Abstract
Ponto–Caspian gobies are among Europe's most invasive freshwater fish species. These small freshwater and brackish water fish have spread rapidly since the end of the last century, invading the major European river systems, including the Rivers Danube, Rhine, Moselle, Meuse, Vistula, Elbe, Nemunas, Neva, Volga, while also establishing in streams, dam reservoirs, lakes, and artificial canals in 17 European countries. Two species have also successfully established in North America. The contribution of Ponto–Caspian gobies to local fish assemblages varies, but locally they are abundant or dominant components of fish assemblages in invaded ecosystems. We have considered their invasive distribution, range of occupied aquatic environments, abundance, and frequency of occurrence, and summarised their role and position in the trophic webs of invaded ecosystems. We focused on four goby species: western tubenose goby Proterorhinus semilunaris, bighead goby Ponticola kessleri, racer goby Babka gymnotrachelus and monkey goby Neogobius fluviatilis. Based on our own research and other published studies, we described the habitat preferences of these species and assessed their ecological impact on co-occurring species, both native and non-native, as predators, prey, competitors, and as hosts and vectors of parasites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The negative economic and biological impact of invasive species, including freshwater fish, is well documented for those species listed as the world's worst invasive species on the Global Invasive Species Database of the IUCN (Lowe et al. 2000). Yet, many more alien species not included on such lists are potentially harmful to the environment based on risk assessment protocols (Kulhanek et al. 2011; Vilizzi et al. 2019, 2021). Among the invasive species in Europe and the Laurentian Great Lakes of North America are Ponto–Caspian gobies. These are small freshwater and brackish water fish that have been rapidly spreading since the end of the previous century. Ponto–Caspian gobies are relatively small bottom-living species, mainly benthophagous, guarders, and cavity nesters (speleophils) with male parental care (Miller 1984; 2003; Balon 1990). They can be distinguished from other freshwater fish species occurring in the Central European bioregion by a particular suite of life-history traits, similar to other invasive fish species, such as the topmouth gudgeon (Pseudorasbora parva), Chinese sleeper (Perccottus glenii), pumpkinseed (Lepomis gibbosus) and brown bullhead (Ameiurus nebulosus) (Grabowska and Przybylski 2014). They are characterised by small-to-medium body length, short lifespan, early maturation, relatively low fecundity, relatively large eggs, multiple spawning, extended reproductive seasons and some form of parental care (Grabowska and Przybylski 2014). The life-history traits of Ponto–Caspian goby species display plasticity during their non-native range expansion, which may be a factor facilitating their invasion success, demonstrated in several studies on the round goby (e.g. Gutowsky and Fox 2012; Hôrková and Kováč 2013; Masson et al. 2018). However, similar studies on other invasive goby species are scarce (Kováč et al. 2009; Placha et al. 2010; Gertzen et al. 2016; Grabowska et al. 2021).
Goby species have migrated through three invasion corridors identified by Bij de Vaate et al. (2002) and have invaded major European river systems, i.e. the Rivers Danube, Rhine, Moselle, Meuse, Vistula, Elbe, Nemunas, Neva, Volga (Slynko et al. 2012; Roche et al. 2013; Manné et al. 2013; Rakauskas et al. 2018; Nogueira Tavares et al. 2020). They have become established in large and small rivers, streams, dam reservoirs, lakes and artificial canals and occur locally at high density (Erős et al. 2005; Polačik et al. 2009; Borcherding et al. 2011), sometimes comprising more than 70% of all fish sampled (Cerwenka et al. 2018).
The largest species is the round goby Neogobius melanostomus, which is the most widespread, often becoming dominant in local fish assemblages within a short time after its arrival. It has been one of nine freshwater fish species designated as a 'globally' high-risk species, i.e. the most potentially invasive freshwater fish species globally (Vilizzi et al. 2019). It is also a high-risk species based on the Basic Risk Assessment (BRA) and the Climate Change Assessment, which additionally require an evaluation of how predicted future climate conditions are likely to affect the BRA regarding the risks of introduction, establishment, dispersal and impact (Vilizzi et al. 2021). Interest in this species has stimulated considerable research in studies on its biology, ecology, genetic diversity and impact on invaded ecosystems, resulting in numerous papers; reviewed by Charlebois et al. (2001) and Kornis et al. (2012).
Less attention has been given to the other goby species from the same region that have expanded to many European waters over the same period. These are the western tubenose goby (Proterorhinus semilunaris), bighead goby (Ponticola kessleri), racer goby (Babka gymnotrachelus), and monkey goby (Neogobius fluviatilis). Depending on the geographical area and individual scoring by an expert, each of these goby species is classified as posing a medium or high risk of being or becoming invasive as part of the BRA assessment (Vilizzi et al. 2021). This finding shows that these species, although not as unequivocally and highly rated for invasiveness as the round goby, have significant potential to impact native organisms. There are 17 European countries where at least one of these four goby species has been recorded as an alien species, and four countries, i.e. Hungary, Slovakia, Germany and Belarus, where all four invasive goby species have established self-sustained populations (Fig. 1a–d). Moreover, in Bulgaria, Romania, Russia and Serbia, they have the status of native species but have expanded their range within the last two decades to regions or river basins where they have not been observed previously. The monkey goby and western tubenose goby have the most extensive invaded range (present in 11 countries as non-native species), followed by the bighead goby (9 countries) and racer goby (8). Based on historical data, the western tubenose goby (designated P. marmoratus before taxonomic revision) was present in the 19th Century in some countries along the Danube (Ahnelt et al. 1998; Švolíková et al. 2021). However, since the 1960s, its further expansion within the Danube system has been observed and is an ongoing process (Švolíková et al. 2021). Thus, it is not clear whether they are native or non-native species in some countries in the region.
Current distribution of A monkey goby, B bighead goby, C racer goby and D western tubenose goby in Europe according to Kvach et al. 2021. Dark colours indicate countries where the goby species are native in some waters but also expand their range in the others. Light colours indicate countries where the goby species are non-native
We considered the invasive distribution of the four Ponto–Caspian gobies (monkey, bighead, racer, and western tubenose), and reviewed the variety of occupied aquatic environments, their abundance and frequency of occurrence, and summarised their role and position in the trophic webs of invaded ecosystems.
Habitats vulnerable to colonisation by gobies
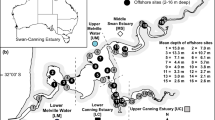
In contrast to North America, where the round goby and western tubenose goby invaded the Laurentian Great Lake system (Kocovsky et al. 2011), European inland waters colonised by the Ponto–Caspian gobies are mostly rivers (e.g. Borcherding et al. 2011; Manné et al. 2013; Roche et al. 2013; Cerwenka et al. 2018; Płąchocki et al. 2020) though they also enter some lakes (Biró 1972; Ulikowski et al. 2021). They are mainly found in shallow nearshore areas, less than 1–1.5 m (Erős et al. 2005; Płąchocki et al. 2020) or 3 m depth (Kocovsky et al. 2011), though their distribution is not restricted to these depths. In the River Danube, six Ponto–Caspian goby species were found in offshore channel habitats at depths greater than 2 m (Szalóky et al. 2015). Occurrences of the racer goby have been recorded across the entire width of the riverbed in rivers with a depth of 3.5 m (Kakareko et al. 2016) and 0.7 m (Kukuła et al. 2019) as well as in a dam reservoir with a depth of 12 m (Kakareko 2011). In lakes in the River Vistula basin, the racer goby has been observed in various zones, including the profundal, though most abundantly in the littoral and sublittoral (Kakareko—pers. obs.)
In inshore and offshore habitats, two environments appear particularly suitable for colonisation by gobies: (1) those with high structural habitat complexity and (2) relatively homogeneous areas of soft substrate composed mainly of sand.
Habitats in the first category, heavily populated by the gobies, comprise stony and rocky substrates and shorelines, including rip-rap embankments. These structures, composed of graded solid elements, abound with crevice microhabitats that provide shelters, feeding (Brandner et al. 2013) and spawning (nesting) sites for the gobies and appear ideal for completing their life cycle. Replacement of natural woody debris by rip-rap is seen as a factor favouring gobies in rivers (Sindilariu et al. 2006). Association with this habitat type have been recorded primarily in the bighead goby (Erős et al. 2005; Wiesner 2005; Jurajda et al. 2005; Polačik et al. 2008; Borcherding et al. 2013b), racer goby (Płąchocki et al. 2020) and western tubenose goby (Jude and DeBoe 1996; Grabowska et al. 2008; Adámek et al. 2010; Janáč et al. 2012). Locations overgrown with macrophytes represent another structurally complex habitat that the gobies can inhabit in large numbers. Western tubenose goby and racer goby prefer such habitats, with the former species being more closely associated with stagnant or slow-moving waters in pools and old river beds (Płąchocki et al. 2020). The study by Płąchocki et al. (2020) is the only report from invaded environments to demonstrate this habitat preference, although the western tubenose goby and racer goby are known to be associated with dense vegetation in their natural range (see Didenko 2013).
Some gobies may also occupy open areas of substrate, scarce or devoid of submerged structural elements such as stones or macrophytes. A species that can occur under such conditions is the monkey goby, and the decisive factor for its occurrence appears to be the high proportion of sand or similar substrates (gravel, mud) (see Erős et al. 2005; Jakovlić et al. 2015; Szalóky et al. 2015; Płąchocki et al. 2020). In rivers or river sections rich in such substrates, the monkey goby was the most abundant (Płąchocki et al. 2020) or second most abundant (Szalóky et al. 2015) goby species. In a laboratory habitat choice experiment, the monkey goby preferred sand over mud or gravel (Kakareko 2011). This finding suggests it has a strong direct association with this substrate type, which is also reflected in the morphology of the species and, more specifically, in traits that may help them burrow into soft substrate (generally sand) to avoid predation, including relatively small head, small ventral lobe, and long caudal peduncle (Čápová et al. 2008; Jakubčinová et al. 2017). The high abundances sometimes achieved by the monkey goby on other substrata, such as mud (Sindilariu et al. 2006), is, therefore, most likely caused by additional factors (e.g. absence of predators or competitors), masking the preference for sand. The racer goby is another species capable of colonising open habitats. However, this species prefers muddy substrates in laboratory choice tests and was found to inhabit extensive open areas (across the entire width of the water body) in a large lowland dam reservoir (Kakareko 2011).
From the above information, it is evident that Ponto–Caspian gobies, in the case of multi-species invasions, are capable of occupying a variety of habitats. This observation suggests that, compared to the round goby, such simultaneous invasions of other goby species may have greater spatial coverage and/or greater resistance to structural changes in the environment and, thereby, may impair river restoration efforts. Evidence suggests that shoreline modifications have a high potential for controlling invasive species (Roche et al. 2021). River channelization, an increase in hard substrates such as groynes and dams, facilitate gobies dispersal, but restoration efforts and more three-dimensional structures, such as large woody debris, increase the abundance of native fishes with a decline in gobies (especially the round goby) (Dorenbosch et al. 2017). Unfortunately, restoring rivers to preserve or rebuild native fish assemblages and the whole littoral ecosystem may be less effective in the presence of gobies other than the round goby.
