Abstract
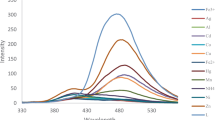
A novel sensor based on acetylferrocene-containing Schiff base (ASB) was synthesized by reaction of α-chloroacetylferrocene and N-(salicylidene)-l-valinmethylester. The structure of the compound was characterized by using elemental analysis and Fourier-transform infrared (FT-IR), 1H nuclear magnetic resonance (NMR), and 13C NMR spectroscopy. Its metal-cation-sensing properties were investigated spectrofluorometrically. ASB served as selective chemosensor for Zn2+ and Cd2+ towards alkali, alkaline-earth, and various heavy-metal ions. It showed significant fluorescence enhancement for Zn2+ and Cd2+ ions, stemming from C=N isomerization and chelation-enhanced fluorescence. The binding modes of the complexes were determined to have 1:1 complexation stoichiometry, and the binding constants were calculated as (6.93 ± 0.25) × 106 M−1 for ASB·Zn 2+ and (7.49 ± 0.18) × 105 M−1 for ASB·Cd 2+ using the nonlinear curve-fitting method.










Similar content being viewed by others
Notes
The CHEF is defined as I max/I 0, where I max corresponds to the maximum emission intensity of the receptor–metal complex, while I 0 is the maximum emission intensity of the free receptor. For recent and relevant examples of heavy- and transition-metal cation (HTM) chemosensors based on chelation-enhanced fluorescence (CHEF).
Guidance for industry Q2B validation of analytical procedures: methodology, Nov 1996.
References
M. Kaur, P. Kaur, V. Dhuna, S. Singh, K. Singh, Dalton Trans. 43, 5707–5712 (2014)
S. Maiti, Z. Aydin, Y. Zhang, M. Guo, Dalton Trans. 44, 8942–8949 (2015)
B. Sen, M. Mukherjee, S. Banerjee, S. Pal, P. Chattopadhyay, Dalton Trans. 44, 8708–8717 (2015)
S. Anbu, R. Ravishankaran, M.F.C.G. Da Silva, A.A. Karande, A.J.L. Pombeiro, Inorg. Chem. 53, 6655–6664 (2014)
V.K. Gupta, N. Mergu, L.K. Kumawat, A.K. Singh, Sens. Actuators B 207, 216–223 (2015)
J. Wang, W. Lin, W. Li, Chem. Eur. J. 18, 13629–13632 (2012)
S.K. Lee, M.G. Choi, J. Choi, S.K. Chang, Sens. Actuators B 207, 303–307 (2015)
Q. Zhao, R. Li, S. Xing, X. Liu, T. Hu, X. Bu, Inorg. Chem. 50, 10041–10046 (2011)
Z. Liu, C. Zhang, W. He, Z. Yang, X. Gao, Z. Guo, Chem. Commun. 46, 6138–6140 (2010)
V.K. Gupta, M.R. Ganjali, P. Norouzi, H. Khani, A. Nayak, S. Agarwal, Crit. Rev. Anal. Chem. 41, 282–313 (2011)
F. Xiao, J. Shen, J. Qu, S. Jing, D.R. Zhu, Inorg. Chem. Commun. 35, 69–71 (2013)
J.T. Hou, B.Y. Liu, K. Li, K.K. Yu, M.B. Wu, X.Q. Yu, Talanta 116, 434–440 (2013)
Y. Ma, F. Wang, S. Kambam, X. Chen, Sens. Actuators B 188, 1116–1122 (2013)
K. Dutta, R.C. Deka, D.K. Das, Spectrochim. Acta A 124, 124–129 (2014)
R. Borthakur, U. Thapa, M. Asthana, S. Mitra, K. Ismail, R.A. Lal, J. Photochem. Photobiol. A Chem. 301, 6–13 (2015)
B. Kashyap, K. Dutta, D.K. Das, P. Phukan, J. Fluoresc. 24, 975–981 (2014)
H. Ye, F. Ge, Y.M. Zhou, J.T. Liu, B.X. Zhao, Spectrochim. Acta A 112, 132–138 (2013)
J.H. Hu, J.B. Li, J. Qi, Y. Sun, Sens. Actuators B 208, 581–587 (2015)
R. Pandey, R.K. Gupta, M. Shahid, B. Maiti, A. Misra, D.S. Pandey, Inorg. Chem. 51, 298–311 (2011)
V.K. Gupta, A.K. Singh, L.K. Kumawat, Sens. Actuators B 195, 98–108 (2014)
L. Wang, H. Li, D. Cao, Sens. Actuators B 181, 749–755 (2013)
Y.S. Mi, Z. Cao, Y.T. Chen, Q.F. Xie, Y.Y. Xu, Y.F. Luo, J.J. Shic, J.N. Xiang, Analyst 138, 5274–5280 (2013)
C.K. Kumar, R. Trivedi, L. Giribabu, S. Niveditha, K. Bhanuprakash, B. Sridhar, J. Organomet. Chem. 780, 20–29 (2015)
P. Chinapang, V. Ruangpornvisuti, M. Sukwattanasinitt, P. Rashatasakhon, Dyes Pigments 112, 236–238 (2015)
J. Shen, T. Liu, Y. Li, W. Ji, S. Jing, D.R. Zhu, G.F. Guan, Inorg. Chem. Commun. 44, 6–9 (2014)
S.J. Ponniah, S.K. Barik, A. Thakur, R. Ganesamoorthi, S. Ghosh, Organometallics 33, 3096–3107 (2014)
V. Uahengo, B. Xiong, P. Zhao, Y. Zhang, P. Cai, K. Hu, G. Cheng, Sens. Actuators B 190, 937–945 (2014)
L. Zhu, D. Zhang, D. Qu, Q. Wang, X. Ma, H. Yian, Chem. Commun. 46, 2587–2589 (2010)
G. Warncke, U. Böhme, B. Günther, M. Kronstein, Polyhedron 47, 46–52 (2012)
O. Dogan, V. Senol, S. Zeytinci, H. Koyuncu, A. Bulut, J. Organomet. Chem. 690, 430–434 (2005)
M.L. Sundararajan, T. Jeyakumar, J. Anandakumaran, B.K. Selvan, Spectrochim. Acta A 131, 82–93 (2014)
J. Müller, G. Kehr, R. Fröhlich, G. Erker, Eur. J. Inorg. Chem. 2005, 2836–2841 (2005)
D. Tomczyk, L. Nowak, W. Bukowski, K. Bester, P. Urbaniak, G. Andrijewski, B. Olejniczak, Electrochim. Acta 121, 64–77 (2014)
J. Wu, W. Liu, J. Ge, H. Zhang, P. Wang, Chem. Soc. Rev. 40, 3483–3495 (2011)
Y.J. Lee, C. Lim, H. Suh, E.J. Song, C. Kim, Sens. Actuators B 201, 535–544 (2014)
L. Wang, W. Qin, X. Tang, W. Dou, W. Liu. J. Phys. Chem. A 115, 1609–1616 (2011)
X. Liu, N. Zhang, J. Zhou, T. Chang, C. Fangab, D. Shangguan, Analyst 138, 901–906 (2013)
H. Bingol, E. Kocabas, E. Zor, A. Coskun, Talanta 82, 1538–1542 (2010)
Acknowledgments
This work was supported by Scientific Research Projects (BAP 13201019) of Selcuk University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Findik, M., Ucar, A., Bingol, H. et al. Fluorogenic ferrocenyl Schiff base for Zn2+ and Cd2+ detection. Res Chem Intermed 43, 401–412 (2017). https://doi.org/10.1007/s11164-016-2630-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2630-8