Abstract
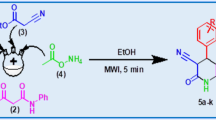
A series of 5-aryl dihydropyrrole was synthesized from the intramolecular cyclization reaction of homopropargyl amine in the presence of AgOAc as catalyst under microwave irradiation reaction conditions. The homopropargyl amine was prepared by the reaction of propargyl bromide with N-tosyl aldimine under a sonochemical Barbier-type reaction condition. Further aromatization reaction of 5-aryl dihydropyrrole in KOtBu/DMSO can afford 2-aryl pyrrole under microwave irradiation reaction conditions.




Similar content being viewed by others
Notes
The addition of 1,2-diiodomoethane was used for the activation of metal, and it reacted with Zn powder in situ to generate Lewis acid ZnI2 and ethene.
The ultrasonic cleaning bath (Elma-T490DH, 50 kHz) should be filled with water containing some 3–5 % detergent. In our laboratory, we used Decon 90, which permits much more even cavitation in bath water.
References
R.J. Sundberg, in Comprehensive Heterocyclic Chemistry, vol. 4, ed. by A.R. Katritzky, C.W. Rees (Pergamon Press, Oxford, 1984), p. 313
G.P. Bean, in Pyrroles, ed. by R.A. Jones (Wiley, New York, 1990), p. 105
R.J. Sundberg, in Comprehensive Heterocyclic Chemistry II, vol. 2, ed. by A.R. Katritzky, C.W. Rees, E.F.V. Scriven (Elsevier, Oxford, 1996), p. 119
G.W. Gribble, in Comprehensive Heterocyclic Chemistry II, vol. 2, ed. by A.R. Katritzky, C.W. Rees, E.F.V. Scriven (Elsevier, Oxford, 1996), p. 207
R.M. Baughman, J.L. Bredas, R.L. Elsenbaumer, L.W. Shacklette, Chem. Rev. 82, 209 (1982)
R.D’. Alessio, A. Bargiotti, O. Carlini, F. Colotta, M. Ferrari, P. Gnocchi, A. Isetta, N. Mongelli, P. Motta, A. Rossi, M. Rossi, M. Tibolla, E. Vanotti, J. Med. Chem. 43, 2557 (2000)
M.A. Galindo, J. Hannant, R.W. Harrington, W. Clegg, B.R. Horrocks, A.R. Pike, A. Houlton, Org. Biomol. Chem. 9, 1555 (2011)
J.T. Gupton, Top Heterocycl. Chem. 2, 53 (2006)
M. Biava, G.C. Porretta, G. Poce, S. Supino, D. Deidda, R. Pompei, P. Molicotti, F. Manetti, M. Botta, J. Med. Chem. 49, 4946 (2006)
R. Martin, A. Jäger, M. Böhl, S. Richter, R. Fedorov, D.J. Manstein, H.O. Gutzeit, H.J. Knölker, Angew. Chem. Int. Ed. 48, 8042 (2009)
M.G. Thomas, M.D. Burkart, C.T. Walsh, Chem. Biol. 9, 171 (2002)
J. Regourd, A.A. Ali, A. Thompson. J. Med. Chem. 50, 1528 (2007)
D. Fehér, R.S. Barlow, P.S. Lorenzo, T.K. Hemscheidt, J. Nat. Prod. 2008, 71 (1970)
M. Rene, J. Anne, J.K. Hans, Synlett 19, 2795 (2011)
H. Yoshimura, K. Kikuchi, S. Hibi, K. Tagami, T. Satoh, T. Yamauchi, A. Ishibahi, K. Tai, T. Hida, N. Tokuhara, M. Nagai, J. Med. Chem. 43, 2929 (2000)
C.P. Gonzalo, J.A. Pomposoa, J.A. Alduncin, M. Salsamendi, A.I. Mikhaleva, L.B. Krivdin, B.A. Trofimov, Electrochim. Acta 52, 4784 (2007)
C. Simon, T. Constantieux, J. Rodriguez, Eur. J. Org. Chem. 2004, 4957 (2004)
H. Shiraishi, T. Nishitani, S. Sakaguchi, Y. Ishii, J. Org. Chem. 63, 6234 (1998)
X. Lin, Z. Mao, X. Dai, P. Lu, Y. Wang, Chem. Commun. 47, 6620 (2011)
O.A. Attanasi, G. Favi, F. Mantellini, G. Moscatelli, S. Santeusanio, J. Org. Chem. 76, 2860 (2011)
E. Ghabraie, S. Balalaie, M. Bararjanian, H.R. Bijanzadeh, F. Rominger, Tetrahedron 67, 5415 (2011)
C.R. Reddy, M.D. Reddy, B. Srikanth, K.R. Prasad, Org. Biomol. Chem. 9, 6027 (2011)
B.M. Trost, J.-P. Lumb, J.M. Azzarelli, J. Am. Chem. Soc. 133, 740 (2011)
A.V. Gulevich, A.S. Dudnik, N. Chernyak, V. Gevorgyan, Chem. Rev. 113, 3084 (2013)
H.C. Brown, U.R. Khire, G. Narla, U.S. Racherla, J. Org. Chem. 60, 544 (1995)
A.S.-Y. Lee, S.-F. Chu, S.-H. Wang, Y.-T. Chang, Tetrahedron Lett. 45, 1551 (2004)
A.S.-Y. Lee, K.-W. Tsao, Y.-T. Chang, S.-F. Chu, Tetrahedron Lett. 48, 6790 (2007)
J.E. Baldwin, J. Chem. Soc. Chem. Commun. 734 (1976)
A.S.-Y. Lee, Y.-T. Chang, F.-Y. Su, J. Chin. Chem. Soc. 61(2), 290 (2014)
A.S.-Y. Lee, Y.-T. Chang, Tetrahedron Lett. 51, 3800 (2010)
A.S.-Y. Lee, C.-H. Chung, S.-F. Chu, Y.-T. Chang, J. Chin. Chem. Soc. 56, 202 (2009)
A.S.-Y. Lee, K.-W. Tsao, Y.-T. Chang, S.-F. Chu, J. Chin. Chem. Soc. 54(2), 519 (2007)
A.S.-Y. Lee, Y.-T. Chang, S.-F. Chu, K.-W. Tsao, Tetrahedron Lett. 47, 7085 (2006)
A.S.-Y. Lee, Y.-T. Chang, S.-H. Wang, S.-F. Chu, Tetrahedron Lett. 43, 8489 (2002)
A.S.-Y. Lee, R.-Y. Cheng, O.-G. Pan, Tetrahedron Lett. 38, 443 (1997)
A.S.-Y. Lee, W.-C. Dai, Tetrahedron 53, 859 (1997)
H.M. Wisniewska, E.R. Jarvo, Chem. Sci. 2, 807 (2011)
B.L. Hayes, Microwave Synthesis (CEM Publishing, London, 2002)
A.S.-Y. Lee, Y.-C. Wu, Y.-T. Chang, B.-C. Wang, Res. Chem. Intermed. 40(6), 2277 (2014)
A.S.-Y. Lee, C.-H. Chung, Y.-T. Chang, P.-L. Chen, J. Appl. Sci. Eng. 15(3), 311 (2012)
Acknowledgments
We thank the National Science Council in Taiwan (NSC 101-2113-M-032-003) and Tamkang University for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, A.SY., Lee, SH.M. & Chang, YT. Microwave assisted synthesis of dihydropyrrole by AgOAc catalyzed intramolecular cyclization reaction of homopropargyl amine. Res Chem Intermed 43, 3493–3503 (2017). https://doi.org/10.1007/s11164-016-2442-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2442-x