Abstract
N. norvegicus supports one of the most commercially-important fisheries in the Mediterranean, and there is considerable interest in developing non-invasive sampling stock assessment methods. Currently, stock assessments are conducted via trawling or by UnderWater TeleVision (UWTV) surveys with limited capacity to provide direct population data due to the burrowing behavior of the species. Here, we used acoustic telemetry to characterize the burrow emergence and movement patterns of N. norvegicus in relation to internal tides and inertial currents in deep-sea habitats of the northwestern Mediterranean. We deployed acoustic tags on 25 adults between May and June 2019, and collected up to 4 months of detection data from each tag. Tagged lobsters spent approximately 12% of their time in door-keeping (i.e., detections at burrow entrance with no displacements) but differences existed among the different behavioral rhythms identified. We observed that mixed day-night, tidal and inertial rhythms in field settings were similar to those observed in laboratory tests. The presence of mixed day-night and tidal periodicity poses the question of why N. norvegicus exhibits flexible responses to environmental cues other than photoperiod. It is possible that tidal regulation of locomotor activity could reduce energy expenditure in relation to hydrodynamic drag. Inertial periodicity occurs in animals with no clear burrowing-oriented activity (always present on the seabed). Possibly, inertial-related movements are the result of a disruption of the biological clock. Our results are discussed in the context of how burrow emergence rhythms may bias UWTV surveys and how novel in situ monitoring approaches address these biases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Managing fishery resources in Marine Protected Areas (MPAs) requires the development of non-invasive sampling methods that, unlike trawling, do not damage habitats or remove biomass (Belgrano et al. 2021; Fernández-Chacón et al. 2021; Grinyó et al. 2022). For this reason, there is a general shift away from using trawl-based stock-assessment methods to those based on imaging (Aguzzi et al. 2022a). However, such methods will still remain limited in their capacity to provide accurate population data unless they also incorporate knowledge of how species’ spatio-temporal movements can alter their detectability (e.g., Aguzzi et al. 2022a). One way to generate this data is to use acoustic telemetry devices to track the long-term movements and behavior of species of interest (Aspillaga et al. 2019; Matley et al. 2022).
The Norway lobster (Nephrops norvegicus) supports one of the most commercially-important fisheries in the EU (STECF 2020; Aguzzi et al. 2022b; Issifu et al. 2022). As such, managing its populations is of strategic importance, and it requires tools for the efficient and reliable stock assessments (Aguzzi et al. 2022a). Currently, stock assessments in the Atlantic and the Mediterranean are conducted either via trawling or by UnderWater TeleVision (UWTV) surveys, which are cameras mounted on towed sledges (Dobby et al. 2021). However, both approaches are unable to provide direct population counts of animals due to the burrowing behavior of N. norvegicus (Aguzzi et al. 2022a). As animals can only be captured in trawls or filmed by UWTV surveys when they are outside their burrows (Aguzzi et al. 2022a), the population-level patterns of burrow emergence have a massive impact on detection of animals (Bell et al. 2006; Aguzzi and Sardà 2008; Sardà and Aguzzi 2012). Typically, individuals emerge between nighttime and crepuscular hours on upper and lower shelf and at midday on slope (e.g., Chapman and Howard 1979; Oakley 1979; Aguzzi et al. 2003). UWTVs surveys address this issue by counting burrows instead of individuals under the assumption that one animal occupies each burrow complex (Dobby et al. 2021). However, burrows may be empty or occupied by dead individuals or other opportunistic species (e.g., Aguzzi et al. 2009a; Sardà and Aguzzi 2012).
While burrow emergence behavior appears largely conserved across populations (Aguzzi et al. 2022a), there is still a lack of high-resolution temporal data (i.e., at hourly frequency, over consecutive days) of this phenomenon. To date, most information comes from laboratory experiments on the locomotor activity rhythms that sustain burrow emergence. In controlled conditions, tests on north-western Mediterranean slope (400 m depth) animals showed displacement rhythms of mixed day-night, tidal-like, and inertial-currents oriented periodicity, plus a non-negligible quantity of arrhythmic individuals (Aguzzi et al. 2004b, a; 2011; Chiesa et al. 2010; Sbragalia et al. 2015). Also, animals in the same tank showed aggressive territoriality, with dominant individuals attempting to evict subordinates from their burrows (Sbragaglia et al. 2017).
