Abstract
The electrochemical behaviour and corrosion resistance of glassy Fe68.6Ni28.2Mn3.2 (at%) specimens were studied in different concentrations of HCl solutions. The results indicated that the corrosion rate increased with increasing concentration of the HCl solutions. Electrochemical impedance spectroscopy results were analysed by fitting the experimental data to an equivalent circuit using the ZSim Demo program, and suitable equivalent circuit models were determined. The results obtained from the impedance and polarization measurements are in good agreement. The thermodynamic parameters were evaluated for the corrosion process and discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bulk metallic glasses (BMGs) or amorphous alloys consist of a chemically homogeneous single-phase solid solution, which does not contain crystalline defects that serve as nucleation sites for corrosion [1, 2]. However, amorphous type alloys have primarily been fabricated in the form of ribbons, thin films and fine wires because of their very high cooling rate requirements. The size and shape limitations and the low thermal stability of these alloys have restricted their fields of application. The use of BMGs as an engineering material is currently attracting our attention for industrial applications. To date, extensive studies have focused on the glass formation, crystallisation processes, and the mechanical and physical properties of BMGs. There is some corrosion data for BMGs in aqueous solutions. The chemical properties of materials are of considerable importance. To enable the use of the new type of BMGs as an engineering material, the glassy alloys must have good corrosion resistance and electrochemical stability in industrial environments. Therefore, it is of considerable importance to investigate the corrosion behaviour and pitting corrosion susceptibility of BMGs [3].
Fe-based BMGs have been attracting considerable attention because they possess some important advantages over the majority of the other bulk amorphous alloy systems, e.g. considerably lower material cost, higher strength (4 GPa), better magnetic properties, and greater thermal stability (the glass transition temperatures are close to or above 900 K) [4–6] .
The corrosion rates of iron-based glassy alloys have been studied in different media [7–15]; however, few studies have focused on the effects of temperature on the corrosion rate [10, 15–18]. The corrosion behaviour of Fe68.6Ni28.2Mn3.2 in aggressive acidic media has not received much attention.
Highly acidic aqueous solutions of hydrogen chloride, HCl, are used as chemical intermediates and in petroleum production, ore reduction, food processing, pickling, and metal cleaning. Because acids are aggressive media, studying the electrochemical behaviour and corrosion resistance of metallic materials is of importance. This work aimed to study the corrosion behaviour and pitting corrosion susceptibility of the Fe68.6Ni28.2Mn3.2 alloy in HCl using electrochemical techniques. The effects of the acid concentration and temperature on the corrosion properties of this iron-based alloy are discussed.
Materials and methods
The size of the and their treatments
The experiments were performed using the Fe68.6Ni28.2Mn3.2 (at%) alloy (Vacoflex 2036) which has the following physical properties: density of 8.1 g/cm3, thermal extension of 15.2 × 10−6 K−1, and a specific electrical resistance of 0.77 μΩ m. Rectangular specimens of the bright face with a surface area of 20 × 10 mm2 and a thickness of 0.25 mm were degreased with alcohol and rinsed several times with bi-distilled water and finally cleaned in an ultrasonic bath. Each experiment was conducted with a freshly stripped sample.
Preparation of the electrolytic environments
Aerated solutions of HCl were used as the corrosion media. Appropriate concentrations of HCl were prepared by dilution using bi-distilled water without further purification. For each run, a freshly prepared solution and cleaned set of electrodes were used. Aerated stagnant solutions at the required temperature (±1 °C) were used as the test solution.
Methods
DC and AC electrochemical measurements were performed using an ACM Gill AC instrument with samples that had a surface area of 2 cm2 at testing temperature. After immersion of the specimen, a stabilisation period of 20 min was observed, which was sufficient to produce the steady-state potential (E ss). A three-electrode cell was used for the electrochemical measurements; the working electrode was the test alloy whereas the counter and reference electrodes were platinum wire and saturated calomel electrodes, respectively. Polarisation measurements were performed at a scan rate of 2 mV s−1, from −800 to 2,000 mV. Electrochemical impedance spectroscopy (EIS) measurements were performed at the frequency ranged from 30 kHz to 0.1 Hz with amplitude of sinusoidal wave of 5 mV. Analysis of the impedance spectra was performed by fitting the experimental data to the equivalent circuit using the ZSim Demo program.
