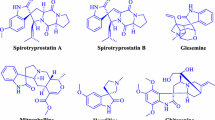
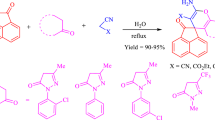
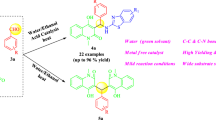
Abstract
A water mediated and thiamine hydrochloride catalysed eco-compatible efficient method was developed for the synthesis of spiro[acenaphthylene-1,2′[1,3]-thiazolidine]-2,4′(1H)-diones via multi-component reaction of acenaphthylene-1,2-dione, substituted anilines, and α-mercaptocarboxylic acid at 80 °C temperature. This transformation involves the formation of two C–N bonds and one C–S bond leading to the creation of a five-member ring in a one-pot operation. Easy availability and recovery of the catalyst, no toxic/organic solvents, and high yield of product make the protocol attractive, sustainable, and economic.
Graphical Abstract





Similar content being viewed by others
References
D.J. Newman, G.M. Cragg, J. Nat. Prod. 70, 461 (2007)
K. Kumar, H. Waldmann, Angew. Chem. Int. Ed. 48, 3224 (2009)
A. Domling, I. Ugi, Angew. Chem. Int. Ed. 39, 3168 (2000)
A. Diguez-Vzquez, C.C. Tzschucke, W.Y. Lam, S.V. Le, Angew. Chem. Int. Ed. 47, 209 (2008)
W. Reifschneider, B. Bisabari-Ershadi, J.E. Dripps, J.B. Bonron, US Patent 5,075,293, 1991, in Chem. Abstr., vol 116 (1991), p. 129249f
G.C. Rovnyak, V.L. Narayanan, R.D. Haugwitz, US Patent 4,053,613, 1975, in Chem. Abstr., vol 88 (1978), p. 22892
A. Verma, S. Saraf, Eur. J. Med. Chem. 43, 897 (2008)
R. Ottana, R. Maccari, M.L. Barreca, G. Bruno, A. Rotondo, A. Rossi, G. Chiricosta, R. Di Paola, L. Sautebin, S. Cuzzocrea, M.G. Vigorita, Bioorg. Med. Chem. 13, 4243 (2005)
P. Vicini, A. Geronikaki, K. Anastasia, M. Incerti, F. Zani, Bioorg. Med. Chem. 14, 3859 (2006)
V. Gududuru, E. Hurh, J.T. Dalton, D.D. Miller, Bioorg. Med. Chem. Lett. 14, 5289 (2004)
R. Ottana, S. Carotti, R. Maccari, I. Landini, G. Chiricosta, B. Caciagli, M.G. Vigorita, E. Mini, Bioorg. Med. Chem. Lett. 15, 3930 (2005)
A.A. Elbarbary, A.I. Khodair, E.B. Pedersen, C. Nielsen, Monatsh. Chem. 125, 593 (1994)
E. Rydzik, A. Szadowska, A. Kaminska, Acta Pol. Pharm. 41, 459 (1984)
H.D. Troutman, L.M. Long, J. Am. Chem. Soc. 70, 3436 (1948)
G.C. Look, J.R. Schullek, C.P. Homes, J.P. Chinn, E.M. Gordon, M.A. Gallop, Bioorg. Med. Chem. Lett. 6, 707 (1996)
M.L. Barreca, J. Balzsarini, A. Chimirri, E. De Clercq, L. De Luca, H.D. Höltje, M. Höltje, A.M. Monforte, P. Monforte, C. Pannecouque, A. Rao, M. Zapalla, J. Med. Chem. 45, 5410 (2002)
R.K. Rawal, R. Tripathi, S.B. Katti, C. Pannecouque, E. De Clercq, Bioorg. Med. Chem. 15, 3134 (2007)
M.L. Barreca, A. Chimirri, L.D. Luca, A. Monforte, P. Monforte, A. Rao, M. Zappala, J. Balzarini, E. De Clercq, C. Pannecouque, M. Witvrouw, Bioorg. Med. Chem. Lett. 11, 1793 (2001)
M.V. Diurno, O. Mazzoni, P.E. Calignano, F. Giordano, A.J. Bolognese, J. Med. Chem. 35, 2910 (1992)
S.R. Sharma, N. Sharma, Ann. Indian Acad Neurol. 11, 231 (2008)
H.L. Teng, H. Huang, C.J. Wang, Chem. Eur. J. 18, 12614 (2012)
B.V. Subba Reddy, G. Karthik, T. Rajasekaran, A. Antony, B. Sridhar, Tetrahedron Lett. 53, 2396 (2012)
Y. Han, Q. Wu, J. Sun, C.G. Yan, Tetrahedron 68, 8539 (2012)
A.C. Wei, M.A. Ali, Y.K. Yoon, R. Ismail, T.S. Choon, R.S. Kumar, Bioorg. Med. Chem. Lett. 23, 1383 (2013)
R.R. Kumar, S. Perumal, S.C. Manju, P. Bhatt, P. Yogeeswari, D. Sriram, Bioorg. Med. Chem. Lett. 19, 3461 (2009)
J. Zheng, Y. Li, Mendeleev Commun. 22, 148 (2012)
