Abstract
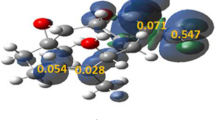
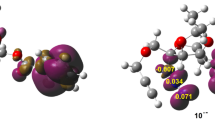
From the structure of a series of spiro (pyrrolidine-2,3′-oxindole) derivatives synthesized by Huisgen reaction of isatin, α-amino acids, and different olefins, different regioselectivities were found. The possible mechanism of the Huisgen reaction of oxindole azomethine ylide and the substituent of olefins was investigated using a B3LYP/6-311G* level of theory, and the results show that the regioselection depends on the energy barrier between the stacking state and the regioisomer. This mechanism can also be applied to the illumination of other Huisgen reactions.






Similar content being viewed by others
References
R. Huisgen, Angew. Chem. Int. Ed. 2, 633 (1963)
S. Rehn, J. Bergman, B. Stensland, Eur. J. Org. Chem. 413 (2004)
S. Abdel-Aziz, El-Ahl, Heteroatom Chem. 13, 324 (2002)
D. Fokas, W.J. Rvan, D.S. Casebier, D.L. Coffen, Tetrahedron Lett. 39, 2235 (1998)
R.S. Manian, J. Jayashankaran, S.S. Kumar, R. Raghunathan, Tetrahedron Lett. 47, 829 (2006)
R.A. Amal, R. Raghunathan, M.R. SrideviKumari, N. Raman, Bioorg. Med. Chem. 11, 407 (2003)
G. Chen, H.P. He, J. Ding, X.J. Hao, Heterocyl. Commun. 15(5), 355 (2009)
G. Chen, Y. Wu, X.F. Gu, Heterocyl. Commun. 17(3–4), 161 (2011)
G. Chen, J. Zhang, Y. Wu, Res. Chem. Intermediat. 38, 413 (2012)
G. Chen, J. Yang, S. Gao, H.P. He, S.L. Li, Y.T. Di, Y. Chang, Y. Lu, X.J. Hao, Mol. Diver. 16(1), 151 (2012)
M.J. Kornet, A.P. Thio, J. Monatsh. Chem. 19, 892 (1976)
M. Oimomi, M. Hamada, T.J. Hara, Antibiotics 27, 987 (1975)
H. Pajouhesh, R. Parsons, F.D. Popp, J. Pharm. Sci. 72, 318 (1983)
F.D. Popp, J. Heter. Chem. 21, 1367 (1984)
J.W. Skiles, D. MeNeil, Tetrahedron Lett. 31, 7277 (1990)
Acknowledgments
This work was financially supported by the grants from Chinese Ministry of Science and Technology (2009CB522303), Important Science & Technology Specific Projects of Innovative Program of Shaanxi Province (2010ZDKG-46), Scientific and Technological Plan Projects of Shaanxi Province of China (2012KJXX-40) and Scientific Research Program Funded by Shaanxi Provincial Education Department (12JK0582, 12JK0589).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, G., Yang, J., Gao, S. et al. Theoretical study of the regioselectivity of the Huisgen reaction. Res Chem Intermed 39, 1245–1250 (2013). https://doi.org/10.1007/s11164-012-0680-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0680-0