Abstract
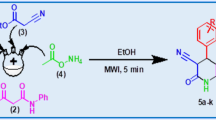
Pyridine has been used for one-pot, two-component synthesis of ethyl 3-substituted-4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate derivatives in moderate to good yields by condensing N-substituted thioureas with diethyl ethoxymalonate under microwave irradiation.




Similar content being viewed by others
References
I. Lieberman, A. Kornberg, E.S. Simms, J. Biol. Chem. 215, 403 (1955)
J. Victor, L.B. Greenberg, D.L. Sloan, J. Biol. Chem. 254, 2647 (1979)
G. Dodin, J.-E. Dubois, J. Am. Chem. Soc. 102, 3049 (1980)
M.A. Lea, A. Luke, A. Assad, M. Patel, P.A. Reddy, Int. J. Biochem. 24, 1453 (1992)
A. Garoufis, S.K. Hadjikakou, N. Hadjiliadis, Coord. Chem. Rev. 253, 1384 (2009)
F. Hueso-Urena, M.N. Moreno-Carretero, J.M. Salas-Peregrin, G. Alvarez de Cienfuegos-Lopez, J. Inorg. Biochem. 43, 17 (1991)
G. Maistralis, N. Katsaros, S.P. Perlepes, D. Kovala-Demertzi, J. Inorg. Biochem. 45, 1 (1992)
F. Hueso-Ureña, M.N. Moreno-Carretero, J.M. Salas-Peregrin, G. Alvarez de Cienfuegos-Lopez, Trans. Met. Chem. 20, 262 (1995)
S. Gore, S. Baskaran, B. Koenig, Green Chem. 13, 1009 (2011)
B.G.S. Torres, F.D.T. Uchôa, R.F.S. Canto, A. Crestani, V. Eifler-Lima, T.D. Costa, Quim. Nova 37, 461 (2014)
N.D. Moirangthem, W.S. Laitonjam, Indian J. Chem. 48B, 1023 (2009)
T.L.V. Ulbricht, C.C. Prince, Chem. Ind. 39, 1221 (1955)
W.W. Calvert, J.T. John, J. Am. Chem. Soc. 78, 5294 (1956)
T. Hudlicky, Chem. Rev. 96, 3 (1996)
P. Biginelli, Gazz. Chim. Ital. 23, 360 (1893)
R.L. Sawant, M.S. Bhatia, B. Chem, Soc. Ethiopia 22, 391 (2008)
E.H. Hu, D.R. Sidler, U.-H. Dolling, J. Org. Chem. 63, 3454 (1998)
Y. Ma, C. Qian, L. Wang, M. Yang, J. Org. Chem. 65, 3864 (2000)
W. Su, J. Li, Z. Zheng, Y. Shen, Tetrahedron Lett. 46, 6037 (2005)
V. Radharani, N. Srinivas, M. Radha Kishan, S.J. Kulkarni, K.V. Raghavan, Green Chem. 3, 305 (2001)
K. Folkers, T.B. Johnson, J. Am. Chem. Soc. 55, 2886 (1933)
S.K. Sharma, P. Kumar, B. Narasimhan, K. Ramasamy, V. Mani, R.K. Mishra, A.B. Majeed, Eur. J. Med. Chem. 48, 16 (2012)
M. Gohain, D. Prajapati, J.S. Sandhu, Synlett 2, 235 (2004)
X. Zhang, Y. Li, C. Liu, J. Wang, J. Mol. Catal. A: Chem. 253, 207 (2006)
C. Friot, A. Reliquet, F. Reliquet, J.C. Meslin, Synthesis 5, 695 (2000)
S.A. Shiba, M.M. Mohamed, M.A. Hassan, A.M. El-Sayed, Phosphorus Sulfur Silicon 158, 91 (2000)
D. Landini, F. Montanari, A. Maia, J. Am. Chem. Soc. 100, 2796 (1978)
J.E. Gorden, R.Z. Kutina, J. Am. Chem. Soc. 99, 3903 (1977)
M.A. Hassan, M.M. Mohamed, S.A. Shiba, M.K. Abou El-Regal, A. Khalil, Phosphorus Sulfur Silicon Relat. Elem. 178, 2497 (2003)
C.H. Soh, Y. Lam, J. Comb. Chem. 12, 286 (2010)
S. Botsi, A. Tsolomitis, Heterocycl. Commun. 13, 229 (2011)
V. Nair, A.R. Sreekanth, N. Abhilash, A.T. Biju, B. Rema Devi, R.S. Menon, N.P. Rath, R. Srinivas, Synthesis 12, 1895 (2003)
A.A. Esmaeili, H. Vesalipoor, R. Hosseinabadi, A.R. Fakhari, M.A. Naseri, E. Ghiamati, Tetrahedron Lett. 52, 4865 (2011)
A.A. Esmaeili, F. Zarifi, A. Moradi, M. Izadyar, A.R. Fakhari, Tetrahedron Lett. 55, 333 (2014)
A. Shaabani, A.H. Rezayan, A. Sarvary, H.R. Khavasi, Tetrahedron Lett. 49, 1469 (2008)
R. Mahesh, S. Mundra, T. Devadoss, L.P. Kotra, Arab. J. Chem. (2014). doi:10.1016/j.arabjc.2014.11.008
S. Mundra, R. Mahesh, J. Young Pharm. 7, 96 (2015)
S.K. Chakka, M. Kalamuddin, S. Sundararaman, L. Wei, S. Mundra, R. Mahesh, P. Malhotra, A. Mohmmed, L.P. Kotra, Bioorg. Med. Chem. 23, 2221 (2015)
I. Papazoglou, P.J. Cox, A.G. Hatzidimitriou, C. Kokotidou, T. Choli-Papadopoulou, P. Aslanidis, Eur. J. Med. Chem. 78, 383 (2014)
Acknowledgments
The authors gratefully acknowledge the financial support from the Department of Biotechnology (BT/IN/Canada/22/AM/2009), New Delhi, India as a part of the ISTP Canada-DBT Collaborative R&D Program. Authors are also thankful to the Birla Institute of Technology & Science (BITS), Pilani, India, and SAIF, Panjab University, Chandigarh, India, for providing the infrastructure facilities and analytical facilities, respectively. The authors are grateful to Dr. L.P. Kotra (University Health Network, Toronto, Canada), Dr. Sai Kumar Chakka (Encycle Therapeutics, Toronto-Canada) and Dr. Asif Mohmmed (ICGEB, Delhi, India) for their valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mundra, S., Mahesh, R. Pyridine-based, microwave-assisted one-pot synthetic protocol for the synthesis of ethyl 3-substituted-4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylates. Res Chem Intermed 42, 4207–4219 (2016). https://doi.org/10.1007/s11164-015-2270-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2270-4