Abstract
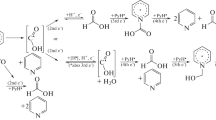
The reduction of 6-amino-5-nitroso-1,3-dimethyluracil (ANDMU) is a key step in caffeine synthesis. Electroreduction technology is a promising green chemical process. The voltammetric behavior of ANDMU at polycrystalline platinum and nickel cathodes was studied. The Nicholson theory was used to resolve the reduction process semi-quantitatively. The results show that ANDMU mainly undergoes an ECE (electron transfer, chemical reaction, electron transfer) process involving four electrons on the platinum and nickel cathodes in which the two electron-transfer steps occur at almost the same potential and the rate of the coupling chemical reaction between two electron-transfer steps is very fast. Apparent kinetic data and diffusion coefficient were determined for the platinum rotating-disk electrode.











Similar content being viewed by others
References
J.R. Chen, Y.X. Duan, Chemical Phamarceutics, Chinese phamarceutical (Science and Technology Press, Beijing, 1996), pp. 110–111
H. Blanke, M. Bugglin, H. Gropp, DE Patent 4425166A1 (1996)
I. Avrutskaya, M. Fioshin, Collect. Czechoslov. Chem. Commun. 47, 196 (1982)
A.N. Dolgachev, I.A. Avrutskaya, M. Fioshin, Elektrokhimiya 15, 571 (1979)
A.N. Dolgachev, I.A. Avrutskaya, M. Fioshin, Elektrokhimiya 15, 927 (1979)
X.E. Hu, H.W. Yang, X.J. Wang, R.S. Bai, J. Appl. Electrochem. 32, 321 (2002)
E. Gileadi, Electrode kinetics for chemists, chemical engineers, and materials scientists (Wiley, New York, 1993)
T.R. Nolen, P.S. Fedkiw, J. Appl. Electrochem. 20, 370 (1990)
A.J. Bard, L.R. Faulkner, Electrochemical methods:fundamentals and applications (Wiley, New York, 1980)
G.S. Laddha, T.E. Degaleesan, Transport phenomena in liquid extraction (McGraw-Hill, New York, 1978)
G.S. Alberts, I. Shain, Anal. Chem. 35, 1859 (1963)
R.S. Nicholson, I. Shain, Anal. Chem. 37, 178 (1965)
R.S. Nicholson, I. Shain, Anal. Chem. 37, 190 (1965)
D.W. Leedy, R.N. Adams, Electroanal. Chem. Interfacial Electrochem. 14, 119 (1967)
R.S. Nicholson, J.M. Wilson, M.L. Olmstead, Anal. Chem. 38, 542 (1966)
R.S. Nicholson, I. Shain, Anal. Chem. 36, 706 (1964)
Acknowledgments
The author acknowledges the National Nature Science Foundation of China (no. 21067002) and Nature Science Foundation of Hainan Province of China (no. 808160) for supporting the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, R., Zheng, X. & Hu, X. Electroreduction of 6-amino-5-nitroso-1,3-dimethyluracil on polycrystalline platinum and nickel cathodes in acid aqueous solution. Res Chem Intermed 38, 1119–1132 (2012). https://doi.org/10.1007/s11164-011-0448-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-011-0448-y