Acoustic tracking technology can be deployed for long time into an MPA, staying safe from the threat of removal from trawling, being hence a promising tool for spatio-temporally monitoring of the burrowing behavior of N. norvegicus (Masmitja et al. 2020; Vigo et al. 2021). This method can reveal the reaction of individuals to light-intensity and water current speed cycles (when moored current sensors are also provided) over consecutive days, disclosing strategic information on species life-traits. Here, our objective was to characterize the burrow emergence activity in terms of a) detection and b) displaced distances in freely motile, acoustically-tracked N. norvegicus and their relationship with internal tides and inertial currents in a north-western Mediterranean deep-sea MPA. To achieve this, we revisited and reanalyzed acoustic detection data by Vigo et al. (2021) with an individual tracking focus, related to chronobiology, to describe burrow emergence behavior as a combination of stationary door-keeping and locomotor free epibenthic wandering. We aimed at relating the periodicity of this latter behavior (which has direct impacts on stock assessments) to the environmental modulation. In particular, we focused on the occurrence of peaks in day-night and inertial (current-related) periodicity in-situ, hence providing a comparative and more reliable dynamic for two decades of laboratory experiments (with animals collected in the same depth range and geographic area).
Materials and Methods
Fieldwork procedures
We used data from 25 acoustically-tagged N. norvegicus adults that were originally sampled as a part of the study by Vigo et al. (2021) (Table 1). Briefly, animals were captured between May 30th to June 7th, 2019 by creels on the muddy bottoms of a north-western Mediterranean slope (315–475 m depth) MPA (Fig. 1). Creels were recovered at night, with deck operations conducted in dim-red light to avoid retinal damage to captured individuals (Shelton et al. 1985). Individuals were transferred in complete darkness to separate 20 × 30 cm cells in a compartmented aquarium with water at 13 °C (i.e., the temperature of the Mediterranean Sea below the 200 m thermocline; Hopkins et al. 1985).
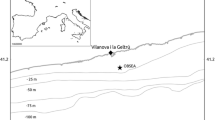
Study area in the Catalan margin (NW Mediterranean, Spain) A on the slope between two large deep-sea submarine canyons, Cap de Creus and the Fonera-Palamós. Spatial distribution of N. norvegicus is indicated as Landings Per Unit Effort (LPUE) along the Catalan coast, obtained by combining vessel monitoring system information and official landing data in the annual average data from period 2008 − 2021. The study area is represented in B indicating the location of the telemetry study inside the MPA. An enlargement of the monitoring zone is also presented in C to show the position of four moored-vertical hydrophone-receiving towers plus the CTD within the MPA. A detailed scheme for the deployed infrastructure is schematized in D to describe the open monitoring space and monitoring infrastructures at the center of which animals were released
Carapace Length (CL ± 0.05 mm) and sex (determined via the thickness of the pleopods) were recorded for all acoustically-tagged individuals (see Table 1). Acoustic transmitters were deployed just prior to their release the day after, the 8th of June 2019 (see Vigo et al. 2021 for additional details). Briefly, acoustic emitters (VEMCO V7-L1-69 k; Innovasea. 18 mm length and a weight of 0.7 gr) were glued to the ventral side of the cephalothorax. Each transmitter spent a specific frequency signal (i.e., the ping) as the Identification Number (ID), every 120 s.
Individuals were released at night at 1–2 m above the seabed, with a release-system consisting of a PVC receptacle with a dissolving latch closure on the door (see Vigo et al. 2021 for additional details). The release site was at the center of a monitoring area composed of 4 vertically moored hydrophones (VEMCO models: VR2W-69 k and VR2AR-69 k) (see Fig. 1). The 4 hydrophones were attached to their individual moorings at 20 m above the sea bottom and 150 m apart from each other, hence creating a detection radius of ~ 350 m.
During the tracking period, we measured several environmental parameters including: salinity, temperature, and pressure (CTD, SBE 37) on one of the hydrophone mooring lines (see Fig. 1). Pressure was used as a proxy for the tidal cycle; e.g., Aguzzi et al. 2020c). In addition to the CTD, a single point current meter (Aquadopp Nortek) was attached to the mooring line.
Detection and positioning of tagged individuals
The detection of each tagged individual (i.e., the presence/absence as a proxy for burrowing behavior) was reported when an acoustic ID signal was identified by at least one hydrophone. Conversely, non-detection indicated that either the animal was concealed in its burrow (with the sediment shielding the sound propagation toward the hydrophones) or that it had moved outside the detection range of the hydrophones. This method allows the high-frequency and continuous monitoring of animals’ presence and displacement by day and night, over consecutive months (see below for the achieved date ranges), until tags and hydrophone batteries are depleted.