Ponto–Caspian gobies as predators
The feeding strategy of gobies is described as generalist and opportunistic in most studies (Kostrzewa and Grabowski 2003; Kakareko et al. 2005; Grabowska et al. 2009; Polačik et al. 2009; Adámek et al. 2010; Borcherding et al. 2013b; Všetičková et al. 2014). Their range of prey is diverse but includes mainly benthic invertebrates, insect larvae and pupae, crustaceans, annelids, and gastropods; fish are found only sporadically (Table 1).
In the diet of the monkey goby, the dominant food items are often chironomid larvae and amphipods, e.g. in the River Vistula (Kakareko et al. 2005; Grabowska et al. 2009), the River Danube (Borza et al. 2009) and the River Rhine (Borcherding et al. 2013b). Trichoptera are another important prey, especially in rivers or sites with a more substantial water current, such as the River Sava (Piria et al. 2016) and the River Bug (Grabowska et al. 2009). To a lesser extent, the monkey goby also consumes molluscs, mainly bivalves, e.g. Sphaeriidae, zebra mussel Dreissena polymorpha and Oligochaeta, especially in more lentic waters (Kakareko et al. 2005; Grabowska et al. 2009). There is an ontogenetic shift in the diet of the monkey goby. For example, young-of-the-year (YOY) monkey goby fed predominantly on chironomid larvae, while larger individuals preferred Crustacea (Borcherding et al. 2013b). Studies of diel feeding by monkey gobies did not show a pattern (Grabowska et al. 2009).
The western tubenose goby feeds mainly on Chironomidae larvae, crustaceans, such as the water louse Assellus aquaticus, and zooplankton in the Nové Mlýny reservoir system (the Dyje River, Czech Republic) or amphipods: Gammarus spp. and Echinogammarus spp. in Lake Erie (Laurentian Great Lakes, USA) (Adámek et al. 2010; Kocovsky et al. 2011; Vašek et al. 2014; Všetičková et al. 2014). Diet shifts with ontogeny (Všetičková et al. 2014), season (Adámek et al. 2010; Všetičková et al. 2014), and sex differences (Všetičková et al. 2014) have been observed. In the Nové Mlýny reservoir system, the diet of < 1-year fish shifted more to chironomids and less to Trichoptera than 1-year fish (Všetičková et al. 2014). It was discovered that western tubenose gobies feed throughout the year, including winter. Feeding intensity increases before and at the beginning of the spawning season (May) and declines markedly during spawning (June) (Adámek et al. 2010). The differences in spawning habits resulted in differences in the amount of food consumed but not in diet composition between males and females. The stomach fullness of females was significantly higher than males throughout March–May, which probably reflects the greater need for females to increase energy reserves before spawning as well as the time allocation of males to territorial defence. Males also consumed significantly less prey than females during nest guarding, i.e. from May to June, while female feeding declined over winter (Všetičková et al. 2014). Ephemeroptera and Trichoptera larvae dominated the western tubenose goby diet in more riverine conditions in the Rivers Dyje and Morava (Vašek et al. 2014; Ondračková et al. 2019). Other prey less frequently consumed were water bugs (Corixidae), Ceratopogonidae, small Crustacea like Copepoda and Cladocera, leeches (Hirudinea), Ephemeroptera nymphs, Odonata larvae (Adámek et al. 2010; Kocovsky et al. 2011; Vašek et al. 2014).
Data for foraging by the racer goby come only from studies from the River Vistula (Grabowska and Grabowski 2005; Kakareko et al. 2005). Their diet depends on the site, e.g. in the Wloclawski Reservoir (River Vistula, Poland), racer gobies fed mainly on amphipods (Pontogammarus robustoides) and Chironomidae larvae at one site (Grabowska and Grabowski 2005), but small molluscs, i.e. various gastropods and Bivalvia (Sphaeriidae) dominated, considering the relative biomass and frequency of occurrence, at another site 1 km away (Kostrzewa and Grabowski 2003). Racer gobies show pronounced nocturnal foraging behaviour (Grabowska and Grabowski 2005).
Based on data from their native range, the bighead goby is the most piscivorous among these four goby species (Vasil’eva and Vasil’ev 2003). A comparison of its diet in the Danube revealed that the native population in the Danube fed mainly on two prey types: fish and amphipods, while non-native populations fed mainly on amphipods and were overall in better body condition (Polačik et al. 2009). Higher fish consumption by the bighead goby in its native range was interpreted as a consequence of lower availability of profitable invertebrate prey rather than opportunistic use of more nutritious prey. Another study revealed that the trophic niche of the bighead goby in the Danube expanded during the growth period with an increase in piscivory (Brandner et al. 2013). The diet also varied over seasons, reflecting food availability in the environment, e.g. fish appeared more frequently in autumn (Števove and Kováč 2013). On the contrary, amphipods dominated the bighead goby diet in the Danube in all seasons (Borza et al. 2009).
In the Rhine (Borcherding et al. 2013b), an ontogenetic shift was observed: YOY bighead goby fed predominantly on chironomid larvae, while larger individuals preferred Crustacea but included a proportion of fish in their diet, which occurred in parallel with a strict habitat shift from gravel and sand when up to 50 mm, to rip-rap structures when larger (Borcherding et al. 2013b). A similar diet of the bighead goby was described in the River Sava (Piria et al. 2016). Generally, apart from chironomid larvae, Crustacea (mainly amphipods) are essential prey for all invasive goby species in the Danube (Polačik et al. 2009), Rhine (Borcherding et al. 2013b), and Vistula (Grabowska and Grabowski 2005; Kakareko et al. 2005; Grabowska et al. 2009). Crustacea in the diet are mainly represented by non-indigenous, invasive species of Ponto–Caspian origin: killer shrimp Dikerogammarus villosus (Borza et al. 2009; Borcherding et al. 2013b; Brandner et al. 2013; Števove and Kováč 2013), Chelicorophium curvispinum (Borza et al. 2009; Števove and Kováč 2013) and P. robustoides (Grabowska and Grabowski 2005; Grabowska et al. 2009). These species have replaced native gammarids in large European rivers (Jażdżewski et al. 2002; Grabowski et al. 2009; Van Riel et al. 2006) and are abundant prey. However, experiments have shown that prey origin does affect racer goby preferences, with a preference for native Gammarus fossarum to the Ponto–Caspian gammarids P. robustoides and D. villosus (Błońska et al. 2015).
There is a common belief that invasive gobies prey heavily on the eggs and young stages of native fish species. Laboratory experiments showed that even the smallest of these four gobies, i.e. the western tubenose goby, predates on larvae of cyprinids (Gebauer et al. 2019), while the bighead goby prefers such prey over amphipods (Borcherding et al. 2013a). The occurrence of fish larvae and juveniles in the stomachs of gobies collected in natural environments are much less frequent than was supposed, even during the spawning season (Vašek et al. 2014). However, fish were found in small amounts in the diet of all four species: racer goby (Kostrzewa and Grabowski 2003; Grabowska and Grabowski 2005), western tubenose goby (Adámek et al. 2010; Vašek et al. 2014), monkey goby (Grabowska et al. 2009) and especially in the case of the bighead goby (Polačik et al. 2009; Borcherding et al. 2013b; Števove and Kováč 2013; Piria et al. 2016) which is most predisposed for such prey given its comparatively broad gape. The gape of bighead gobies allows them to catch and swallow relatively large prey, including fish, at earlier life stages. For comparison, the forceps-like mouth of round gobies may be more suitable for picking small food items (Borza et al. 2009). Piscivory generally appears more frequently in autumn (Števove and Kováč 2013), with YOY at high densities. Intraguild predation, i.e. feeding on other goby species or cannibalism occurred much more often than predation on native fish species (Borza et al. 2009; Borcherding et al. 2013b; Brandner et al. 2013; Števove and Kováč 2013; Piria et al. 2016). Among native fish species, mainly cyprinids, including chub Squalius cephalus (Piria et al. 2016), ruffe Gymnocephalus cernuus and European perch Perca fluviatilis (Brandner et al. 2013) were identified from stomachs. For example, bighead goby in the Rhine consumed N. melanostomus (25%), cyprinids (15%), P. kessleri (10%), European perch (5%) and other fishes (45%) (Brandner et al. 2013).
Considering the role of gobies as predators, a wide variety of aquatic macroinvertebrates are potentially threatened. In places where the benthos is not rich and abundant, the presence of gobies at high densities might be problematic for populations of benthic biota. A field experiment revealed a negative impact of the western tubenose goby on aquatic invertebrate density and community composition, and this impact increased in summer (Mikl et al. 2017a). In addition, a preference for larger invertebrates resulted in an overall reduction in invertebrate body size in the environment (Mikl et al. 2017a).
Ponto–Caspian gobies as competitors
In expanding their range, gobies also engage in interspecific interactions, especially with native species, in recipient ecosystems. Their aggressive behaviour and efficient monopolisation of resources was demonstrated in laboratory studies (e.g., Kessel et al. 2011; Kakareko et al. 2013; Grabowska et al. 2016). However, their direct impact under natural conditions is more difficult to confirm. The most vulnerable are native benthic species that display similar habitat, diet or spawning requirements, e.g. native bullheads. Both, gobies and bullheads feed on benthic macroinvertebrates (e.g. Mills and Mann 1983; Tomlinson and Perrow 2003; Grabowska and Grabowski 2005; Kornis et al. 2012). Similar ecological requirements, e.g. reproductive behaviour that includes male territoriality, parental care, and nest defence (e.g. Mills and Mann 1983; Tomlinson and Perrow 2003; Kornis et al. 2012), can lead to competition under circumstances of co-occurrence at high-density and resource deficiency, especially during the breeding period.
Research indicates an adverse effect of gobies on European and river bullhead populations, Cottus gobio and Cottus perifretum, respectively (e.g. Kessel et al. 2011; Kakareko et al. 2013) (Table 2). A study performed by Baer et al. (2017) showed a decline in the number of European bullhead specimens caught (from 57 individuals in 2011 to one in 2014) following the successive invasions of western tubenose, bighead, and round goby in the River Rhine. A decrease in average river bullhead density followed by an increase in western tubenose and bighead goby populations was reported in the River Meuse, Netherlands (Kessel et al. 2016). Similarly, increasing western tubenose goby abundance coincided with a decline of European bullheads at a sampling point on the River Moselle in Germany (Von Landwust 2006). Contrasting field observations were presented in two different long-term surveys conducted in the rip-rap zones of the River Danube in Austria, where the presence of round and western tubenose gobies (Janáč et al. 2018), as well as round, bighead, and racer gobies (Ramler and Keckeis 2019), did not affect European bullhead abundance as both species avoided each other, i.e. they were not present together at the same site (Janáč et al. 2018; Ramler and Keckeis 2019). In situ, underwater observation confirmed that habitat segregation between larger specimens of European bullhead and racer goby, noted in the River Brda in Poland (Kakareko et al. 2016), may reduce competition under natural conditions. However, an adverse effect was suggested in the case of smaller bullhead.
