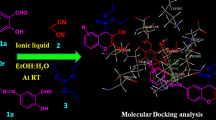
We have explored a number of protic ionic liquids (PILs) as a catalyst for the synthesis of biscoumarins by condensation of 4-hydroxycoumarin with an aromatic aldehyde. Methylimidazolium- and triethylammonium-based PILs were synthesized by simple neutralization reaction with protic acids. Triethylammonium hydrogen sulfate [Et3NH][HSO4] was found to be the best among the studied PILs concerning the yield of products and reaction time period. Different biscoumarin derivatives were synthesized based on 4-hydroxycoumarin and various substituted aromatic aldehydes at optimum reaction conditions. Obtained products were separated just by simple filtration. The facile method does not require additional purification for formed products. The catalyst has shown better yields along with outstanding recyclability, providing an environmental benign protocol for the synthesis of biscoumarin derivatives.
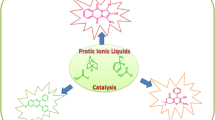
Graphical Abstract
Screening of simple protic ionic liquids as a catalyst in the synthesis of biscoumarins, out of which [Et3NH][HSO4] was found to be best among the studied PILs.





Similar content being viewed by others
References
N.V. Plechkova, K.R. Seddon, Chem. Soc. Rev. 37, 123 (2008)
T. Welton, Chem. Rev. 99, 2071 (1999)
S. Zhu, R. Chen, Y. Wu, Q. Chen, X. Zhang, Z. Yu, Chem. Biochem. Eng. Q. 23, 207 (2009)
R. Sheldon, Chem. Commun. 2399 (2001)
M.A.P. Martins, C.P. Frizzo, D.N. Moreira, N. Zanatta, H.G. Bonacorso, Chem. Rev. 108, 2015 (2008)
A.R. Hajipour, F. Rafiee, Org. Prep. Proced. Int. 42, 285 (2010)
T.L. Greaves, C.J. Drummond, Chem. Rev. 108, 206 (2008)
P.P. Salvi, A.M. Mandhare, A.S. Sartape, D.K. Pawar, S.H. Han, S.S. Kolekar, C. R. Chim. 14, 883 (2011)
M. Picquet, I. Tkatchenko, I. Tommasi, P. Wasserscheid, J. Zimmermann, Adv. Synth. Catal. 345, 959 (2003)
K.E. Johnson, R.M. Pagni, J. Bartmess, Monatsh. Chem. 138, 1077 (2007)
B. Tamami, A. Sardarian, E. Ataollahi, Turk. J. Chem. 40, 422 (2016)
S.A. Siddiqui, T.M. Potewar, R.J. Lahoti, K.V. Srinivasan, Synthesis, 2849 (2006)
S.S. Palimkar, S.A. Siddiqui, T. Daniel, R.J. Lahoti, K.V. Srinivasan, J. Org. Chem. 68, 9371 (2003)
Z. Du, Z. Li, S. Guo, J. Zhang, L. Zhu, Y. Deng, J. Phys. Chem. B 109, 19542 (2005)
Z. Fei, D. Zhao, T.J. Geldbach, R. Scopelliti, P.J. Dyson, Chem. Eur. J. 10, 4886 (2004)
Z. Duan, Y. Gu, J. Zhang, L. Zhu, Y. Deng, J. Mol. Catal. A: Chem. 250, 163 (2006)
J. Gui, H. Ban, X. Cong, X. Zhang, Z. Hu, Z. Sun, J. Mol. Catal. A: Chem. 225, 27 (2005)
C. Wang, W. Zhao, H. Li, L. Guo, Green Chem. 11, 843 (2009)
A.G. Ying, H.D. Liang, R.H. Zheng, C.H. Ge, H.J. Jiang, C.L. Wu, Res. Chem. Intermed. 37, 579 (2011)
R.V. Hangarge, D.V. Jarikote, M.S. Shingare, Green Chem. 4, 266 (2002)
S. Rezayati, F. Sheikholeslami-Farahani, F. Rostami-Charati, S.A.S. Abad, Res. Chem. Intermed. 42, 4097 (2016)
G. Karthikeyan, P.T. Perumal, Can. J. Chem. 83, 1746 (2005)
Z.Y. Yu, Q.S. Fang, J. Zhou, Z.B. Song, Res. Chem. Intermed. 42, 2035 (2016)
E. Janus, I. Goc-Maciejewska, M. Łożyński, J. Pernak, Tetrahedron Lett. 47, 4079 (2006)
D. Patil, D. Chandam, A. Mulik, P. Patil, S. Jagadale, R. Kant, V. Gupta, M. Deshmukh, Catal. Lett. 144, 949 (2014)
G. Zhao, T. Jiang, H. Gao, B. Han, J. Huang, D. Sun, Green Chem. 6, 75 (2004)
H.-P. Zhu, F. Yang, J. Tang, M.-Y. He, Green Chem. 5, 38 (2003)