To reveal the detailed micro-structural features of the investigated alloy under the testing conditions, X-ray photo-electron spectra (XPS) from the surface of the alloy was measured, after the electrochemical test in 3.0 M of the test solution at 30 °C.
Results and discussion
Electrochemical techniques (such as impedance–polarisation) provide good information about the corrosion rate, corrosion behaviour, and susceptibly to localise attack of the alloy in different media.
AC electrochemical results
The EIS measurements help to isolate the individual components describing the particular processes and properties of the phases and interfaces, such as the electrolyte resistance, charge transfer resistance (the fast process), double layer capacitance, adsorption and diffusion (the slowest process) [19].
The Nyquist diagrams, constructed from the EIS measurements on the Fe68.6Ni28.2Mn3.2 alloy in HCl solutions (Fig. 1a) at 30 °C, exhibited a capacitive arc at high and intermediate frequencies (HF and MF) followed by a diffusion arc at low frequencies (LF) in the 1.0 M HCl solution. At the low frequency limit, an inductive loop in 12 M HCl was observed.
The first capacitive arc corresponds to the combined effect of the double-layer capacitance and metal dissolution, and its diameter is related to the charge transfer resistance (Rct) at the metal/solution interface. Evidently, the increase in the concentration of HCl [i.e. (Cl−)] decreased the diameter of the first capacitive arc and consequently increased the dissolution rates for the alloy. Nevertheless, the Rct values were likely representing the dissolution of not only Fe (the primary and active alloying element) but also other alloying elements.
The short tail in the LF in the 1.0 M HCl solution indicates that the ion diffusion process (mass transport process) occurs on the electrode, and it reveals the existence of a porous film on the surface of the metal, where the diameters of the pores control the diffusion of ions through the film [15]. The inductive behaviour observed at low frequencies for the alloy in the highest concentration of HCl is generally attributed to the formation of Fe(I) and Fe(II) adsorbed intermediate species in the active region for Fe in the studied solutions [20, 21]. Several authors have suggested that this inductive behaviour may be attributed to the relaxation process obtained by adsorbing species such as Cl −ads and H +ads onto the surface of the electrode [22–24].
The corresponding Bode plots in Fig. 1b, reveal that the polarisation resistance (R P), charge transfer resistance (R ct) and relaxation times decrease as the HCl concentration increases. The phase angle (max) values approach 90° in HCl solutions, which is the characteristic response of alloy surface capacitance.
A constant phase element (CPE) is used in the model in place of a capacitor to compensate for the non-homogeneity of the metal surface, which causes a greater depression in the Nyquist semicircle diagram. The CPE is a special element whose admittance value is a function of the angular frequency (ω), and whose phase is independent of the frequency. The admittance and impedance of the CEP are, respectively, expressed as:
where Y 0 (Q) is the magnitude of the CPE, j is an imaginary number (j 2 = −1), ω is the angular frequency (in rad s−1), n is the phase angle of the CPE and n = α/(π/2). The HF loops have a depressed semicircular appearance, 0.5 ≤ n ≤ 1, which is often referred to as the frequency dispersion and results from the non-homogeneity or the roughness of the solid surface.
The quality of fitting to the equivalent circuit (EC) was judged firstly by the Chi-square (χ 2) values and secondly by the error distribution versus frequency, and then comparing the experimental with the simulated data.
The corrosion data obtained through the best fitting impedance spectra with a suitable equivalent circuit are (error less than 4 %) summarized in Table 1.