Possible mechanisms of competitive interactions between gobies and bullhead were revealed under experimental conditions. For example, in a series of laboratory experiments, higher aggressiveness gave a competitive advantage to the racer goby over European bullhead regarding foraging and shelter occupation (Kakareko et al. 2013; Jermacz et al. 2015; Grabowska et al. 2016). Furthermore, racer gobies displayed aggressive behaviour towards bullhead and reduced the time spent by the native species in the shelter (Grabowska et al. 2016), including under various flow conditions (Jermacz et al. 2015). Additionally, the invasive goby was faster to reach food and limited the bullhead's feeding time (Kakareko et al. 2013). The aggression of the racer goby and successful control of resources depended on the size of competitors (Grabowska et al. 2016) and were intensified during the spawning period (Kakareko et al. 2013; Grabowska et al. 2016) but was also observed outside the breeding period (Jermacz et al. 2015). Likewise, western tubenose and bighead gobies outcompeted river bullhead in the preferred shelter habitat (Kessel et al. 2011). On the other hand, monkey and western tubenose gobies proved uncompetitive against European bullhead (Błońska et al. 2016).
Although the decline of native bullhead populations is often associated with the Ponto–Caspian goby expansion, it might result from other environmental changes to which bullhead are vulnerable, such as increased siltation and channel modification, and water pollution (Knaepkens et al. 2002). Nevertheless, the presence of gobies, their aggressiveness and competition for shelters observed under experimental conditions suggest interference with native bullhead during reproduction, which can have an adverse impact on the reproductive success of native bullheads (Grabowska et al. 2016).
Habitat competition with gobies was also tested in laboratory studies for another native species, the stone loach Barbatula barbatula (Kessel et al. 2011; Błońska et al. 2017). Studies performed outside the reproductive period showed no effect on habitat occupancy of stone loach in the presence of western tubenose, bighead, round, and monkey goby intruders (Kessel et al. 2011), while in spring (spawning season), western tubenose goby males, but not females, significantly reduced the time spent by stone loach in a shelter (Błońska et al. 2017), potentially increasing their exposure to predation. However, there is no evidence of an adverse impact of gobies on stone loach from field surveys (Piria et al. 2016).
The effect of gobies on other benthic species is less pronounced and often ambiguous based on an observed decrease in abundance or decline of some native species in coincidence with the invasion of gobies, or simply their presence/absence at a site. For example, a decline in the gudgeon Gobio gobio population, when co-occurring with the monkey goby, was observed in the River Sava, which suggests a potential negative impact (Jakovlić et al. 2015). However, other research conducted at similar sampling sites did not confirm these observations (Piria et al. 2016). Changes in the abundance of small individuals of the barbel Barbus barbus corresponded with bighead goby population fluctuations (Ramler and Keckeis 2019). Barbel abundance increased in response to a decline in bighead goby abundance, and barbel avoided rip-rap and groyne fields as a response to bighead goby presence in the River Danube (Ramler and Keckeis 2019). During the same surveys, negative associations between young nase Chondrostoma nasus and racer goby were reported (Ramler and Keckeis 2019), but it resulted from differences in habitat preferences between these two species rather than an adverse impact of the racer goby.
Food competition is another suggested interaction between gobies and native fish species. Several studies indicated high dietary overlap between gobies and native species (Kocovsky et al. 2011), e.g., bighead goby with Balon’s ruffe Gymnocephalus baloni (Copp et al. 2008) and European perch (Borcherding et al. 2019) as well as western tubenose goby with European perch (Adamek et al. 2010). On the other hand, there was no significant dietary overlap between the racer goby and perch and ruffe in the River Vistula. There was spatial segregation through different prey dominating the diet of native percids and racer goby during their main foraging period (Grabowska and Grabowski 2005). Similar results for the same native species and other non-native gobies (round, monkey, and bighead gobies) in the River Sava have been obtained (Piria et al. 2016). While most studies have focused on adult individuals as competitors, much less is known about food competition between juvenile stages. Borcherding et al. (2019) studied multi-species juvenile assemblages in the Lower Rhine, where native predators co-occurred with invasive gobies under limited food resources and high dietary overlap. They showed how the competitive advantage of round and monkey goby over young pikeperch and European perch forced native species into a competitive bottleneck, i.e., native predators were exposed as potential prey or competitors to alien gobies before reaching a size or ontogenetic stage that enabled them to prey on gobies. This example showed how complicated and multidimensional the interaction between alien and native species is in the environment.
Although most research and management activities focus on interspecific interactions between gobies and native species, they also interact with each other (Gaye-Siessegger et al. 2022). The round goby is usually the latest invader among Ponto–Caspian gobies, but its impact is the most noticeable, including on other gobies; the western tubenose and bighead goby are the most affected. A decline in the abundance of both species after the arrival of round goby was noted in the River Neckar (a tributary of the Rhine, Gaye-Siessegger et al. 2022). The greater aggressiveness and competitive ability of the round goby facilitate the replacement of western tubenose (summarised by Švolíková et al. 2021) and bighead gobies (Janáč et al. 2018) in some locations.
Although there was no effect of the round goby on the western tubenose goby at the YOY stage (Janáč et al. 2016), a significant size difference in adulthood and the shorter life cycle of the western tubenose goby makes the species a relatively poor competitor in later life stages (Švolíková et al. 2021). Indeed, under laboratory conditions, direct competition for a shelter resource between mature western tubenose gobies and immature round gobies showed higher aggression of round gobies against size-matched individuals (Cartwright et al. 2019). The bighead goby was a weaker competitor in direct interactions with the round goby, despite size differences, in laboratory experiments (Borcherding et al. 2013a). Field surveys suggest that adapting habitat use patterns can limit competition with the round goby (Ramler and Keckeis 2019), though not always successfully, as a continuous decline in the abundance of tubenose and bighead gobies was observed (Janáč et al. 2018; Gaye-Siessegger et al. 2022). Generally, at sites where the round goby is present, it is the dominant goby species (Cerwenka et al. 2018; Janáč et al. 2018). The arrival of round gobies correlated with the decline in abundance of other alien gobies that established populations earlier was observed after the arrival of the round goby (Šlapanský et al. 2017; Cerwenka et al. 2018). The racer goby appears much less abundant than the other goby species in the Danube and the Rhine, probably due to a lack of preferred habitat (Haertl et al. 2012) and competition with other goby species, especially the round goby. A specific situation exists in assemblages of Ponto–Caspian gobies in the River Vistula system where the round goby is absent, except for the lowermost section (close to the Vistula mouth), and does not affect other goby species in contrast to their native region and the Danube–Rhine corridor. The racer goby was the first invasive Ponto–Caspian goby species recorded, but the later arrival of the monkey goby and western tubenose goby (Grabowska et al. 2008) did not significantly reduce its abundance.
Intraguild interactions among monkey, tubenose and/or bighead gobies are less pronounced, and no significant population decline was reported under co-occurrence. During the spawning season, under limited shelter conditions, displacement of racer gobies by monkey gobies was observed, probably due to the larger body size of the latter species (Kakareko 2011). Potential competition between the monkey goby and the newest invader from the Ponto–Caspian region, the Caucasian dwarf goby Knipowitscha cf. caucasica, is suggested due to their dietary overlap (Borcherding et al. 2021). Potential spatial competition between western tubenose and racer gobies, considering their habitat preferences and results of laboratory experiments, was suggested by Płąchocki (2017).
To summarize, several circumstances shape the result of interspecific interactions. They include the goby species involved in competitive interactions, season, the relative size of competitors, and limitation of resources in the environment. As benthic species, gobies may pose the biggest threat to other native benthic species, especially during the reproductive season when males seek a shelter to build a nest that they later aggressively defend (Błońska et al. 2016; Grabowska et al. 2016). Prolonged batch spawning of gobies from spring until early autumn increases this shelter space occupation and may negatively impact indigenous species that also depend on shelter availability, like the bullhead or stone loach (Błońska et al. 2016, 2017; Grabowska et al. 2016) with a potential relaxation of competition outside the reproductive season (Kessel et al. 2011; Błońska et al. 2016). Ponto–Caspian goby species are not equally competitive (Błońska et al. 2016), and the size of the adult or ontogenic stage influences the outcome of competitive contests (Błońska et al. 2016; Grabowska et al. 2016, Kessel et al. 2011; Cartwright et al. 2019). Mechanisms decreasing interspecific competition include habitat segregation (Kakareko et al. 2016), prey partitioning (Grabowska and Grabowski 2005; Števove and Kováč 2013) or lower consumption rates (Borcherding et al. 2019) are sometimes encountered. Resource availability also influences the intensity of species interactions (Borcherding et al. 2019), but it is difficult to prove in natural conditions; thus, mesocosm studies would shed more light on this question.
Ponto–Caspian gobies as prey
Invasive species can impact ecosystems by altering established predator–prey relationships in the native community and thereby modifying the structure of food webs in complex ways (David et al. 2017). This effect includes cases where invasive species impact native predators and their new prey. This outcome can take various forms, depending on whether the new species become attractive prey for predators and how they interact with native prey, which in turn may decrease (Venable et al. 2019) or increase (Castorani and Hovel 2015; Noonburg and Byers 2005) predator pressure on native prey species. The Ponto–Caspian gobies are mainly represented by small-sized individuals (< 10 cm of total length) in invaded environments (Kakareko 2011; Plachá et al. 2010), being bottom dwellers and considered poor swimmers (Teletchea and Beisel 2018). They, therefore, potentially represent attractive prey. However, the representation of Ponto–Caspian gobies, other than round gobies, in the diet of predators in invaded waters is still poorly understood. The few papers addressing this problem deal mainly with predatory fish (Table 3). There is surprisingly little information on the contribution of invasive gobies to the diet of non-fish predators. The gobies have been recorded as a rare component of the diet of the cormorant Phalacrocorax carbo sinensis (Wziątek et al. 2010) and Eurasian otter Lutra lutra (Mirzajani et al. 2021).
Studies on predatory fish show that invasive goby species can become a substantial or dominant dietary item for some native piscivores within a few years after establishment (Płąchocki et al. 2012; Rakauskas et al. 2018). Such rapid incorporation in the diet supports the idea that gobies have a high potential to integrate relatively quickly into food webs they invade. Additionally, it shows that native predators can efficiently learn how to exploit gobies as new prey resources, despite there being no native counterparts of this family in European freshwaters where surveys were conducted. Laboratory studies (Augustyniak et al. 2022; Kłosiński et al. 2022) suggest that the gobies might be relatively easily accessible prey for some predators. It has been shown that the monkey and racer goby, compared to their native analogues, i.e. gudgeon G. gobio and European bullhead C. gobio, do not exhibit more effective defence behaviour in the face of a direct threat from European perch (Augustyniak et al. 2022). In other laboratory studies, the behavioural responses of the monkey goby and gudgeon to predation cues (prey skin extracts) were less pronounced in invasive than native species (Kłosiński et al. 2022). These findings support the idea that invasive gobies have no advantage over native prey in avoiding predation.