C.P. Homes, J.P. Chinn, C.G. Look, E.M. Gordon, M.A. Gallop, J. Org. Chem. 60, 7328 (1995)
S.K. Srivastava, S.L. Srivastava, S.D. Srivastava, J. Indian Chem. Soc. 77, 104 (2000)
R.C. Sharma, D. Kumar, J. Indian Chem. Soc. 77, 492 (2000)
T. Srivastava, W. Haq, S.B. Katti, Tetrahedron 58, 7619 (2002)
V. Gududuru, V. Nguyen, J. Dalton, D.D. Miller, Synlett 13, 2357 (2004)
R.P. Umesh, V.J. Dhanaji, R.B. Manisha, A.M. Ramrao, Tetrahedron Lett. 52, 1689 (2012)
F. Naser, E. Sattar, Chin. Chem. Lett. 24, 389 (2013)
T.H. Istvan, T.A. Paul, Chem. Rev. 107, 2167 (2007)
V.K. Ahluvalia, R.S. Varma, Green Solvents for Organic Synthesis (Alpha Sciences International, Abingdon, 2009)
C.J. Li, L. Chen, Chem. Soc. Rev. 35, 68 (2006)
S. Chitra, N. Paul, S. Muthusbramanian, P. Manisankar, Green Chem. 13, 2777 (2011)
P. Klumphu, B.H. Lipshutz, J. Org. Chem. 79, 888 (2014)
J.H. Clark, Nat. Chem. 1, 123 (2009)
K. Eskandari, B. Karami, S. Khodabakhshi, J. Chem. Res. 38, 600 (2014)
C. Noonan, L. Baragwanath, S.J. Connon, Tetrahedron Lett. 49, 4003 (2008)
P. Jung, D.K. Nitsche, A. Demir, A.S. Siegert, P. Lingen, B. Baumann, M. Dünkelmann, P.M. Müller, J. Am. Chem. Soc. 124, 12084 (2002)
Y. Chen, W. Shan, M. Lei, L. Hu, Tetrahedron Lett. 53, 5923 (2012)
K. Pradhan, P. Bhattacharyya, S. Paul, A.R. Das, Tetrahedron Lett. 53, 5840 (2012)
J. Liu, M. Lei, L. Hu, Green Chem. 14, 840 (2012)
S. Fatma, D. Singh, P. Ankit, P. Mishra, M. Singh, J. Singh, Tetrahedron Lett. 55, 2201 (2014)
M. Rajopadhye, F.D. Popp, J. Heterocycl. Chem. 21, 289 (1984)
J. Azizian, A.V. Morady, K. Jadidi, M. Mehrdad, Y. Sarrafi, Synth. Commun. 30, 537 (2000)
M. Jain, P. Khanna, A. Saxena, S. Bhagat, C.E. Olsen, S.C. Jain, Synth. Commun. 36, 1863 (2006)
U.C. Mashelkar, D.M. Rane, R.S. Kenny, J. Heterocycl. Chem. 45, 865 (2008)
M. Pandey, D.S. Raghuvanshi, K.N. Singh, J. Heterocycl. Chem. 46, 49 (2009)
R. Sakhuja, S.S. Panda, L. Khanna, S. Khurana, S.C. Jain, Bioorg. Med. Chem. 21, 5465 (2011)
V.V. Mulwad, A.A. Mir, J. Korean Chem. Soc. 52, 649 (2008)
A. Dandia, R. Singh, S. Khan, S. Kumari, P. Soni, Tetrahedron Lett. 15, 4438 (2015)
A. Dandia, R. Singh, J. Joshi, S. Kumari, J. Fluor. Chem. 156, 283 (2013)
A. Dandia, A.K. Laxkar, R. Singh, Tetrahedron Lett. 53, 3012 (2012)
A. Dandia, R. Singh, S. Bhaskaran, S.D. Samant, Green Chem. 13, 1852 (2011)
A. Dandia, R. Singh, J. Joshi, S. Maheshwari, P. Soni, RSC Adv. 3, 18992 (2013)
A. Dandia, R. Singh, S. Bhaskaran, Ultrason. Sonochem. 17, 399 (2010)
A. Dandia, R. Singh, S. Khaturia, C. Me-rienne, G. Morgantc, A. Loupy, Bioorg. Med. Chem. 14, 2409 (2006)
A. Dandia, R. Singh, S. Khaturia, Bioorg. Med. Chem. 14, 1303 (2006)
I.R. Siddiqui, P. Rai, A. Rahila, A. Srivastaca, New J. Chem. 37, 3798 (2013)
M. Lei, L. Ma, L. Hu, Synth. Commun. 42, 2981 (2012)
Acknowledgments
R.S. thanks DST, New Delhi for start-up Grant (YSS/2015/000972). We are thankful to the Central Drug Research Institute (CDRI), Lucknow, and MNIT, Jaipur for the spectral analyses and elemental analyses.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, R., Ganaie, S.A. An eco-compatible synthesis of novel spiro[acenaphthylene-1,2′[1,3]-thiazolidine]-2,4′(1H)-diones using thiamine hydrochloride as efficient catalyst in aqueous medium. Res Chem Intermed 43, 45–55 (2017). https://doi.org/10.1007/s11164-016-2604-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2604-x