The displacement of acoustic-tagged individuals (i.e., as a proxy for the expression of spatially-oriented locomotor activity) was computed by triangulating simultaneous detections by a minimum of three hydrophones. Each detected acoustic tag signal had a different timestamp (in terms of its position and the time of flight). Thus, the exact position of the sound emitting source was calculated by applying least squares regression to the time difference of the arrival of signal at the different hydrophones (see Masmitja et al. 2020, for additional details). To do so, we had to treat the internal clock-drift effect of each hydrophone (i.e., the same ping being received at different hydrophones at slightly different recorded time stamps, with the signal erroneously interpreted as a change in position). This was solved by attaching an additional acoustic tag to each of the 4 moorings (i.e., 1 m above each hydrophone) for synchronization.
Data processing
We obtained tracking data for just under 4 months: 8th of June at 23:00 to 23rd of September 2020 at 17:00. Time series for detections and displacement (as proxy for burrowing behavior) were compiled by summing 120 s readings by one hour. To compile the displacement time series, we discarded all the triangulated positions with velocities above a maximum threshold of 0.5 m/s, since they were biologically unrealistic (see Vigo et al. 2021 for details). For environmental readings, we extracted hourly averages from 30-min frequency readings.
Given the open nature of the monitoring area, with animals free to enter and exit the hydrophones' detection ranges, the detection, and displacement time series were interspersed with data gaps of variable length. Therefore, the longest time series segment, corresponding to each individual, was selected for further analysis. In order to do so, we established a length threshold of minimum three continuous days (i.e., the minimum reliable data segment length to statistically assess the occurrence of periodicity in behavior; Dunlap et al. 2003).
We considered a door-keeping behavior when individuals were detected and, at the same time, they presented less than 0 m or 0.5 m of displacement during one hour. Detection and movements time series were aligned at corresponding hours for each individual, having detection data for every 2 min. When the displacement was between 0 and 0.5 m and detections were present (no 0 s in every 2 min), the hours were counted. We could then estimate the relative time of door-keeping behavior, by calculating the percentage over the all the time series length. Data with 0 detections were also counted and estimated in relative percentages, to describe the duration individuals spent inside their burrow (no door-keeping behavior nor emergence). We also estimated the relative time of activity, when individuals were outside the burrow and presenting movement. Obtained temporal measurements were also related to each type of rhythm that the individuals presented in periodogram analysis (see below), to look for potential behavioral differences.
To avoid redundancy, the occurrence of significant periodicity was screened only in time series of displacement data. We used the Lomb-Scargle Periodogram of the “Lomb” package of the R statistical software (Ruf 1999; R Core Team). Periodicity was screened within 10 and 27-h intervals (i.e., accounting for day-night photic and tidal or inertial hydrographic cycles). Given the high variability in behavioral data usually reported in field studies, the classification of periodicity was as follows: significant (i.e., at α = 0.001) periodogram peaks between 22.5 and 24.4-h indicated day-night rhythms; peaks above 24.5-h tidal rhythms; and finally, peaks between 16 and 20-h inertial periods (based on the latitude of the studied area; reviewed by Aguzzi et al. 2009b).
Secondly, a waveform analysis was conducted to assess the phase of detection and displacement rhythms. That analysis was performed at the 24-h (i.e., day-night) cycle scale, since fluctuations in photoperiod and natural light intensity largely modulate the burrow emergence behavior of N. norvegicus (Aguzzi et al. 2003; 2009b; 2015). Resulting waveforms for detections and displacements were superimposed to evidence the occurrence of door-keeping behavior (animals were detected at their tunnel entrance, but no displacement registered; Fig. 2).