Dietary studies of predatory fish indicate that factors like size and species (of both predator and prey) and habitat type are important in shaping the role of gobies in the diet of predators. Gobies were found to be consumed chiefly by mid-size predators (10–30 cm standard length, SL) in age classes ≥ 1 + , although they were also found in the diet of smaller individuals (5–10 cm SL) (Specziár 2011; Všetičková et al. 2018). Generally, small predators (YOY), due to gape size limitations, focused on invertebrates as a prey source. In contrast, larger individuals (> 30 cm SL) preyed primarily on larger, more profitable prey, including native cyprinid fish. In the River Dyje (Mikl et al. 2017a, b), including the Mušov Reservoir (Všetičková et al. 2018), rip-rap bank structures seem to shape the role of the gobies in food webs. This type of habitat has been successfully colonised by the western tubenose goby (and round goby), which in turn translates into a higher proportion of the gobies in the diet of predators closely associated with it (burbot Lota lota, ≥ 1 + European perch P. fluviatilis, ≤ 2 + wels catfish Silurus glanis) compared to those foraging more in open waters (pike Esox lucius, Volga pikeperch Sander volgensis, pike-perch Sander lucioperca) (Mikl et al. 2017a, b; Všetičková et al. 2018). It should be noted that of these predators, wels catfish and European perch exhibited positive selection towards western tubenose goby, while burbot preferred the round goby (Mikl et al. 2017a, b). In Lake Balaton (Specziár 2011), as in rivers lacking rip-rap banks (Płąchocki et al. 2012; Rakauskas et al. 2018), where fish were collected in open areas of soft substrate, the monkey goby was the most abundant goby species in the diet of piscivorous fishes. Under these conditions, the monkey goby was the preferred prey for top predators such as pike-perch, Volga pikeperch (Specziár 2011) and pike (Rakauskas et al. 2018). There are suggestions that native predators, such as asp Aspius aspius, burbot, European perch, pike-perch, and wels catfish, benefit from the presence of the gobies, as co‐occurrences of these prey and predatory fish have been noticed, although this pattern has not been found to affect predator abundance (Mueller et al. 2018; Ramler and Keckeis 2019).
These findings show that invasive gobies are relatively easily accessible prey for native piscivorous fish and have become an essential component of their diet in habitats with contrasting structural complexity (rip-rap, open, soft-bottom areas). Furthermore, the availability of gobies as prey varies enormously according to the species of both goby and predator. Therefore, complex changes in trophic interactions within fish assemblages can be expected in multispecies goby invasions.
Ponto–Caspian gobies as hosts/vectors of parasites and pathogens
Ponto–Caspian gobies are hosts of a wide range of parasites in both their native and non-native ranges, with up to 167 parasite taxa recorded in the racer, monkey, bighead, and western tubenose goby species (Kvach and Ondračková 2020). A reduced parasite fauna in non-native compared to the native range was reported for the racer (28 vs. 43 parasite spp. in non-native vs. native range, respectively), monkey (53 vs. 110 spp.) and bighead gobies (37 vs. 49 spp.), while comparable parasite numbers between ranges are recognised for the western tubenose goby (58 vs. 60 spp.; summarised in Kvach and Ondračková 2020). The susceptibility of Ponto–Caspian gobies to local parasite species has been observed in all non-native regions where gobies established after expansion or translocation via four major European invasion corridors (Panov et al. 2009) or via transoceanic invasion into the Great Lakes in North America and the North and Baltic Seas in Europe. Most species reported to infect gobies in both ranges include fungi, protozoan and metazoan parasites (Kvach and Ondračková 2020), while reports for viruses and bacteria are rare (see Tarján et al. 2014 for exception). Although porcine circovirus type 2 was detected in the internal organs of monkey gobies in the Middle Danube, it is unclear whether this represents an active infection in the fish or (more probably) just a passive carrier status (Tarján et al. 2014).
Several reports of parasite co-introduction along with goby hosts have been documented, including eight parasite species (Table 4), all showing specificity or preference for Gobiidae, limiting their ability to switch and, consequently, a potential threat to native host species. The microsporidian Loma acerinae was co-introduced with the monkey and/or western tubenose gobies into the Middle Dnieper basin (Kvach et al. 2014; Zaichenko 2015), Vistula basin (Kvach et al. 2014), and Lower Volga (Kvach et al. 2015). Three coccidian species, i.e. Eimeria daviesae, Goussia kessleri, and Goussia szekelyi were co-introduced along with monkey and/or bighead gobies into the Middle Danube in Hungary (Molnár 2006). The myxosporean Sphaeromyxa sevastopoli was reported from the western tubenose goby in Lake St. Clair in the United States (Pronin et al. 1997). The monogenean Gyrodactylus proterorhini, infecting all four goby species, was introduced into the river basins of the Vistula (Kvach et al. 2014; Mierzejewska et al. 2014), Rhine (Huyse et al. 2015; Ondračková et al. 2015a, 2021), Middle Danube (Ondračková et al. 2009, 2021), and Middle Dnieper (Zaichenko 2015). Finally, the co-introduction of cestodes along with the monkey goby was reported for Ligula pavlovskii in Southern Hungary (Vitál et al. 2021) and Proteocephalus gobiorum the Middle Dnieper (Kvach et al. 2014; Zaichenko 2015).
Of the parasites co-introduced into the non-native ranges of the Ponto–Caspian gobies, only G. proterorhini infected species other than gobies. For example, in the Wloclawski Reservoir (Vistula basin), 9% of European perch were infected (Ondračková et al. 2021). Nevertheless, parasites experimentally transferred to European perch and pike-perch did not survive 24 h, indicating that natural infections of G. proterorhini are accidental without a significant risk for native fish species (Ondračková 2016).
Interactions between parasites and non-native hosts are highly variable, reflecting, among others, behavioural, ecological, immunological, and physical barriers that might cause parasite transmission to fail among natives and invaders (Prenter et al. 2004). Where introduced, Ponto–Caspian gobies are commonly involved in parasite life cycles, varying in the position in the parasite life cycle and showing different parasite load levels. Release from parasites and pathogens, a part of the so-called Enemy Release Hypothesis, has been proposed as one of the factors supporting the invasion success of non-native species in new areas (Torchin et al. 2003). Significantly reduced parasite fauna compared to the native range has predominantly been reported in distant and disconnected non-native areas, such as North American Great Lakes for the tubenose goby (e.g. Pronin et al. 1997; Kvach and Stepien 2008; Kvach and Ondračková 2020), while parasite load reduction in non-native populations of the bighead goby interconnected with the native Danubian range was not apparent (Ondračková et al. 2012). In addition, parasite release is apparent mainly during the period immediately following fish host introduction and establishment (Gendron et al. 2012), as was found for all goby species introduced into the Lower Rhine (Ondračková et al. 2015a).
Over time, non-native species are parasitized by local parasite species, which they acquire in the new environment (Poulin and Mouillot 2003). All four goby species have acquired new parasites in their non-native range, mainly larval endoparasites exhibiting low host specificity (Kvach et al. 2014; Ondračková et al. 2009, 2021). Due to the lack of evolutionary host-parasite adaptation, novel host-parasite interactions can lead to high infection intensities and insufficient immune responses to parasite species that the host has not yet encountered (Lively and Dybdhal 2000). For example, high prevalence and abundance in the racer, monkey, and tubenose gobies were found for larval trematodes Bucephalus polymorphus (Ondračková et al. 2015b), a parasite whose life cycle is associated with the invasive zebra mussel (Kvach and Mierzejewska 2011). Data from tributaries of the River Danube show that both the prevalence and intensity of B. polymorphus infection significantly increased in local cyprinids serving as natural intermediate hosts just after the introduction of tubenose and round gobies (Ondračková et al. 2015b).
Gobies can also be susceptible to non-native parasites unrelated to the Ponto–Caspian region. For example, high infection intensities of glochidia, bivalve larval stages of the invasive Asian mussel Sinanodonta woodiana, were observed in early life stages of tubenose (and round) gobies in the Rivers Morava and Dyje, potentially supporting the dispersal of the non-native mussel species along these rivers (Šlapanský et al. 2016). Furthermore, all four goby species have also been found as paratenic hosts of the nematode Anguillicola crassus (Kvach and Ondračková 2020), an invasive Asian parasite of the European eel Anguilla anguilla, exhibiting severe pathological effects on its definitive host (Palstra et al. 2007). However, the prevalence and infection intensities were relatively low in western tubenose and bighead gobies (Koubková and Baruš 2000; Ondračková et al. 2009, 2019) in the Danube basin compared to case in the round goby in the River Rhine, where over 30% of fish were infected (Emde et al. 2014). Furthermore, infection of the Asian cestode Schyzocotyle acheilognathi, spreading from its native range and causing fish mortalities in aquaculture (Kuchta et al. 2018), has been reported from the monkey goby in the River Ros in the Dnieper basin (Zaichenko 2015). As a specific example, the bighead (along with round) gobies contribute to the expansion of non-native acanthocephalan Pomphorhynchus laevis in the River Rhine (Hohenadler et al. 2018). Using comparative and genetic methods, Hohenadler et al. (2018) found that P. laevis outcompeted and suppressed the endemic Pomphorhynchus tereticollis from the mainstream of the river. All these examples indicate that non-native gobies may contribute to the distribution of invasive parasites, supporting the invasional meltdown theory (Simberloff and Von Holle 1999).
Introduction of non-native species that are either susceptible to local parasite species, supporting the increase of the parasite numbers in the environment (parasite spill-back, Kelly et al. 2009) or serving as a sink for other parasites, i.e. diluting the infection (Gendron and Marcogliese 2017), may consequently lead to changes in parasite communities of local fish species. Hohenadler et al. (2019) compared parasite communities of native fish species at sites affected and unaffected by the invasion of Ponto–Caspian gobies in Germany. They found increased prevalence and abundance of the native nematode Raphidascaris acus and non-native acanthocephalan Pomphorhynchus laevis in native fish species at localities invaded by bighead (and round) gobies, indicating spillback (for R. acus) and spill over (for P. laevis) effects in the River Rhine associated with the goby introduction.
Conclusion
Our review summarizes published evidence that Ponto–Caspian gobies have successfully adapted to environmental conditions, including their abiotic and biotic components, in non-native areas (see Sakai et al. 2001 for a review). The contribution of Ponto–Caspian gobies to local fish assemblages and their impacts on the ecosystem varies depending on the river basin, habitat type, duration of established population and presence of co-occurring fish species, including other gobies and the order of their arrival into the invaded ecosystem.
Although Ponto–Caspian alien gobies inhabit inshore and offshore habitats, they are most commonly found in shallow waters (< 1–3 m). Although it is often stated that Ponto–Caspian alien gobies predominate in rivers at sections where anthropogenic river embankments, i.e. rip-rap habitats, are present, goby species vary in their habitat preferences and utilise many other types of habitats that are encountered in rivers and lakes, like open sandy bottoms (e.g. monkey goby, racer goby) or densely vegetated bottom (e.g. tubenose goby), where the round goby is absent or scarce. This pattern suggests that multi-species invasions of Gobiidae (other than round goby) cover a wider range of habitats than in the case of round goby invasions, which can translate into better establishment of invaders in the environment and, e.g. impair restoration efforts of river systems.