The impedance spectra in 2.0, 6.0 and 9.0 M HCl solutions were analysed using the equivalent circuit model (EC) as shown in Fig. 2a, where the circuit description code (CDC) is Rs (RQ). In the 1.0 M HCl, Nyquist diagram was analysed in terms of an EC, which is shown in Fig. 2b, b′ with a CDC of R s {Q [R ct(W)]}, where CPE(Q) is the double-layer capacitance that characterises the charge separation between the metal and the electrolyte interface and W is the Warburg impedance. W can be represented as:
where σ is the Warburg coefficient (Ω cm2 s−1/2).
a Simple complex plane impedance plot, together with the equivalent circuit. b, b′ Complex plane impedance plot, together with the equivalent circuit model fitting after the immersion in 1.0 M HCl at 30 °C and a simple electrified interface. c Complex plane impedance plot, together with the equivalent circuit models fitting for spectra consist inductive loop
The model shown in Fig. 2b includes two parallel resistance and capacitance combinations and a Warburg impedance, which are considered to be a diffusion process. The Warburg impedance can be obtained by the value of I mag axis at low frequency, where b f, is defined in the following equation and is qualitatively represented as the Warburg impedance. Larger values of b f indicate that it is more difficult for the ions to diffuse through the pores within the formed passive films.
The low value of bf (0.00160 Ω−1 cm−2) corresponding to the low frequency indicates the low porosity of the film formed on the iron-based alloy in the 1.0 M HCl solution.
In Fig. 2c, it can be observed that, in addition to the characteristic elements of (Rct), CPE and the ohmic resistance of the electrolyte (R s), the inductive contribution (L element) and the Rct2 resistance connected in series must be incorporated for the iron-based alloy when inductive behaviour is observed.
A decrease of approximately 81.5 % in the Rct values can be observed in Table 1 by increasing the concentrations (aggressivety of Cl−) from 1.0 to 12.0 M for the Fe68.6Ni28.2Mn3.2 alloy. The value of R ct at in a 12.0-M solution of HCl is approximately 5.4 times lower (225 Ω cm2) than the corrosion resistivity of the studied alloy is a 1.0-M HCl solution. The Q values, which are associated with the CPE, and the corrosion rate in mm year−1 moved in opposite directions with increasing Cl− over the alloy surface. The n values (~0.88) indicated that the CPE is slightly associated with the film capacitance processes, especially at high acid concentrations, which is suggested to be due to a pitting process. Scully [25] reported that in chloride solutions pits may develop at the deepest set of pores where the operative current density is highest because the film is thinnest (or non-existent), and therefore the flux of cations and the chloride ion migration rates are at their maximum values.
DC electrochemical results
The majority of corrosion studies employ the active to noble polarisation sequence because it provides information about the corrosion or the passivation of a fresh surface. However, the reverse, noble-active, polarisation is essential in the study of localised corrosion or the breakdown of passivity.
The effect of the concentrations of HCl acid on the forward and the reverse polarisation curves of the iron-based alloy are presented in Fig. 3. The most striking characteristic is that these curves indicated that this alloy actively corroded without exhibiting anodic passivation in either the forward or reverse scan in any of the studied concentrations of acid. The evolution of hydrogen gas was observed during the cathodic reaction in each case. The corrosion rate (I corr) increased with increasing HCl. The presence of a positive hysteresis loop at 0.6- and 12.0-M concentrations of acid indicates that the alloy suffered a localised attack (inductive loop in the impedance spectrum). In these solutions the specimens have a shiny electrode polished surface after the polarisation experiments. The test solution turned from colourless to a light green yellowish due to the dissolution of Ni and Fe. The sample completely corroded at the interface in the 12-M solution, and therefore the intensity of the colour increases. The rapid dissolution of the iron-based alloy at the interface in the highest concentration of HCl solution is attributed to localised corrosion (crevice or pitting) as shown in the Nyquist spectra and polarisation curves.
The observed poor corrosion resistance of Fe68.6Ni28.2Mn3.2 glassy alloy in HCl solutions must be related to the aggressivity of Cl− and their elemental compositions (i.e. the lack of passivated elements such as Cr and P). The low concentration of Ni in this alloy does not provide passivation in the presence of Cl−. The absence of grain boundaries, segregates or crystalline defects in these glasses is evidently not sufficient on its own to provide good corrosion resistance.