Waveform analysis outputs (mean values ± SE) on time series of detections (red) and displacements (black) for a group of 4 representative individuals per each group of activity rhythms: A (ID: 14,468) day-night; B (ID: 14,472) tidal; and finally, C (ID: 14,475) inertial. MESORs are the dashed horizontal lines reported only for those locomotor patterns with evident activity increase over the 24-h (see Table 1). The black arrows indicate the start and end of activity (i.e., both detection –individual outside the burrow– and displacement –individual moving and not engaging in door-keeping behavior– above the MESOR). In D, a picture showing one N. norvegicus at the entrance of its tunnel as performing a door-keeping behavior and another individual completely outside the burrow in the monitored MPA. Green lasers indicate a length of 10 cm. The picture was taken by the ROV “LIROPUS 2000” in the study area during the same oceanographic survey (see Vigo et al. 2020; 2023)
For each individual, the selected detection and displacement time series were divided into 24-h sub-segments (Aguzzi et al. 2006). All values corresponding to each sub-segment (i.e., measurements at the same time of the day over the entire length of the selected period) were averaged. The resulting mean (± SE) values were plotted to determine the waveform and depict the mean fluctuation over a standard period of 24 h. The phase was then assessed according to the Midline Estimating Statistic of Rhythm (MESOR) method (Aguzzi et al. 2006). The MESOR value was estimated by re-averaging all waveform values, and the resulting value was represented as a horizontal threshold line onto the waveform itself. The Onset and Offset of waveform peaks were estimated by considering the first and the last value above the MESOR, respectively, indicating the peak’s temporal limits.
Periodogram analyses were also applied to pressure, temperature, salinity, and water speed time series. Those time series showing significant periodicity at α = 0.05 were considered for further waveform analysis.
An integrated chart of waveform peaks (Aguzzi et al. 2012; 2015) was used to visualize the temporal relationships of detection and displacement rhythms among all individuals and in relation to the hydrographic and day-night cycles. The duration of the waveforms’ peaks as delimited by Onset and Offset values (see above) is presented in the form of continuous horizontal bars depicting the peak temporal amplitude for an individual. The temporal amplitudes for all individuals were represented together and in association with the results of the same waveform analysis for oceanographic data (including the day hours as proxy for the day-night cycle).
Comparisons of rhythmic behavior by sex and body size
Individuals were assigned one of four behavioral levels based on their dominant displayed periodicity: “arrhythmic”, “day-night”, “inertial” and “other” (which included short semi-diurnal and long tidal periods). The counts of male and female individuals displaying each behavior were treated as Poisson events (O’Hara and Kotze 2010) described by a discrete probability distribution, with each combination of sex and behavioral label having an expected rate λ (non-negative real number).
We selected a Bayesian approach instead of conventional, frequency-based analysis, as it provides a full distribution instead of a single value for the estimations of the λ parameter, after reallocating the credibility of probabilities across all possible values. In addition, the credible intervals which express the uncertainty do not depend on a large sample size (i.e., are N-independent). Finally, it allows quantifying the support in favor of the null hypothesis, instead of only against it (Kruschke 2021).
For count ratio comparisons (i.e., if the sex ratio of the entire sample was reflected in each behavioral category), we used a Bayesian analogue (i.e., Bayes Factor) of the classic contingency table analysis. This method tests the independence assumption in a contingency table under a Poisson sampling plan without a fixed total size N (Gunel and Dickey 1974). The analysis was performed in the R package “BayesFactor” (Morey and Rouder 2022). Then, probability density distributions for each λ were estimated from 104 posterior draws which were generated by a Markov Chain Monte Carlo (MCMC; McGrayne 2011) sampling process. This procedure simulates a parameter in sequential steps, and the value of step i depends only on the value at step i-1 (i.e., the previous state, independently of the initial step 0 and the process that led to i-1). The distributions of all λ rates were visualized in density plots alongside their 90% credible intervals and real observed counts.
Finally, the potential effect of CL on rhythmic behavior was analyzed using box-plots and comparing mean, median and variation of observed CL per behavioral label.
Results
Detection and displacement time series
Detection and displacement time series for all animals are reported in Figure S1 and S2, with a representative section per individual considered for quantitative and time series analyses (see below). Detection rates varied among the 25 tracked individuals, resulting in time series of different lengths (see Table 1): 8 animals ≤ 1 week; 5 animals ≤ 1 month; 2 animals ≤ 2 months; and finally, 10 individuals < 2 months (of those latter, 4 were present over the whole period of recording). The average displacement distance was 216.0 m/day (min: 90.7 m/day, max: 424.7 m/day).
The individuals showed a relative time of 12.92% and 11.81%, respectively, presenting a door-keeping behavior identified. The mean time spent inside the burrows, when detections and movement were 0, represented 38.31% of their time series, while the mean relative time in activity was about 49.88%. The values for each individual are shown in Table 2. The mean values, estimated for each type of rhythm (Table 3), indicated that the day-night periodicity was the rhythm in which individuals presented less activity outside the burrows (35.94%), with a similar door-keeping behavior between the inertial individuals (16.18 and 15.25% respectively).