All goby species appear to be flexible in the type of prey utilised; thus, a wide variety of aquatic macroinvertebrates are potentially threatened, while foraging on native fish eggs, larvae and juveniles is less intensive than expected, except for the most piscivorous bighead goby. Under certain circumstances, the opportunistic feeding strategy of gobies may affect macroinvertebrate communities and native fish species, especially in the case of benthophagous fishes, through exploitative competition. Food competition may occur among adults and juveniles of gobies and native fish species, often using the same nursery area. Moreover, the results of experiments showed that the aggressive behaviour of gobies, especially males during the reproductive period, can cause spatial competition with some native species, especially bullhead. However, field observations of co-occurring racer goby and European bullhead suggested that resource/space partitioning allows for avoidance of competition in natural conditions. These findings demonstrate that to understand the real impact of invasive gobies, other than the round goby, more field-oriented studies are needed, ideally combined with field experiments or mesocosm experiments.
There are few studies on the role of gobies as prey for predators, and these are mainly concerned with piscivorous fish and rarely other predators (birds, mammals). However, gobies have been shown to rapidly (within a few seasons) become components of the diets of piscivorous fish, indicating their potential to change trophic networks. Laboratory studies suggest that gobies are relatively easy to hunt and thus attractive prey for piscivorous fish. Field studies have shown that the importance of gobies as prey for the predators is determined by factors such as size and species (of both predator and prey) and habitat type (e.g. rip-rap, open, soft-bottom areas) in newly invaded areas. Therefore, multispecies goby invasions in highly diverse environments, such as rivers, should be expected to cause complex changes in trophic interactions within fish assemblages. However, the nature of these changes is still unexplored and should be assessed in long-term studies.
The invasion of Ponto–Caspian gobies has resulted in the co-introduction of eight new parasites from the same region, but all of them show specificity or preference for Gobiidae and appear no threat, or at most a limited threat, to native host species. Records of parasite co-introduction are somewhat rare and usually cover one geographical area. Exceptions are L. acerinae and G. proterorhini; these species have been introduced with hosts to several non-native areas, but only within Europe. Although low parasite prevalence and abundance were observed in the initial invasion stage, gobies acquired a wide range of local parasite species with low host specificity shortly after establishment. They included not only native parasites but also invasive species of other than Ponto–Caspian origin. Non-native gobies, therefore, can potentially increase the total parasite numbers in the environment and may contribute to the spread of some invasive parasites, such as the Asian nematode A. crassus, the cestode S. acheilognathi, or the non-native acanthocephalan P. laevis and the glochidia of Asian mussel S. woodiana.
Based on the documented impact of the round goby on recipient ecosystems (Kornis et al. 2012), other Ponto–Caspian goby invaders were expected to display a similar detrimental effect. However, published studies do not support this prediction, although some field-based and experimental studies provide indications that the presence of Ponto–Caspian gobies, other than the round goby, can also be non-neutral for invaded ecosystems (Fig. 2). The intensity of the interactions between gobies and native species and the overall outcome of their presence in invaded ecosystems depends on several variables. When the goby species discussed in this review were studied in co-occurrence with the round goby, its impact on native species was much more pronounced, which obscured the impact of the other goby invaders. Thus, we recommend long-term studies in a variety of environmental contexts and fish communities for future research. We also consider the presence or absence of the round goby and other invasive fish species critical in understanding the impacts of Ponto–Caspian gobies in their non-native range.
References
Adámek Z, Jurajda P, Prášek V, Sukop I (2010) Seasonal diet pattern of non-native tubenose goby (Proterorhinus semilunaris) in a lowland reservoir (Mušov, Czech Republic). Knowl Manag Aquat Ecosyst 397:02. https://doi.org/10.1051/kmae/2010018
Ahnelt H, Bănărescu P, Spolwind R, Harka A, Waidbacher H (1998) Occurrence and distribution of three gobiid species (Pisces, Gobiidae) in the middle and upper Danube region–examples of different dispersal patterns? Biologia 53(5):665–678
Augustyniak M, Kołacka K, Kobak J, Hliwa P, Kłosiński P, Poznańska-Kakareko M, Jermacz Ł, Kakareko T (2022) Differences in predator-avoidance behavior between two invasive gobies and their native competitors. Current Zool 82:456. https://doi.org/10.1093/cz/zoac082
Baer J, Hartmann F, Brinker A (2017) Invasion strategy and abiotic activity triggers for non-native gobiids of the River Rhine. PLoS ONE 12(9):e0183769. https://doi.org/10.1371/journal.pone.0183769
Balon EK (1990) Epigenesis of an epigeneticist: the development of some alternative concepts on the early ontogeny and evolution of fishes. Guelph Ichthyol Rev 1:1–48
Bij de Vaate A, Jazdzewski K, Ketelaars HA, Gollasch S, Van der Velde G (2002) Geographical patterns in range extension of Ponto–Caspian macroinvertebrate species in Europe. Can J Fish Aquat Sci 59(7):1159–1174. https://doi.org/10.1139/f02-098
Biró P (1972) Neogobius fluviatilis in Lake Balaton–a Ponto–Caspian goby new to the fauna of Central Europe. J Fish Biol 4:249–255. https://doi.org/10.1111/j.1095-8649.1972.tb05671.x
Błońska D, Grabowska J, Kobak J, Jermacz Ł, Bącela-Spychalska K (2015) Feeding preferences of an invasive Ponto–Caspian goby for native and non-native gammarid prey. Freshwater Biol 60(10):2187–2195. https://doi.org/10.1111/fwb.12647
Błońska D, Kobak J, Kakareko T, Grabowska J (2016) Can the presence of alien Ponto–Caspian gobies affect shelter use by the native European bullhead? Aquat Ecol 50(4):653–665. https://doi.org/10.1007/s10452-016-9584-1
Błońska D, Kobak J, Grabowska J (2017) Shelter competition between the invasive western tubenose goby and the native stone loach is mediated by sex. J Limnol 76(2):221–229. https://doi.org/10.4081/jlimnol.2016.1557
Borcherding J, Staas S, Krüger S, Ondračková M, Šlapanský L, Jurajda P (2011) Non-native Gobiid species in the lower River Rhine (Germany): recent range extensions and densities. J Appl Ichthyol 27(1):153–155. https://doi.org/10.1111/j.1439-0426.2010.01662.x
Borcherding J, Hertel A, Breiden S (2013a) Activity and competitive behaviour of invasive Neogobius melanostomus and Ponticola kessleri (Gobiidae) from the River Rhine. Germany Ethol Ecol Evol 25(4):351–365. https://doi.org/10.1080/03949370.2013.806361
Borcherding J, Dolina M, Heermann L, Knutzen P, Krüger S, Matern S, van Treeck R, Gertzen S (2013b) Feeding and niche differentiation in three invasive gobies in the Lower Rhine Germany. Limnologica 43(1):49–58. https://doi.org/10.1016/j.limno.2012.08.003
Borcherding J, Heubel K, Storm S (2019) Competition fluctuates across years and seasons in a 6-species-fish community: empirical evidence from the field. Rev Fich Biol Fish 29(3):589–604. https://doi.org/10.1007/s11160-019-09567-x
Borcherding J, Aschemeier D, Bruhy J, Heermann L, Lindner J, Schröder SL, Wagner K, Staas S (2021) The Caucasian dwarf goby, a new alien Gobiidae spreading at the lower Rhine Germany. J Appl Ichthyol 37(3):479–482. https://doi.org/10.1111/jai.14196
Borza P, Erős T, Oertel N (2009) Food resource partitioning between two invasive gobiid species (Pisces, Gobiidae) in the littoral zone of the River Danube Hungary. Int Rev Hydrobiol 94(5):609–621. https://doi.org/10.1002/iroh.200911134
Brandner J, Auerswald K, Cerwenka AF, Schliewen UK, Geist J (2013) Comparative feeding ecology of invasive Ponto–Caspian gobies. Hydrobiologia 703(1):113–131. https://doi.org/10.1007/s10750-012-1349-9
Čápová M, Zlatnická I, Kováč V, Katina S (2008) Ontogenetic variability in the external morphology of monkey goby, Neogobius fluviatilis (Pallas, 1814) and its relevance to invasion potential. Hydrobiologia 607(1):17–26. https://doi.org/10.1007/s10750-008-9361-9
Cartwright A, Gebauer R, Vanina T, Stejskal V, Drozd B (2019) Shelter competition between mature non-indigenous western tubenose goby (Proterorhinus semilunaris) and immature invasive round goby (Neogobius melanostomus) for plants and rocks. Biol Invasions 21(8):2723–2734. https://doi.org/10.1007/s10530-019-02006-9
Castorani MCN, Hovel KA (2015) Invasive prey indirectly increase predation on their native competitors. Ecology 96(7):1911–1922. https://doi.org/10.1890/14-1538.1
Cerwenka AF, Brandner J, Schliewen U, Geist J (2018) Population trends of invasive alien gobies in the upper Danube River: 10 years after first detection of the globally invasive round goby (Neogobius melanostomus). Aquat Invasions 13(4):525–535. https://doi.org/10.3391/ai.2018.13.4.10
Charlebois PM, Corkum LD, Jude DJ, Knight C (2001) The round goby (Neogobius melanostomus) invasion: current research and future needs. J Great Lakes Res 27(3):263–266. https://doi.org/10.1016/S0380-1330(01)70641-7
Copp GH, Kováč V, Zweimüller I, Dias A, Nascimento M, Balážová M (2008) Preliminary study of dietary interactions between invading Ponto–Caspian gobies and some native fish species in the River Danube near Bratislava (Slovakia). Aquat Invasions 3(2):193–200. https://doi.org/10.3391/ai.2008.3.2.10