In the HCl solutions, the following mechanism is proposed for the corrosion of iron and steel [26, 27]. According to this mechanism, the anodic dissolution of iron is as follows:
Ismail et al. [28], reported that species such as Ni(H2O)ads, NiCl −ads and/or NiClOH −ads adsorbed onto the surface of NiCu alloys in chloride solution (NaCl + HCl, pH 2) via the reactions:
These adsorbed species occupy a fraction of the electrode surface. In the active region, the next step may be the dissolution reaction where the adsorbed species by Cl− and/or H+ attack as follows:
In the presence of Cl−, the desorption of the intermediate species produces new active sites on the electrode surface. So, the formation of the passive film is hindered, leading to a localised metal dissolution.
X-ray photo-electron spectroscopy analysis (XPS)
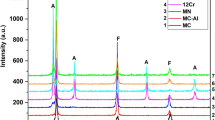
To clarify the surface composition of the alloy Fe68.6Ni28.2Mn3.2 alloy in HCl solutions, the surface of the alloy was analysed after the impedance and polarisation measurements in 3.0 M of HCl using XPS.
The peak binding energies of the Ni 2p 3/2 electron were estimated as 852.7–852.9 eV for both before (as-received) treatment and after treatment (after the experiment) at different scattering times in HCl solutions as shown in Fig. 4. The identical values in both cases indicate that Ni is not involved in the passive film formed on the iron-based alloy in HCl solutions. However, the peak binding energy of the Cl 2p 3/2 electron was observed at 199.1 eV, which indicates the presence of Cl− on the alloy surface as NiCl2, a soluble compound. The Fe 2p 3/2 electron was observed at 710.8 eV, which indicates that Fe is present as FeCl3, which is also soluble.
Temperature effect
Temperature can modify the interaction between the metal and the electrolyte. It is well known that temperature has a considerable effect on the electrochemical behaviour and the corrosion rate of metals. Limited publications on the corrosion behaviour on metallic glasses alloys at different temperatures have been reported. The objective of this section is to study the influence of temperature on the pitting and general corrosion behaviour of the Fe68.6Ni28.2Mn3.2 glassy alloy in the 3.0 M acidic solution. The concentration of 3.0 M of acid was selected because this concentration allows the electrochemical behaviour of the iron-based glassy alloy to be studied at different temperatures before the specimen is destroyed, and especially at high temperature (low aggressivity in the studied temperature range).
Impedance diagrams for the glassy Fe68.6Ni28.2Mn3.2 alloy in 3.0 M HCl at various temperatures in the range of 30–60 °C are presented in Fig. 5. The Nyquist diagrams show a single loop (Randle circuit) at 30 °C with an open end at low frequency. This arc is related to the capacitive behaviour of the alloy which is under charge transfer control with a diameter that decreases with increasing temperature. The results in Table 2 indicate that increasing the temperatures decrease the resistivity of the Fe68.6Ni28.2Mn3.2 alloy (R ct values) during the test.
Most likely, the acceleration of the anodic process occurs more quickly at higher temperatures and this reflects the inductive loop at the end of the Nyquist plots at temperatures greater than 30 °C, which indicates that the Faradic process occurs (localised attack) on the iron-based alloy. The corrosion rate values also increase and reach a value of approximately 0.597 mm year−1 at 60 °C.
Single peaks can be clearly observed in the Bode-phase plots which become narrow as the temperature of the bulk acid increases, (shorter time constants, faster reaction). This phenomenon may be explained by the fact that the time lag between the formation and dissolution of a film over the metal surface becomes shorter and shifts toward the dissolution process with increasing temperature. If n is accepted to be a measure of the surface inhomogeneity, then its decrease should be associated with a certain increase in heterogeneity, which results from roughening of the metal surface. The latter may be caused by an enhanced dissolution of metal, which occurs at high temperatures during exposure to the solution. However, the high values of 0.5 > n > 1 represent the capacitive behaviour of the alloy surface.
The curves in Fig. 6 reveal that the cathodic polarisation curves were linear and shifted towards a higher current density region with increasing test solution temperature, which is associated with the considerable evolution of hydrogen. The increasing temperature is associated with the increase in the corrosion current density (I corr). The highest current were observed at 60 °C with a maximum amplitude of 5.478 × 10−2 mA/cm2 (58.09 % greater than at 30 °C); this behaviour may due to pitting attack by Cl−, as shown in impedance plots, which reveal good agreement between the polarisation and impedance measurements.
