Abstract
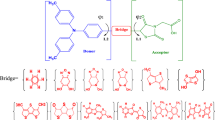
The use of computational methods such as density functional theory (DFT) in material design has attracted considerable attention aimed at achieving efficient dye-sensitized solar cells (DSSCs). A series of novel (2Z)-2-cyano-2-[2-[(E)-2-[5-[(E)-2-(4-dimethylaminophenyl)vinyl]-2-thienyl]vinyl]pyran-4-ylidene]acetic acid derivatives were simulated using DFT and time-dependent DFT for calculations of molecular properties, electronic properties, optical properties, population analysis, global reactivity indices and light harvesting efficiency (LHE). The results showed that incorporation of an F/CH3 substituent on the acceptor unit increased/decreased the charge density on the acceptor unit, thereby increasing/lowering its tendency to accept electrons from the donor unit through a π-conjugated linker due to the electron-withdrawing/electron-donating effect of F/CH3; this ultimately increased/decreased the highest occupied molecular orbital and lowest occupied molecular orbital (HOMO–LUMO) energy band gap (Eg). The para-amino substituents on the donor unit drastically increased the natural bond orbital (NBO) charges of both the donor and acceptor units of the dyes, i.e. para-CH3 < para-NH2 < para-N(CH3)2; this agreed well with the ordering of the band gap. Generally, dyes with para-N(CH3)2 on the donor subunit have longer light absorption wavelengths, a low Eg and a high LHE, which could lead to enhancement of the photocurrent and charge transfer in DSSCs.




Similar content being viewed by others
References
J. Bisquert, D. Cahen, G. Hodes, S. Ruhle, A. Zaban, J. Phys. Chem. 108, 8106 (2004)
B. O’regan, M. Grätzel, Nature 353, 737 (1991)
I. Chung, B. Lee, J. He, R.P.H. Chang, M.G. Kanatzids, Nature 485, 486 (2012)
M. Gratzel, Photochem. Photobiol. 4, 145 (2003)
M.K. Nazeeruddin, C. Klein, P. Liska, M. Gratzel, Chem. Rev. 249, 1460 (2005)
N. Vlachopoulos, P. Liska, J. Augustynski, M. Grätzel, J. Am. Chem. Soc. 110, 1216 (1988)
K. Kalyanasundaram, M. Grätzel, Coord. Chem. Rev. 177, 347 (1998)
A. Hagfeldt, M. Grätzel, Acc. Chem. Res. 33, 269 (2000)
H. Kafafy, H. Wu, M. Peng, H. Hu, K. Yan, R.M. El-Shishtawy, D. Zou, Int. J. Photoenergy (2014). doi:10.1155/2014/548914
Z.S. Wang, Y. Cui, K. Hara, Y. Dan-oh, C. Kasada, A. Shinpo, Adv. Mater. 19, 1138 (2007)
A. Burke, L. Schmidt-Mende, S. Ito, M. Gratzel, Chem. Commun. 3, 234 (2007)
W.H. Howie, F. Claeyssens, H. Miura, L.M. Peter, J. Am. Chem. Soc. 130, 1367 (2008)
R.M. El-Shishtawy, M.A. Abdullah, G.A. Saadullah, A.K.E. Shaaban, J. Mol. Model. (2014). doi:10.1007/s00894-014-2241-5
Y. Numata, A. Islam, H. Chen, L. Han, Energy Environ. Sci. 5, 8548 (2012)
S. Ahmad, E. Guillen, L. Kavan, M. Gratzel, M.K. Nazeeruddin, Energy Environ. Sci. 6, 3439 (2013)
W. Zhu, Y. Wu, S. Wang, W. Li, X. Li, J. Chen, Z.-S. Wang, H. Tian, Adv. Funct. Mater. 21, 756 (2011)
J. Tang, W. Wu, J. Hua, J. Li, X. Li, H. Tian, Energy Environ. Sci. 2, 982 (2009)
B.-G. Kim, C.-G. Zhen, E.J. Jeong, J. Kieffer, J. Kim, Adv. Funct. Mater. 22, 1606–1612 (2012)
B.-G. Kim, K. Chung, J. Kim, Chem. Eur. J. 19, 5220 (2013)
W. Fan, D. Tan, W.-Q. Deng, Chem. Phys. Chem. 13, 2051 (2012)