David P, Thebault E, Anneville O, Duyck PF, Chapuis E, Loeuille N (2017) Impacts of invasive species on food webs: a review of empirical data. Adv Ecol Res 56:1–60. https://doi.org/10.1016/bs.aecr.2016.10.001
Didenko AV (2013) Gobiids of the Dniprodzerzhynsk reservoir (Dnieper River, Ukraine): distribution and habitat preferences. Acta Ichthyol Piscat 43(4):257–266. https://doi.org/10.3750/AlP2013.43.4.01
Dorenbosch M, Kessel NV, Liefveld W, Schoor M, Velde G, Leuven RS (2017) Application of large wood in regulated riverine habitats facilitates native fishes but not invasive alien round goby (Neogobius melanostomus). Aquat Invasions 12(3):405–413. https://doi.org/10.3391/ai.2017.12.3.13
Emde S, Rueckert S, Kochmann J, Knopf K, Sures B, Klimpel S (2014) Nematode eel parasite found inside acanthocephalan cysts—a “Trojan horse” strategy? Parasite Vector 7:504. https://doi.org/10.1186/s13071-014-0504-8
Erős T, Sevcsik A, Tóth B (2005) Abundance and night-time habitat use patterns of Ponto–Caspian gobiid species (Pisces, Gobiidae) in the littoral zone of the River Danube Hungary. J Appl Ichthyol 21(4):350–357. https://doi.org/10.1111/j.1439-0426.2005.00689.x
Gaye-Siessegger J, Bader S, Haberbosch R, Brinker A (2022) Spread of invasive Ponto–Caspian gobies and their effect on native fish species in the Neckar River (South Germany). Aquat Invasions 17(2):207–223. https://doi.org/10.3391/ai.2022.17.2.05
Gebauer R, Veselý L, Vanina T, Buřič M, Kouba A, Drozd B (2019) Prediction of ecological impact of two alien gobiids in habitat structures of differing complexity. Can J Fish Aquat Sci 76(11):1954–1961. https://doi.org/10.1139/cjfas-2018-034
Gendron AD, Marcogliese DJ (2017) Enigmatic decline of a common fish parasite (Diplostomum spp.) in the St. Lawrence River: evidence for a dilution effect induced by the invasive round goby. Int J Parasitol Parasites Wildl 6:402–411. https://doi.org/10.1016/j.ijppaw.2017.04.002
Gendron AD, Marcogliese DJ, Thomas M (2012) Invasive species are less parasitized than native competitors, but for how long? The case of round goby in the Great Lakes-St. Lawrence Basin. Biol Invasions 14:367–384. https://doi.org/10.1007/s10530-011-0083-y
Gertzen S, Fidler A, Kreische F, Kwabek L, Schwamborn V, Borcherding J (2016) Reproductive strategies of three invasive Gobiidae co-occurring in the Lower Rhine (Germany). Limnologica 56:39–48. https://doi.org/10.1016/j.limno.2015.10.005
Grabowska J, Grabowski M (2005) Diel-feeding activity in early summer of racer goby Neogobius gymnotrachelus (Gobiidae): a new invader in the Baltic basin. J Appl Ichthyol 21(4):282–286. https://doi.org/10.1111/j.1439-0426.2005.00676.x
Grabowska J, Przybylski M (2014) Life-history traits of non-native freshwater fish invaders differentiate them from natives in the Central European bioregion. Rev Fish Biol Fish 25(1):165–178. https://doi.org/10.1007/s11160-014-9375-5
Grabowska J, Pietraszewski D, Ondračková M (2008) Tubenose goby Proterorhinus marmoratus (Pallas, 1814) has joined three other Ponto–Caspian gobies in the Vistula River (Poland). Aquat Invasions 3(2):261–265. https://doi.org/10.3391/ai.2008.3.2.20
Grabowska J, Grabowski M, Kostecka A (2009) Diet and feeding habits of monkey goby (Neogobius fluviatilis) in a newly invaded area. Biol Invasions 11(9):2161–2170. https://doi.org/10.1007/s10530-009-9499-z
Grabowska J, Kakareko T, Błońska D, Przybylski M, Kobak J, Copp GH (2016) Interspecific competition for a shelter between non-native racer goby and native European bullhead under experimental conditions–effects of season, fish size and light conditions. Limnologica 56:30–38. https://doi.org/10.1016/j.limno.2015.11.004
Grabowska J, Tarkan AS, Błońska D, Karakuş NT, Janic B, Przybylski M (2021) Prolific pioneers and reserved settlers. Changes in the life-history of the western tubenose goby (Proterorhinus semilunaris) at different invasion stages. Sci Total Environ 750:142316. https://doi.org/10.1016/j.scitotenv.2020.142316
Grabowski M, Bacela K, Konopacka A, Jazdzewski K (2009) Salinity-related distribution of alien amphipods in rivers provides refugia for native species. Biol Invasions 11(9):2107–2117. https://doi.org/10.1007/s10530-009-9502-8
Gutowsky LFG, Fox MG (2012) Intra-population variability of life-history traits and growth during range expansion of the invasive round goby Neogobius melanostomus. Fish Manag Ecol 19(1):78–88. https://doi.org/10.1111/j.1365-2400.2011.00831.x
Haertl M, Cerwenka AF, Brandner J, Borcherding J, Geist J, Schliewen UK (2012) First record of Babka gymnotrachelus (Kessler, 1857) from Germany. Spixiana 35(1):155–159
Hohenadler MAA, Nachev M, Thielen F, Taraschewski H, Grabner D, Sures B (2018) Pomphorhynchus laevis: an invasive species in the river Rhine? Biol Invasions 20(1):207–217. https://doi.org/10.1007/s10530-017-1527-9
Hohenadler MAA, Nachev M, Freese M, Pohlmann JD, Hanel R, Sures B (2019) How Ponto–Caspian invaders affect local parasite communities of native fish. Parasitol Res 118:2543–2555. https://doi.org/10.1007/s00436-019-06399-3
Hôrková K, Kováč V (2013) Different life-histories of native and invasive Neogobius melanostomus and the possible role of phenotypic plasticity in the species’ invasion success. Knowl Manag Aquat Ecosyst. https://doi.org/10.1051/kmae/2013081
Huyse T, Vanhove MPM, Mombaerts M, Volckaert FAM, Verreycken H (2015) Parasite introduction with an invasive goby in Belgium: double trouble? Parasitol Res 114:2789–2793. https://doi.org/10.1007/s00436-015-4544-6
Jakovlić I, Piria M, Šprem N, Tomljanović T, Matulić D, Treer T (2015) Distribution, abundance and condition of invasive Ponto–Caspian gobies Ponticola kessleri (Günther, 1861), Neogobius fluviatilis (Pallas, 1814), and Neogobius melanostomus (Pallas, 1814) in the Sava River basin Croatia. J Appl Ichthyol 31(5):888–894. https://doi.org/10.1111/jai.12803
Jakubčinová K, Simonović P, Števove B, Čanak Atlagić J, Kováč V (2017) What can morphology tell us about ecology of four invasive goby species? J Fish Biol 90(5):1999–2019. https://doi.org/10.1111/jfb.13283
Janáč M, Valová Z, Jurajda P (2012) Range expansion and habitat preferences of non-native 0+ tubenose goby (Proterorhinus semilunaris) in two lowland rivers in the Danube basin. Fund Appl Limnol 181:73–85. https://doi.org/10.1127/1863-9135/2012/0321
Janáč M, Valová Z, Roche K, Jurajda P (2016) No effect of round goby Neogobius melanostomus colonisation on young-of-the-year fish density or microhabitat use. Biol Invasions 18(8):2333–2347. https://doi.org/10.1007/s10530-016-1165-7
Janáč M, Roche K, Šlapanský L, Polačik M, Jurajda P (2018) Long-term monitoring of native bullhead and invasive gobiids in the Danubian rip-rap zone. Hydrobiologia 807(1):263–275. https://doi.org/10.1007/s10750-017-3398-6
Jażdżewski K, Konopacka A, Grabowski M (2002) Four Ponto–Caspian and one American gammarid species (Crustacea, Amphipoda) recently invading Polish waters. Contrib Zool 71(4):115–122. https://doi.org/10.1163/18759866-07104001
Jermacz Ł, Kobak J, Dzierżyńska A, Kakareko T (2015) The effect of flow on the competition between the alien racer goby and native European bullhead. Ecol Freshw Fish 24(3):467–477. https://doi.org/10.1111/eff.12162
Jude DJ, DeBoe SF (1996) Possible impact of gobies and other introduced species on habitat restoration efforts. Can J Fish Aquat Sci 53(S1):136–141. https://doi.org/10.1139/f96-001
Jurajda P, Černý J, Polačik M, Valová Z, Janáč M, Blažek R, Ondračková M (2005) The recent distribution and abundance of non-native Neogobius fishes in the Slovak section of the River Danube. J Appl Ichthyol 21(4):319–323. https://doi.org/10.1111/j.1439-0426.2005.00688.x
Kakareko T (2011) Wpływ wybranych czynników na rozmieszczenie ipreferencje siedliskowe babkiłysej (Neogobius gymnotrachelus Kessler,1857) i babki szczupłej (Neogobius fluviatilis Pallas, 1811), obcych gatunków ryb w Polsce [The impact of selected factors on the distribution and habitat preferences of the racer goby (Neogobius gymnotrachelus Kessler, 1857) and the monkey goby (Neogobius fluviatilis Pallas, 1811), non-native fish species in Poland]. Wydawnictwo Naukowe Uniwersytetu Mikołaja Kopernika, Toruń, Poland
Kakareko T, Żbikowski J, Żytkowicz J (2005) Diet partitioning in summer of two syntopic neogobiids from two different habitats of the lower Vistula River Poland. J Appl Ichthyol 21(4):292–295. https://doi.org/10.1111/j.1439-0426.2005.00683.x
Kakareko T, Kobak J, Grabowska J, Jermacz Ł, Przybylski M, Poznańska M, Pietraszewski D, Copp GH (2013) Competitive interactions for food resources between invasive racer goby Babka gymnotrachelus and native European bullhead Cottus gobio. Biol Invasions 15(11):2519–2530. https://doi.org/10.1007/s10530-013-0470-7
Kakareko T, Kobak J, Poznańska M, Jermacz Ł, Copp GH (2016) Underwater evaluation of habitat partitioning in a European river between a non-native invader, the racer goby and a threatened native fish, the European bullhead. Ecol Freshw Fish 25(1):60–71. https://doi.org/10.1111/eff.12191
Kelly DW, Patterson RA, Townsend CR, Poulin R, Tompkins DM (2009) Parasite spillback: a neglected concept in invasion ecology? Ecology 90:2047–2056. https://doi.org/10.1890/08-1085.1
Kessel NV, Dorenbosch M, Boer MD, Leuven RSEW, Velde GVD (2011) Competition for shelter between four invasive gobiids and two native benthic fish species. Curr Zool 57(6):844–851. https://doi.org/10.1093/czoolo/57.6.844
Kessel NV, Dorenbosch M, Kranenbarg J, van der Velde G, Leuven RSEW (2016) Invasive Ponto–Caspian gobies rapidly reduce the abundance of protected native bullhead. Aquat Invasions 11(2):179–188. https://doi.org/10.3391/ai.2016.11.2.07
Kłosiński P, Kobak J, Augustyniak M, Pawlak R, Jermacz Ł, Poznańska-Kakareko M, Kakareko T (2022) Behavioural responses to con- and heterospecific alarm cues by an alien and a coexisting native fish. Hydrobiologia 849:985–1000. https://doi.org/10.1007/s10750-021-04761-0
