Abstract
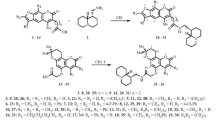
Use of the Delépine reaction for synthesis of 4-aminocoumarin from 4-chlorocoumarin was not successful. The product was 4-iminocoumarin instead of 4-aminocoumarin. The 4-iminocoumarin was characterized by elemental analysis and spectral studies (FT-IR, 1H NMR, 13C NMR). Density functional theory calculations for 4-iminocoumarin were performed using molecular structure with optimized geometry. Molecular orbital calculations provided a detailed description of the orbitals, including spatial characteristics, nodal patterns, and the contributions of individual atoms.




Similar content being viewed by others
References
A.A. Al-Amiery, A.A. Mohammed, H. Ibrahim, World Acad. Sci. Eng. Technol. 57, 433 (2009)
A.A. Al-Amiery, Y. Al-Majedy, H. Abdulreazak, H. Abood, Bioinorg. Chem. Appl. 2011, 1–6 (2011)
A.A. Al-Amiery, Y. Al-Majedy, H. Ibrahim, A. Temimi, Org. Med. Chem. Lett. 2, 1–7 (2012)
A.A. Al-Amiery, A. Kadhum, A. Mohamad, Bioinorg. Chem. Appl. 2012, 1–5 (2012)
A.A. Al-Amiery, A.A. Mohammed, G.M. Alhyali, J. Appl. Sci. Res. 7(4), 470 (2011)
A.A. Al-Amiery, Res. Chem. Intermed. 38, 745–759 (2012)
A. El-Agrody, M. Abd El-Latif, N. El-Hady, A. Fakery, A. Bedair, Molecules 6, 519 (2001)
S. Kovalenko, I. Bylov, K. Sytnik, V. Chernykh, Y. Bilokin, Molecules 5, 1146 (2000)
S. Parmer, R. Kumar, J. Med. Chem. 11, 635 (1968)
Y.D. Reddy, V.V. Somayajulu, J. Indian Chem. Soc. 58, 599 (1981)
T.O. Soine, J. Pharm. Sci. 53, 231 (1964)
M.M. Dutta, B.N. Goswani, J.C.S. Kataky, J. Heterocycl. Chem. 23, 793 (1986)
A.A. Al-Amiery, A. Musa, A. Kadhum, A. Mohamad, Molecules 16, 6833 (2011)
A.A. Al-Amiery, A. Kadhum, A. Mohamad, Molecules 17, 5713 (2012)
A. Kadhum, A.A. Al-Amiery, A. Musa, A. Mohamad, Int. J. Mol. Sci. 12, 5747 (2011)
A. Kadhum, A. Mohamad, A.A. Al-Amiery, M. Takriff, Molecules 16, 6969 (2011)
A.A. Al-Amiery, R. Al-Bayati, K. Saour, M. Radi, Res. Chem. Intermed. 38, 559 (2012)
V. Kelly, E. Ellis, M. Manson, Cancer Res. 60, 957 (2000)
S. Kirkiacharian, D. Thuy, S. Sicsic, R. Bakhchinian, R. Kurkjian, T. Tonnaire, Farmaco 57, 703 (2002)
D. Suzuki, M. Yu, L. Xie, S. Morris, K. Lee, Med. Res. Rev. 23, 322 (2003)
V. Zagorevskii, N. Dudykina, Zh. Obsch. Khim. 32, 2384 (1962)
I. Manolov, N. Danchev, Eur. J. Med. Chem. Chim. Ther. 30, 531 (1995)
H. Uno, M. Kurokawa, H. Nishimura, Chem. Pharm. Bull. 24, 644 (1976)
I. Ivanov, S. Karagiosov, I. Manolov, Arch. Pharm. (Weinh.) 61, 324 (1991)
A.P. Chavan, J. Chem. Res. 3, 179 (2006)
B. Stamboliyska, V. Janevska, B. Shivachev, P. Nikolova, G. Stojkovic, B. Mikhova, E. Popovski, Arkivoc X, 62 (2010)
M. Delépine, Bull. Soc. Chim. Fr. 13, S. 352–361 (1895)
R. Alexander, Name Reactions in Organic Chemistry, 2nd edn. (Academic Press, New York, 1961)
D.P. Spalding, H.S. Mosher, F.C. Whitmore, J. Am. Chem. Soc. 72, 5338 (1950)
Acknowledgments
The authors gratefully acknowledge Universiti Kebangsaan Malaysia for support of this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Amiery, A.A., Kadhum, A.A.H., Al-Majedy, Y.K. et al. The legend of 4-aminocoumarin: use of the Delépine reaction for synthesis of 4-iminocoumarin. Res Chem Intermed 39, 1385–1391 (2013). https://doi.org/10.1007/s11164-012-0694-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0694-7