Abstract
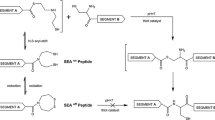
A variety of fluorophores were introduced at the N-termini of short peptides for use as biological-probes. The fluorescent peptides were cross-linked with a diacetylenic cross-linking agent between the amino acid side chains of ornithine (Orn) residues to produce peptides with high helix content.




Similar content being viewed by others
References
K. Fujimoto, M. Kajino, M. Inouye, Development of a series of cross-linking agents that effectively stabilize α-helical structures in various short peptides. Chem. Eur. J. 14, 857–863 (2008)
K. Fujimoto, N. Oimoto, K. Katsuno, M. Inouye, Effective stabilisation of α-helical structures in short peptides with acetylenic cross-linking agents. Chem. Commun. 1280–1281 (2004)
P.S. Arora, A.Z. Ansari, Chemical biology: a Notch above other inhibitors. Nature 462, 171–173 (2009)
D.A. Guarracino, P.S. Arora, Making strides in peptide-based therapeutics. Chem. Biol. 16, 919–920 (2009)
L.K. Henchey, A.L. Jochim, P.S. Arora, Contemporary strategies for the stabilization of peptides in the α-helical conformation. Curr. Opin. Chem. Biol. 12, 692–697 (2008)
T. Mineno, T. Ueno, Y. Urano, H. Kojima, T. Nagano, Creation of superior carboxyfluorescein dyes by blocking donor-excited photoinduced electron transfer. Org. Lett. 8, 5963–5966 (2006)
M. Kajino, K. Fujimoto, M. Inouye, Side-chain cross-linked short α-helices that behave like original proteins in biomacromolecular interactions. J. Am. Chem. Soc. 133, 656–659 (2011)
H. Maeda, T. Maeda, K. Mizuno, K. Fujimoto, H. Shimizu, M. Inouye, Alkynylpyrenes as improved pyrene-based biomolecular probes with the advantages of high fluorescence quantum yields and long absorption/emission wavelengths. Chem. Eur. J. 12, 824–831 (2006)
T. Hirano, T. Osaki, S. Fujii, D. Komatsu, I. Azumaya, A. Tanatani, H. Kagechika, Fluorescent visualization of the conformational change of aromatic amide or urea induced by N-methylation. Tetrahedron Lett. 50, 488–491 (2009)
O.V. Oskolkova, R. Saf, E. Zenzmaier, A. Hermetter, Fluorescent organophosphonates as inhibitors of microbial lipases. Chem. Phys. Lipids 125, 103–114 (2003)
A. Eisfeld, J.S. Briggs, The J- and H-bands of organic dye aggregates. Chem. Phys. 324, 376–384 (2006)
A. Munoz de la Pena, A.T. Ndou, J.B. Zung, I.M. Warner, Stoichiometry and formation constants of pyrene inclusion complexes with β- and γ-cyclodextrin. J. Phys. Chem. 95, 3330–3334 (1991)
Acknowledgments
This work was partially supported by Grants-in-Aid for Scientific Research (B) (22350072) and for Young Scientists (B) (23750184) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Fujimoto, K., Kajino, M. & Inouye, M. Versatile synthesis of fluorescent, cross-linked peptides as biological probes with the advantage of high helix content. Res Chem Intermed 39, 311–319 (2013). https://doi.org/10.1007/s11164-012-0651-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0651-5