Knaepkens G, Bruyndoncx L, Bervoets L, Eens M (2002) The presence of artificial stones predicts the occurrence of the European bullhead (Cottus gobio) in a regulated lowland river in Flanders (Belgium). Ecol Freshw Fish 11(3):203–206. https://doi.org/10.1034/j.1600-0633.2002.00013.x
Kocovsky PM, Tallman JA, Jude DJ, Murphy DM, Brown JE, Stepien CA (2011) Expansion of tubenose gobies Proterorhinus semilunaris into western Lake Erie and potential effects on native species. Biol Invasions 13(12):2775–2784. https://doi.org/10.1007/s10530-011-9962-5
Kornis MS, Mercado-Silva N, Vander Zanden MJ (2012) Twenty years of invasion: a review of round goby Neogobius melanostomus biology, spread and ecological implications. J Fish Biol 2:235–285. https://doi.org/10.1111/j.1095-8649.2011.03157.x
Kostrzewa J, Grabowski M (2003) Opportunistic feeding strategy as a factor promoting the expansion of racer goby (Neogobius gymnotrachelus Kessler, 1857) in the Vistula basin. Lauterbornia 48:91–100
Koubková B, Baruš V (2000) Metazoan parasites of the recently established tubenose goby (Proterorhinus marmoratus: Gobiidae) population from the South Moravian reservoir Czech Republic. Helminthologia 37(2):89–95
Kovac V, Copp GH, Sousa RP (2009) Life-history traits of invasive bighead goby Neogobius kessleri (Gunther, 1861) from the middle Danube River, with a reflection on which goby species may win the competition. J Appl Ichthyol 25(1):33. https://doi.org/10.1111/j.1439-0426.2009.01189.x
Kuchta R, Choudhury A, Scholz T (2018) Asian fish tapeworm: the most successful invasive parasite in freshwaters. Trends Parasitol 34(6):511–523. https://doi.org/10.1016/j.pt.2018.03.001
Kukuła K, Ortyl B, Bylak A (2019) Habitat selection patterns of a species at the edge–case study of the native racer goby population in Central Europe. Sci Rep 9(1):1–11. https://doi.org/10.1038/s41598-019-56264-7
Kulhanek SA, Ricciardi A, Leung B (2011) Is invasion history a useful tool for predicting the impacts of the world’s worst aquatic invasive species? Ecol Appl 21(1):189–202. https://doi.org/10.1890/09-1452.1
Kvach Y, Mierzejewska K (2011) Non-indigenous benthic fishes as new hosts for Bucephalus polymorphus Baer, 1827 (Digenea: Bucephalidae) in the Vistula River basin Poland. Knowl Manag Aquat Ecosyst 400:02. https://doi.org/10.1051/kmae/2010034
Kvach Y, Ondračková M (2020) Checklist of parasites for Ponto–Caspian gobies (Actinopterygii: Gobiidae) in their native and non-native ranges. J Appl Ichthyol 36:472–500. https://doi.org/10.1111/jai.14036
Kvach Y, Stepien CA (2008) Metazoan parasites of introduced round and tubenose gobies in the Great Lakes: support for the “enemy release hypothesis.” J Great Lakes Res 34:23–35. https://doi.org/10.3394/0380-1330(2008)34[23:MPOIRA]2.0.CO;2
Kvach Y, Kornyychuk Y, Mierzejewska K, Rubtsova N, Yurakhno V, Grabowska J, Ovcharenko M (2014) Parasitization of invasive gobiids in the eastern part of the Central trans-European corridor of invasion of Ponto–Caspian hydrobionts. Parasitol Res 113:1605–1624. https://doi.org/10.1007/s00436-014-3791-2
Kvach Y, Boldyrev V, Lohner R, Stepien CA (2015) The parasite community of gobiid fishes (Actinopterygii: Gobiidae) from the Lower Volga River region. Biologia 70(7):948–957. https://doi.org/10.1515/biolog-2015-0108
Kvach Y, Zamorov V, Pupins M (2021) Review of invasive Ponto–Caspian gobiids: current range and history of expansion. Daugavpils University Academic Press Saule, Daugavpils, p 92
Lively CM, Dybdahl MF (2000) Parasite adaptation to local host genotypes. Nature 405:679–681. https://doi.org/10.1038/35015069
Lowe S, Browne M, Boudjelas S, De Poorter M (2000) 100 of the World’s worst invasive alien species a selection from the global invasive species database. Published by The Invasive Species Specialist Group (ISSG) a Specialist Group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN), First published as special lift-out in Aliens 12, December 2000. Updated and reprinted version Nov 2004, pp 12
Manné S, Poulet N, Dembski S (2013) Colonisation of the Rhine basin by non-native gobiids: an update of the situation in France. Knowl Manag Aquat Ecosyst 411:02. https://doi.org/10.1051/kmae/2013069
Masson L, Masson G, Beisel JN, Gutowsky LFG, Fox MG, MacIsaac H (2018) Consistent life history shifts along invasion routes? An examination of round goby populations invading on two continents. Divers Distrib 24(6):841–852. https://doi.org/10.1111/ddi.12726
Mierzejewska K, Kvach Y, Stańczak K, Grabowska J, Woźniak M, Dziekońska-Rynko J, Ovcharenko M (2014) Parasites of non-native gobies in the Włocławek Reservoir on the lower Vistula River, first comprehensive study in Poland. Knowl Manag Aquat Ecosyst 414:01. https://doi.org/10.1051/kmae/2014011
Mikl L, Adámek Z, Všetičková L, Janáč M, Roche K, Šlapanský L, Jurajda P (2017a) Response of benthic macroinvertebrate assemblages to round (Neogobius melanostomus, Pallas 1814) and tubenose (Proterorhinus semilunaris, Heckel 1837) goby predation pressure. Hydrobiologia 785(1):219–232. https://doi.org/10.1007/s10750-016-2927-z
Mikl L, Adámek Z, Roche K, Všetičková L, Šlapanský L, Jurajda P (2017b) Invasive Ponto–Caspian gobies in the diet of piscivorous fish in a European lowland river. Fund Appl Limnol 190(2):157–171. https://doi.org/10.1127/fal/2017/1024
Miller PJ (1984) The tokology of gobioid fishes. In: Potts GW, Wootton RJ (eds) Fish reproduction: strategies and tactics. Academic Press INC, London, pp 119–153
Miller PJ (2003) The freshwater fishes of Europe. Vol. 8/I Mugilidae, Atherinidae, Atherionopsidae, Blennidae, Odontobutidae Gobii–dae 1. AULA-Verlag, Wiesbaden, Germany
Mills CA, Mann RHK (1983) The bullhead Cottus gobio, a versatile and successful fish. Freshwater Biological Association, Ambleside, pp 76–88
Mirzajani A, Naderi S, Ganeh A, Hadipour E, Salahi M, Javidpour J (2021) Trophic flexibility of Eurasian otter (Lutra lutra) in Anzali Wetland, Iran, assessed by fecal and stable isotope analysis. Aquat Ecol 55:401–415. https://doi.org/10.1007/s10452-021-09832-x
Molnár K (2006) Some remarks on parasitic infections of the invasive Neogobius spp. (Pisces) in the Hungarian reaches of the Danube River, with a description of Goussia szekelyi sp. n. (Apicomplexa: Eimeriidae). J Appl Ichtohyol 22:1–6. https://doi.org/10.1111/j.1439-0426.2006.00742.x
Mueller M, Pander J, Geist J (2018) Comprehensive analysis of> 30 years of data on stream fish population trends and conservation status in Bavaria, Germany. Biol Conserv 226:311–320. https://doi.org/10.1016/j.biocon.2018.08.006
Nogueira Tavares C, Brauns M, Hille S, Krenek S, Borcherding J, Weitere M (2020) Tracing the colonization process of non-native gobies into a large river: the relevance of different dispersal modes. Biol Invasions 22(8):2421–2429. https://doi.org/10.1007/s10530-020-02281-x
Noonburg EG, Byers JE (2005) More harm than good: when invader vulnerability to predators enhances impact on native species. Ecology 86(10):2555–2560
Ondračková M (2016) Gyrodactylus proterorhini in its non-native range: distribution and ability to host-switch in freshwaters. Parasitol Res 115:3153–3162. https://doi.org/10.1007/s00436-016-5073-7
Ondračková M, Dávidová M, Blažek R, Gelnar M, Jurajda P (2009) The interaction between an introduced fish host and local parasite fauna: Neogobius kessleri in the Middle Danube River. Parasitol Res 105:201–208. https://doi.org/10.1007/s00436-009-1384-2
Ondračková M, Šimková A, Civáňová K, Vyskočilová M, Jurajda P (2012) Parasite diversity and microsatellite variability in native and introduced populations of four Neogobius species (Gobiidae). Parasitology 139:1493–1505. https://doi.org/10.1017/S0031182012000844
Ondračková M, Valová Z, Hudcová I, Michálková V, Šimková A, Borcherding J, Jurajda P (2015a) Temporal effects on host-parasite associations in four naturalized goby species living in sympatry. Hydrobiologia 746:233–243. https://doi.org/10.1007/s10750-014-1967-5
Ondračková M, Hudcová I, Dávidová M, Adámek Z, Kašný M, Jurajda P (2015b) Non-native gobies facilitate the transmission of Bucephalus polymorphus (Trematoda). Parasite Vector 8:382. https://doi.org/10.1186/s13071-015-0999-7
Ondračková M, Všetičková L, Adámek Z, Kopeček L, Jurajda P (2019) Ecological plasticity of tubenose goby, a small invader in South Moravian waters. Hydrobiologia 829:217–235. https://doi.org/10.1007/s10750-018-3833-3
Ondračková M, Janáč M, Borcherding J, Grabowska J, Bartáková V, Jurajda P (2021) Non-native gobies share predominantly immature parasites with local fish hosts. J Vertebr Biol 70(4):21050. https://doi.org/10.25225/jvb.21050
Palstra AP, Heppener DFM, Van Ginneken VJT, Székely C, Van den Thillart GEEJM (2007) Swimming performance of silver eels is severely impaired by the swim-bladder parasite Anguillicola crassus. J Exp Mar Biol Ecol 352(1):244–256. https://doi.org/10.1016/j.jembe.2007.08.003
Panov VE, Alexandrov B, Arbačiauskas K, Binimelis R, Copp GH, Grabowski M, Lucy F, Leuven RS, Nehring S, Paunović M, Semenchenko V, Son MO (2009) Assessing the risks of aquatic species invasions via European inland waterways: from concepts to environmental indicators. Integr Environ Assess Manag 5:110–126. https://doi.org/10.1897/IEAM_2008-034.1
Piria M, Jakšić G, Jakovlić I, Treer T (2016) Dietary habits of invasive Ponto–Caspian gobies in the Croatian part of the Danube River basin and their potential impact on benthic fish communities. Sci Total Environ 540:386–395. https://doi.org/10.1016/j.scitotenv.2015.05.125
Plachá M, Balážová M, Kováč V, Katina S (2010) Age and growth of non-native monkey goby Neogobius fluviatilis (Teleostei, Gobiidae) in the River Ipeľ Slovakia. Folia Zool 59(4):332–340. https://doi.org/10.25225/fozo.v59.i4.a10.2010