The absence of an anodic passivation oxide layer caused a direct diffusion of the acid to the alloy/solution interface which resulted in a rapid dissolution of the alloy component. It is well known that the chloride ion, as an active ion, can be adsorbed onto the surface of metals before a passive film is formed, which prevents passivation and promotes the dissolution of metals and decreases the corrosion resistance as the temperature increases [29], or may for adsorbed species such as FeCl −ads [26, 27] or Ni(H2O)ads, NiCl −ads and/or NiClOH −ads [28].
The impedance and polarisation curves of the iron-based alloy indicate that the corrosion process is accompanied by a charge transfer over potential, i.e. it is of the activation type. The apparent activation energy (ΔE a) of the corrosion process of the glassy Fe68.6Ni28.2Mn3.2 alloy in 3.0 M of the studied acid was obtained from the linear variation of the rate of the corrosion reaction assigned as the Icorr, or the reciprocal of the charge transfer resistance, 1/Rct, with temperature, which obeys the familiar Arrhenius equation. This linear relationship can be represented as:
where R is the universal gas constant, A is Arrhenius constant and T is the absolute temperature. The activation energy was calculated from the slope of log Icorr or log 1/Rct against 1/T plots with using the least squares method (LSM) by the Sigma Plot Program. The enthalpy (∆H*) and entropy (ΔS*) values for the corrosion of the glassy alloy were calculated from the transition-state equation:
where h is Plank’s constant, and N is Avogadro’s number. A plot of log (I corr/T) or log (1/R ct/T) versus 1/T produced a straight line with a slope of −ΔH*/2.303R, and the intercept was log (R/Nh) + ΔS*/2.303R, from which the values of ΔH* and ΔS* were deduced. The calculated thermodynamic parameters from the linear regression with coefficients are close to 1 (standard deviation, R 2 about 0.98) are given in Table 3. It is known that the value of ΔE a denotes the energy barrier for the chemical reaction. A lower activation energy means that there is a lower energy barrier for metal corrosion. By forming a passive film on the alloy, the corrosion reaction will be further pushed to surface sites that are characterised by higher values of ΔE a, which indicates that the corrosion of the alloy occurs on the uncovered part of the surface that has low activation energies.
The data in Table 3 reveal that the differences between ΔE a and ΔH* are close to the average value of RT at the studied temperatures (2.64 kJ mol−1) [30], which implies that the corrosion process of the studied alloy is a mono-molecular reaction as shown in the following equation:
where P is the pressure, V is the volume and n is the number of electrons involved in the reaction. The entropy of activation, ΔS*, is negative in all the acid solutions. This result indicates that the activated complex in the rate-determining step requires very low energy to produce the reaction results. It is clear from Table 3 that the HCl solution has the lower ∆S* values (more negative), which reflect the highest order corrosion reaction, which means that the system is close to equilibrium and the corrosion reaction very fast.
Conclusions
The Fe68.6Ni28.2Mn3.2 ribbons were investigated in acidic aqueous solutions to obtain information about their electrochemical and corrosion resistivity. The following conclusions can be drawn from this study:
-
The corrosion rate of the Fe68.6Ni28.2Mn3.2 ribbons was increased with increasing concentration of acid.
-
HCl solutions hindered the formation of a passive film formed on the Fe68.6Ni28.2Mn3.2 alloy in solutions open to air at 30 ºC.
-
Based on the EIS and polarisation measurements, the alloy exhibits susceptibility for pitting corrosion only at high concentrations of HCl.
-
The low value of the apparent activation energy (ΔE a) indicates that the corrosion of the alloy occurs at the uncovered region of the surface which has low activation energies.