R. Zheng, X. Wang, H. Xu, J. Du, Synth. Commun. 36, 1503 (2006)
S. Guo, Z. Du, S. Zhang, D. Li, Z. Li, Y. Deng, Green Chem. 8, 296 (2006)
J. Li, Y.-P. Sui, J.-J. Xin, X.-L. Du, J.-T. Li, H.-R. Huo, H. Ma, W.-H. Wang, H.-Y. Zhou, H.-D. Zhan, Z.-J. Wang, C. Li, F. Sui, X. Li, Bioorg. Med. Chem. Lett. 25, 5520 (2015)
J. Li, Z. Hou, F. Li, Z.-D. Zhang, Y. Zhou, X.-X. Luo, M.-K. Li, J. Mol. Struct. 1075, 509 (2014)
Y.-P. Sui, H.-R. Huo, J.-J. Xin, J. Li, X.-J. Li, X.-L. Du, H. Ma, H.-Y. Zhou, H.-D. Zhan, Z.-J. Wang, C. Li, F. Sui, M.-K. Li, Molecules 20, 17614 (2015)
J.-J. Xin, J. Li, Z.-D. Zhang, X.-B. Hu, M.-K. Li, J. Mol. Struct. 1084, 200 (2015)
K.M. Khan, S. Iqbal, M.A. Lodhi, G.M. Maharvi, Z. Ullah, M.I. Choudhary, A. Rahman, S. Perveen, Bioorg. Med. Chem. 12, 1963 (2004)
K.M. Khan, F. Rahim, A. Wadood, N. Kosar, M. Taha, S. Lalani, A. Khan, M.I. Fakhri, M. Junaid, W. Rehman, M. Khan, S. Perveen, M. Sajid, M.I. Choudhary, Eur. J. Med. Chem. 81, 245 (2014)
Z.N. Siddiqui, M.T.N. Musthafa, A. Ahmad, A.U. Khan, Arch. Pharm. 344, 394 (2011)
I. Kostova, G. Momekov, Eur. J. Med. Chem. 41, 717 (2006)
A. Das Gupta, S. Samanta, R. Mondal, A.K. Mallik, Bull. Korean Chem. Soc. 33, 4239 (2012)
M. Kidwi, V. Bansal, P. Mothsra, S. Saxena, R.K. Somvanshi, S. Dey, T.P. Singh, J. Mol. Catal. A: Chem. 268, 76 (2007)
J.N. Sangshetti, N.D. Kokare, D.B. Shinde, Green Chem. Lett. Rev. 2, 233 (2009)
K. Tabatabaeian, H. Heidari, A. Khorshidi, M. Mamaghani, N.O. Mahmoodi, J. Serb. Chem. Soc. 77, 407 (2012)
F. Shirini, M. Abedini, S.A. Kiaroudi, Phosphorus, Sulfur Silicon Relat. Elem. 189, 1279 (2014)
B. Karmakar, A. Nayak, J. Banerji, Tetrahedron Lett. 53, 4343 (2012)
A.R. Kiasat, L. Hemat-Alian, Res. Chem. Intermed. 41, 873 (2015)
J. Albadi, A. Mansournezhad, S. Salehnasab, Res. Chem. Intermed. 41, 5713 (2015)
M. Nikpassand, L.Z. Fekri, L. Karimian, M. Rassa, Curr. Org. Synth. 12, 358 (2015)
R. Rezaei, M.R. Sheikhi, Res. Chem. Intermed. 41, 1283 (2015)
J.M. Khurana, A. Lumb, A. Chaudhary, B. Nand, J. Heterocycl. Chem. 51, 1747 (2014)
J.M. Khurana, S. Kumar, Monatsh. Chem. 141, 561 (2010)
K.P. Boroujeni, P. Ghasemi, Z. Rafienia, Monatsh. Chem. 145, 1023 (2014)
W. Li, Y. Wang, Z. Wang, L. Dai, Y. Wang, Catal. Lett. 141, 1651 (2011)
A. Tzani, A. Douka, A. Papadopoulos, E.A. Pavlatou, E. Voutsas, A. Detsi, ACS Sustain. Chem. Eng. 1, 1180 (2013)
M.A. Zolfigol, A.R. Moosavi-Zare, M. Zarei, C. R. Chim. 17, 1264 (2014)
N. Tavakoli-Hoseini, M.M. Heravi, F.F. Bamoharram, A. Davoodnia, M. Ghassemzadeh, J. Mol. Liq. 163, 122 (2011)
Z.N. Siddiqui, K. Khan, ACS Sustain. Chem. Eng. 2, 1187 (2014)
R. Rezaei, F. Moezzi, M.M. Doroodmand, Chin. Chem. Lett. 25, 183 (2014)
Acknowledgements
Authors SKP and MMV under UGC-BSR Meritorious Students fellowship and SCB under RGNF are grateful to the University Grants Commission (UGC), India, for financial support and DST-FIST, New Delhi, India, for instrument facilities at the Department of Chemistry, Shivaji University, Kolhapur.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patil, S.K., Awale, D.V., Vadiyar, M.M. et al. Simple protic ionic liquid [Et3NH][HSO4] as a proficient catalyst for facile synthesis of biscoumarins. Res Chem Intermed 43, 5365–5376 (2017). https://doi.org/10.1007/s11164-017-2932-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-2932-5