Płąchocki D (2017) Preferencje siedliskowe i rozmieszczenie babkirurkonosej (Proterorhinus semilunaris) w płytkowodnych środowiskach dolnej Wisły [habitat preferences and distribution of the tubenose goby (Proterorhinus semilunaris) in shallow waters of the lower Vistula River] Unpublished doctoral dissertation. Nicolaus Copernicus University, Toruń, Poland
Płąchocki D, Kobak J, Kakareko T (2012) First report on the importance of alien gobiids in the diet of native piscivorous fishes in the lower Vistula River (Poland). Oceanol Hydrobiol Stud 41(2):83–89. https://doi.org/10.2478/s13545-012-0020-4
Płąchocki D, Kobak J, Poznańska-Kakareko M, Kakareko T (2020) Environmental factors associated with the occurrence of the Ponto–Caspian gobies in a lowland river belonging to the central European invasion corridor. River Res Appl 36(1):25–35. https://doi.org/10.1002/rra.3543
Polačik M, Janác M, Trichkova T, Vassilev M, Keckeis H, Jurajda P (2008) The distribution and abundance of the Neogobius fishes in their native range (Bulgaria) with notes on the non-native range in the Danube River. Acta Zool Bulg 60:77–88. https://doi.org/10.1127/lr/18/2008/193
Polačik M, Janáč M, Jurajda P, Adámek Z, Ondračková M, Trichkova T, Vassilev M (2009) Invasive gobies in the Danube: invasion success facilitated by availability and selection of superior food resources. Ecol Freshw Fish 18(4):640–649. https://doi.org/10.1111/j.1600-0633.2009.00383.x
Poulin R, Mouillot D (2003) Host introductions and the geography of parasite taxonomic diversity. J Biogeogr 30:837–845. https://doi.org/10.1046/j.1365-2699.2003.00868.x
Prenter J, MacNeil C, Dick JTA, Dunn AM (2004) Roles of parasites in animal invasions. Trends Ecol Evol 19:385–390. https://doi.org/10.1016/j.tree.2004.05.002
Pronin NM, Fleischer GW, Baldanova DR, Pronina SV (1997) Parasites of the recently established round goby (Neogobius melanostomus) and tubenose goby (Proterorhinus marmoratus) (Gobiidae) from the St. Clair River and Lake St. Clair, Michigan, USA. Folia Parasit 44:1–6
Rakauskas V, Virbickas T, Skrupskelis K, Kesminas V (2018) Delayed expansion of Ponto–Caspian gobies (Pisces, Gobiidae, Benthophilinae) in the Nemunas River drainage basin, the northern branch of the central European invasion corridor. BioInvasions Rec 7(2):143–152. https://doi.org/10.3391/bir.2018.7.2.05
Ramler D, Keckeis H (2019) Occurrence of non-native fishes in the Danube east of Vienna (Austria) and potential interactions of invasive gobiids with native fishes. J Appl Ichthyol 35(4):850–862. https://doi.org/10.1111/jai.13916
Roche KF, Janač M, Jurajda P (2013) A review of Gobiid expansion along the Danube-Rhine corridor–geopolitical change as a driver for invasion. Knowl Manag Aquat Ecosyst 411:01. https://doi.org/10.1051/kmae/2013066
Roche K, Šlapanský L, Travnik M, Janáč M, Jurajda P (2021) The importance of rip-rap for round goby invasion success–a field habitat manipulation experiment. J Vertebr Biol 70(4):21052. https://doi.org/10.25225/jvb.21052
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Weller SG (2001) The population biology of invasive species. Annu Rev Ecol Syst 32:305–332
Simberloff D, Von Holle B (1999) Positive interactions of non-indigenous species: invasional meltdown? Biol Invasions 1:21–32. https://doi.org/10.1023/A:1010086329619
Sindilariu PD, Freyhof J, Wolter C (2006) Habitat use of juvenile fish in the lower Danube and the Danube Delta: implications for ecotone connectivity. Hydrobiologia 571(1):51–61. https://doi.org/10.1007/s10750-006-0216-y
Šlapanský L, Jurajda P, Janáč M (2016) Early life stages of exotic gobiids as new hosts for unionid glochidia. Freshw Biol 61:979–990. https://doi.org/10.1111/fwb.12761
Šlapanský L, Janáč M, Roche K, Mikl L, Jurajda P (2017) Expansion of round gobies in a non-navigable river system. Limnologica 67:27–36. https://doi.org/10.1016/j.limno.2017.09.001
Slynko YV, Kiyashko VI (2012) Analysis of effectiveness of pelagic fish species invasions into the Volga River reservoirs. Russ J Biol Invasions 3(2):129–138. https://doi.org/10.1134/S2075111712020099
Specziár A (2011) Size-dependent prey selection in piscivorous pikeperch Sander lucioperca and Volga pikeperch Sander volgensis shaped by bimodal prey size distribution. J Fish Biol 79(7):1895–1917. https://doi.org/10.1111/j.1095-8649.2011.03127.x
Števove B, Kováč V (2013) Do invasive bighead goby Neogobius kessleri and round goby N. melanostomus (Teleostei, Gobiidae) compete for food? Knowl Manag Aquat Ecosyst 410:08. https://doi.org/10.1051/kmae/2013064
Švolíková KS, Števove B, Križek P, Mosná P, Fedorčák J, Kováč V (2021) Tubenose goby–a discreet invader from the past goes higher. J Vertebr Biol 70(4):21042. https://doi.org/10.25225/jvb.21042
Szalóky Z, Bammer V, György ÁI, Pehlivanov L, Schabuss M, Weiperth A, Erős T (2015) Offshore distribution of invasive gobies (Pisces: Gobiidae) along the longitudinal profile of the Danube River. Fund Appl Limnol 187(2):127–133. https://doi.org/10.1127/fal/2015/0768
Tarján ZL, Pénzes JJ, Tóth RP, Benkő M (2014) First detection of circovrus-like sequences in amphibians and novel putative circoviruses in fishes. Acta Vet Hung 62(1):134–144. https://doi.org/10.1556/avet.2013.061
Teletchea F, Beisel J-N (2018) Alien fish species in France with emphasis on the recent invasion of gobies. In: Sajal R (ed) Biological resources of water. IntechOpen, London, pp 75–92
Tomlinson ML, Perrow MR (2003) Ecology of the Bullhead. Conserving Natura 2000 rivers ecology series no. 4. English Nature, Peterborough
Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM (2003) Introduced species and their missing parasites. Nature 421:628–630. https://doi.org/10.1038/nature01346
Ulikowski D, Traczuk P, Kapusta A, Kalinowska K (2021) New records of alien monkey goby, Neogobius fluviatilis (Pallas, 1814), in the waters of the Great Masurian Lakes system (north-eastern Poland). BioInvasions Rec 10:924–933. https://doi.org/10.3391/bir.2021.10.4.17
Van Riel MC, Van der Velde G, Bij de Vaate A (2006) To conquer and persist: colonization and population development of the Ponto–Caspian amphipods Dikerogammarus villosus and Chelicorophium curvispinum on bare stone substrate in the main channel of the River Rhine. Arch Hydrobiol 166(1):23–40. https://doi.org/10.1127/0003-9136/2006/0166-0023
Vašek M, Všetičková L, Roche K, Jurajda P (2014) Diet of two invading gobiid species (Proterorhinus semilunarisand Neogobius melanostomus) during the breeding and hatching season: no field evidence of extensive predation on fish eggs and fry. Limnologica 46:31–36. https://doi.org/10.1016/j.limno.2013.11.003
Vasil’eva ED, Vasil’ev VP (2003) Neogobius kessleri (Günther, 1861). In: Miller PE (ed) The freshwater fishes of Europe Mugilidae, Atherinidae, Atherinopsidae, Blenniidae, Odontobutidae, Gobiidae. AULA-Verlag, Wiesbaden, Germany, pp 280–292
Venable CP, Adams TS, Langkilde T (2019) Aversion learning in response to an invasive venomous prey depends on stimulus strength. Biol Invasions 21(6):1973–1980. https://doi.org/10.1007/s10530-019-01949-3
Vilizzi L, Copp GH, Adamovich B, Almeida D, Chan J, Davison PI, Zeng Y (2019) A global review and meta-analysis of applications of the freshwater Fish Invasiveness Screening Kit. Rev Fish Biol Fisher 29(3):529–568. https://doi.org/10.1007/s11160-019-09562-2
Vilizzi L, Copp GH, Hill JE, Adamovich B, Aislabie L, Akin D, Semenchenko V (2021) A global-scale screening of non-native aquatic organisms to identify potentially invasive species under current and future climate conditions. Sci Total Environ 788:147868. https://doi.org/10.1016/j.scitotenv.2021.147868
Vitál Z, Boross N, Czeglédi I, Priszner B, Erős T, Molnár K, Cech G, Székely C, Sándor D, Takács P (2021) First genetically verified occurrence of Ligula pavlovskii outside its native range and characteristics of its infection in Neogobius fluviatilis. J Great Lakes Res 47(1):236–241. https://doi.org/10.1016/j.jglr.2020.10.008
Von Landwust C (2006) Expansion of Proterorhinus marmoratus (Teleostei, Gobiidae) into the River Moselle (Germany). Folia Zool 55(1):107–111
Všetičková L, Janáč M, Vašek M, Roche K, Jurajda P (2014) Non-native western tubenose gobies Proterorhinus semilunaris show distinct site, sex and age-related differences in diet. Knowl Manag Aquat Ecosyst 414:10. https://doi.org/10.1051/kmae/2014022
Všetičková L, Mikl L, Adámek Z, Prášek V, Roche K, Jurajda P (2018) The diet of reservoir perch before, during and after establishment of non-native tubenose goby. Knowl Manag Aquat Ecosyst 419:4. https://doi.org/10.1051/kmae/2017052
Wiesner C (2005) New records of non-indigenous gobies (Neogobius spp.) in the Austrian Danube. J Appl Ichthyol 21(4):324–327. https://doi.org/10.1111/j.1439-0426.2005.00681.x
Wziątek B, Martyniak A, Stańczak K, Hliwa P (2010) Presja kormorana czarnego (Phalacrocorax carbo sinensis L., 1758) na ichtiofaunę Zbiornika Włocławskiego i gospodarkę rybacko-wędkarską w latach 2005–2009. [The impact of cormorant (Phalacrocorax carbo sinensis L., 1758) on the fish fauna and the economy of fishing and angling in the Włocławek Reservoir in 2005−2009]. Komun Ryb 5(118):16–19
Zaichenko NV (2015) Parazyty bychkovykh ryb v deyakykh kontynentalnykh vodnykh obyektakh. Naukovi Zapysky Ternopilsk Pedagog Univ (biologiya) 2(63):22–28
Acknowledgements
We thank anonymous reviewers for their comments that considerably improved the manuscript. We thank Prof. Carl Smith (Faculty of Biology & Environmental Protection, University of Lodz) for revising the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grabowska, J., Błońska, D., Ondračková, M. et al. The functional ecology of four invasive Ponto–Caspian gobies. Rev Fish Biol Fisheries 33, 1329–1352 (2023). https://doi.org/10.1007/s11160-023-09801-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11160-023-09801-7