Periodogram analyses for displacement time series evidenced the occurrence of 4 major groups of animals, showing locomotor patterns matching frequency ranges within day-night, tidal, and inertial ranges, or no significant periodic activity (see Table 1). Assuming that day-night periodicities are in the range of 22.5 and 24.4-h, 12 animals (48%) followed that rhythmic pattern with a major activity phase at daylight hours (see Table 1). An example is described by the waveform analysis output for an individual (ID 14468) as representative of this group, in Fig. 2A. That animal shows a significant increment in displacement (i.e., an increase above the MESOR) from 6:00 to 19:00. Superimposing detection on the displacement waveform (see Fig. 2A), moments of stationary door-keeping behavior (i.e., animals waiting at the entrance of their tunnels) were revealed, when displacements were greatly reduced. This is evident for that individual, which showed a baseline level of detections around 19 per hour, when displacements were close to 0 (i.e., approximately from 21:00 to 04:00).
One animal (4%) followed a tidal (i.e., period > 24.5-h) activity pattern (see ID 14472 in Table 1 and Fig. 2B). That individual shows a broad increase in displacement activity at daytime comprised by several peaks occurring between from 5:00 to 18:00), with drops at 06:00, 11:00, and 17:00. Detections also increased broadly at daytime, being present also during the night, when displacements dropped to zero from 22:00 to 04:00 (again, for the occurrence of stationary door-keeping behavior).
3 animals (12%) displayed inertial (i.e., periodicity between 16 and 20-h) displacement rhythmicity (see Table 1). An example is reported in waveform analysis for an individual (ID 14475) within this category in Fig. 2C. No temporally-structured increment of displacement activity appears in relation to the light cycle. The temporal trend of detections follows a similar pattern. Since the waveforms for both data sets are similar in that animal and the others for this same group, it appears that door-keeping (and hence burrowing) behavior does not occur at all.
Finally, 1 animal (4%) followed a semi-diurnal (i.e., period < 16-h) activity pattern (see ID 14461 in Table 1). Differently, other 8 individuals (32%) were arrhythmic (i.e., displayed no significant periodicity in their displacement behavior).
Environmental time series
Time series for environmental data are reported in Figure S3. Water temperature fluctuated between a minimum and a maximum of 13.66 and 13.92 °C, respectively (with an averaged value and standard deviation of 13.82 ± 0.04 °C). Salinity varied between 38.55 and 38.65 as maximum and minimum (with an averaged value of 38.63 ± 0.01). Current speed occurred in the minimum and maximum range of 0.01 and 0.21 m/s (with an averaged value of 0.06 ± 0.03 m/s). Finally, the water column pressure fluctuated between 357.45 and 358.03 dbar (with an averaged value of 357.70 ± 0.10 dbar).
Periodogram analysis indicated the presence of significant periodicity for pressure, salinity, and water speed (see Table 1 for period values and Figure S4 for periodogram plots). Pressure showed a mixed semi-diurnal / diurnal periodicity pattern, with the other three occurring predominantly within the inertial range. Temperature did not show any significant periodicity and was therefore discarded from waveform analysis.
Waveform analysis for current speed and water pressure showed phase opposition (Fig. 3). The waveform for speed indicated the occurrence of a significant peak (mean values above the MESOR) from 23:00 to 10:00. Conversely, a significant increase in the fluctuation status for the water column pressure occurs from 17:00 to 01:00.
Waveform analysis for current speed and water column pressure as recorded during the acoustic monitoring period. MESORs are the dashed horizontal lines (see Table 1)
Comparisons by sex and body size
The Bayes Factor comparisons for differences in counts per behavioral category between male and female N. norvegicus (i.e., in relation to the expected ones based on the initial sample, roughly a ratio of 2:1) did not reveal a strongly pronounced deviation from the null hypothesis of independence, overall. In particular, the outcome of the analysis was a Bayes Factor of 1.33.
In order to get more insight of the pairwise comparisons within each behavioral category, the posterior distributions of the corresponding Poisson rates λ were derived and visualized. Most categories showed the same pattern except for the few individuals with inertial-based periodic activity, where more females were encountered (see Table 1) and there is a marked overlap of the distributions of λ for male and female (Fig. 4). In regard to the CL comparisons, the box-plots (Figure S5) showed very similar characteristics.