C.K. Tai, Y.J. Chen, H.W. Chang, P.L. Yeh, B.C. Wang, Comput. Theor. Chem. 971, 42 (2011)
H.W. Ham, Y.S. Kim, Thin Solid Films 518, 6558 (2010)
C.-R. Zhang, Z.-J. Liu, Y.-H. Chen, Y.-Z. Wu, W. Feng, D.-B. Wang, Curr. Appl. Phys. 10, 77 (2010)
T. Ruiz-Anchondo, N. Foore-Holguin, D. Glossman-Mitinik, Molecules. 493, 323 (2010)
T. Liu, H.X. Zhang, X. Zhou, B.H. Xia, Eur. J. Inorg. Chem. 8, 1268 (2008)
M.P. Balanay, D.H. Kim, J. Mol. Struct. (THEOCHEM) 910, 20 (2009)
B.F. Minaev, V.B. Gleb, V.A. Minaeva, Dyes Pigments 92, 531 (2011)
G.V. Baryshnikova, B.F. Minaeva, V.A. Minaeva, Z. Ning, Q. Zhang, Opt. Spektrosk. 112, 193 (2012)
B.F. Minaev, G.V. Baryshnikov, A.A. Slepets, Opt. Spektrosk. 112, 899 (2012)
G.V. Baryshnikov, B.F. Minaev, E.V. Myshenko, V.A. Minaeva, Opt. Spektrosk. 115, 555 (2013)
G.A. Samuel, P. Jason, P. Joshi, Q. Qiquan, Y. Youngjae, J. Photochem. Photobiol. A Chem. 224, 116 (2004)
T. Zhidan, L. Yunchang, T. Baozhu, Z.H. Jinlong, Res. Chem. Intermed. 30, 495 (2013)
J.H. Kim, H. Lee, Chem. Mater. 14, 2270 (2002)
J.L.H. Xue, X. Gu, Z. Yang, B. Xu, W. Tian, J. Phys. Chem. C 113, 12911 (2009)
Y.A. Son, S.Y. Gwon, S.Y. Lee, S.H. Kim, Spectrochim. Acta A Mol. Biomol. Spectrosc. 75, 225 (2010)
E.K.U. Gross, J.F. Aobson, M. Petersilka, Top. Curr. Chem. 181, 81 (1996)
M.E. Reda, A.M. Asiri, S.G. Aziz, S.A.K. Elroby, J. Mol. Model. 20, 2241 (2014)
Y. Luo, D. Jonsson, P. Norman, K. Ruud, O. Vahtras, B. Minaev, H. Ågre, A. Rizzo, K.V. Mikkelsen, Int. J. Quantum Chem. 70, 219 (1998)
F. Furche, K. Burke, Time-dependent density functional theory in quantum chemistry, in Annual reports in computational chemistry, vol. 1, ed. by A. Spellmeyer (Elsevier, Amsterdam, 2005), pp. 19–30
A.D. Becke, J. Phys. Chem. 98, 5648 (1993)
C. Lee, W. Yang, R.G. Parr, Phys. Rev. B 37, 785 (1988)
D. Jacquemin, E.A. Perpète, I. Ciofini, C. Adamo, Acc. Chem. Res. 42, 326 (2008)
M. Pastore, E. Mosconi, F. de Angelis, M. Gratzel, J. Phys. Chem. C 114, 7205 (2010)
Spartan 14, Wavefunction, INC, Irvine CA 92612, USA
M.K. Nazeeruddin, A. Kay, I. Rodicio, R. Humphrybaker, E. Muller, P. Liska, N. Vlachopoulos, M. Gratzel, J. Am. Chem. Soc. 115, 6382 (1993)
Y. Kurashige, T. Nakajima, S. Kurashige, K. Hirao, Y. Nishikitani, J. Phys. Chem. A 111, 5544 (2007)
M.-W. Lee, J.-Y. Kim, H.J. Son, J.Y. Kim, B.-S. Kim, H. Kim, D.-K. Lee, K. Kim, D.-H. Lee, M.J. Ko, Sci. Rep. (2015). doi:10.1038/srep07711
C.-R. Zhang, L. Liu, J.-W. Zhe, N.-Z. Jin, Y. Ma, L.-H. Yuan, M.-L. Zhang, Y.-Z. Wu, Z.-J. Liu, H.-S. Chen, Int. J. Mol. Sci. 14, 5461 (2013)
J. Preat, C. Michaux, D. Jacquemin, E.A. Perpete, J. Phys. Chem. C 113, 16821 (2009)
Z. Zhou, H.V. Navangul, J. Phys. Org. Chem. 3, 784 (1990)
T. Koopmans, Physica 1, 104 (1934)
R.G. Parr, L. Szentpaly, S. Liu, J. Am. Chem. Soc. 121, 1922 (1999)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Semire, B., Oyebamiji, A. & Odunola, O.A. Design of (2Z)-2-cyano-2-[2-[(E)-2-[5-[(E)-2-(4-dimethylaminophenyl)vinyl]-2-thienyl]vinyl]pyran-4-ylidene]acetic acid derivatives as D-π-A dye sensitizers in molecular photovoltaics: a density functional theory approach. Res Chem Intermed 42, 4605–4619 (2016). https://doi.org/10.1007/s11164-015-2303-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2303-z