References
K. Hashimoto, K. Osada, T. Masumoto, S. Shimodaira, Corros. Sci. 16, 71 (1976)
M. Hukovic, N. Radic, Z. Grubac, A. Tonejcv, Electrochim. Acta 47, 2387 (2002)
Ch. Qin, W. Zhao, A. Inoue, Int. J. Mol. Sci. 12, 2275 (2011)
S. Guo, Y. Shen Nonferrous, Met. Soc. China. 21(11), 2433 (2011)
A. Makino, T. Kubota, C. Chang, M. Makabe, A. Inoue, Mater. Trans. 48(11), 3024 (2007)
Z.P. Lu, C.T. Liu, J.R. Thompson, W.D. Porter, Phys. Rev. Lett. 92, 245503 (2004)
M. Naka, K. Hashimoto, T. Mosumoto, J. Jpn. Inst. Metals 38, 835 (1974)
S. Pang, T. Zhang, K. Asami, A. Inoue, J. Mater. Res. 17, 701 (2002)
S.T. Arab, K.M. Emran, Bull. Electrochem. 21(11), 513 (2005)
S.T. Arab, K.M. Emran, Int. J. Appl. Chem. 3(1), 69 (2007)
J. Jayaraj, K.B. Kim, H.S. Ahn, E. Fleury, Mater. Sci. Eng. A 449, 517 (2007)
H. Fang, X. Hui, G. Chen, J. Alloys Compd. 464, 292 (2008)
S.T. Arab, Mater. Res. Bull. 43(3), 510 (2008)
X. Li, J. Chen, K. Zhang, Mater. Des. 30(7), 2665 (2009)
S.T. Arab, K.M. Emran, Mater. Lett. 63, 1022 (2008)
L. Zhang, Y. Jin, B. Peng, Y. Zhang, X. Wang, Q. Yang, J. Yu, Appl. Surf. Sci. 255, 1686 (2008)
A. Kawashima, Y. Yokoyama, A. Inoue, Corros. Sci. 52(9), 2950 (2010)
S.T. Arab, K.M. Emran, H.A. Al-Turaif, J. Saudi Chem. Soc. (2011) (in press) http://ac.els-cdn.com/S1319610311001190/1-s2.0-S1319610311001190-main.pdf?_tid=6566f8a8-9555-11e2-beec-00000aacb35f&acdnat=1364220658_a10d83c57323410d34a329ec604f2bbf
M. Hukovic, S. Omanvic, J. Electroanal. Chem. 455, 181 (1998)
M. Keddam, O.R. Mattos, H. Takenouti, Electrochim. Acta 31, 1147 (1986)
L. Bai, B.E. Conway, J. Electrochem. Soc. 138, 2897 (1991)
M. Keddam, O.R. Mattos, H. Takenouti, J. Electrochem. Soc. 128, 257 (1981)
M.A. Veloz, I. Gonzalez, Electrochim. Acta 48, 135 (2002)
M.H. Hussin, M.J. Kassim, Int. J. Electrochem. Sci. 6, 1396 (2011)
J.C. Scully, Passivity of metals and semiconductors, in Proc. 5th Int. Symp. Passivity, ed. by M. Froment (Elsevier, Amsterdam, 1983), p. 253
M. Morad, J. Morvan, J. Pagetti (1995) in Proceedings of the eighth european symposium on corrosion inhibitors (8SEIC), Sez V, Suppl. N. 10, Ann. Univ. Ferrara, NS (p. 159)
I. Naqvi, A.R. Saleemi, S. Naveed, Int. J. Electrochem. Sci. 6, 146 (2011)
K.M. Ismail, A.M. Fathi, W.A. Badawy, Corros. Sci. 48(8), 1912 (2006)
Th.M. Mukhlouf, M. Wahdan, Polish J. Chem. 69, 1072 (1995)
E. Noor, Int. J. Electrochem. Sci. 2, 996 (2007)
Acknowledgments
The author would like to acknowledge Professor Sanaa T. Arab and Dr. Hamad A. Al-Turaif, King Abdulaziz University. The author also wishes to express his gratitude to Dr. Hartmann Thomas from the Vacuumschmelze Company for supplying the iron-based alloy for this research. This paper was presented as a poster presentation in the “Fifth Saudi Science Conference” Umm Al-Qura—Saudi Arabia, 16–18 April 2012.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Emran, K.M. Effects of concentration and temperature on the corrosion properties of the Fe–Ni–Mn alloy in HCl solutions. Res Chem Intermed 41, 3583–3596 (2015). https://doi.org/10.1007/s11164-013-1473-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-013-1473-9