Integrated chart of waveforms peaks for biological and oceanographic data
The temporal phase relationship, obtained by comparing peaks of all waveforms (i.e., values above the MESOR) for detections and displacement time series, is presented in Fig. 5. For displacement time series, significant increases (constituting waveform peaks) are concentrated at daytime (i.e., from 6:00 to 19:00) with a marked reduction at night. Detections follow the same pattern at daytime, but significant waveform peak values occur also at nighttime (bimodal). Nocturnal detections without displacement indicate the occurrence of door-keeping behavior as a proxy for burrowing activity (i.e., because animals are detected but do not leave the entrance). That overall temporal dynamic highlights those differences reported at the level of individuals’ waveforms (see Fig. 2).
The comparison of both behavioral time series with waveform peaks for current velocity and water column pressure (see Fig. 3) suggests under which levels of hydrodynamic fluctuations burrow emergence behavior may be inhibited. If we consider the time interval 06:00–19:00 (see Fig. 5), as the time window where the majority of displacement peaks occurred, animals globally increased their displacement activity when current speed starts decreasing (between around 0.064 – 0.058 m/s). The general daytime activity also coincides with the midday minimum in water column pressure at 12.00–13:00. That activity is reduced when pressure drops below 357.70 dbar (06:00) and rises above 357.74 dbar (19:00).
Discussion
Long-lasting and high-frequency acoustic-based tracking of N. norvegicus in deep-sea benthic habitats allowed us to study its burrow emergence rhythms over a long period of time. These observations provide new information on how this species’ biological clock in relation to burrow occupation and door-keeping (Atkinson and Naylor 1976; Arechiga and Atkinson 1980; Moller and Naylor 1980; Chiesa et al. 2010; Aguzzi et al. 2011), as well as emergence and non-burrow occupation behavior, that might affect stock assessments (Aguzzi et al. 2022a). Simultaneously, these results also explain at individual level, the historically-reported population shelf and slope catchability patterns on a day-night and tidal basis (Oakley 1979; Bell et al. 2006; Aguzzi and Sardà 2008; Sardà and Aguzzi 2012).
Tagged lobsters did not show long periods of concealment into their burrows (i.e., no long-lasting absence of detections in Figure S1 are evident), being instead engaged in enduring or repeating door-keeping activities (i.e., detections with no displacements). The lobsters patrol the seabed around their tunnel entrances (Aguzzi et al. 2006) as has been recently confirmed in the same MPA study area by ROV surveys carried out over the 24-h (Vigo et al. 2023). Here, variable ranges of displacement were detected over consecutive days (i.e., see the MESOR estimate as comparison among displacement time series of variable length in days). These observations support the idea of marked inter-individual variability in locomotor activity rhythms as observed in laboratory experiments (e.g., Aguzzi et al. 2004b, a; Katho et al. 2013; Sbragalia et al. 2013, 2014; García et al. 2019).
We observed that the proportion of individuals conducting mixed day-night, tidal and inertial rhythms in field settings were similar to those observed in laboratory tests (Aguzzi et al. 2013). Laboratory experiments also reveal the occurrence of mixed periodicity that points out the plasticity of the species’ biological clock; i.e., able to adapt to a wide range of photoperiod lengths (for latitude and seasons) as well as intensities (for depth) (Aguzzi et al. 2011). Those rhythms are occurring in the Mediterranean disphotic slope environment, where even though the monochromatic blue-green light (i.e., wavelength between 400–700 nm) is dampened by several orders of magnitude, it still elicits a turnover of the community on a 24-h basis (Aguzzi et al. 2003; 2015). Therefore, while the ecological basis of the day-night rhythmic expression can be more apparent, the interpretation of tidal and inertial fluctuations is less straightforward. A large proportion of animals were diurnal, while one individual displayed a bimodal (tidal-like) periodicity, which at the surveyed depth seems to be similar to crepuscular rhythms of shallower shelf populations. That pattern in displacement could be recognized in the periodogram analysis as tidally-oriented, being instead of borderline periodicity with the day-night one. Finally, inertial periodicity has been detected as per the laboratory results and field hydrographic comparisons by Aguzzi et al. (2009a), but the ecological reasons remain harder to explain.
The presence of mixed day-night and tidal periodicity poses the question of why animals exhibit flexible responses to different environmental cues other than photoperiod. If N. norvegicus followed an optimal interval in environmental light intensity to perform emergence (Chapman et al. 1975) as a visual predator and scavenger (e.g., Farmer 1975; Oakley 1979) or to avoid incoming predators (Richardson 1996; Chapman et al. 2000; Aguzzi et al. 2009b; Albalat et al. 2016), it should be less sensitive to currents. Alternatively, our data indicate that emergence behavior is influenced by tidal patterns (Newland et al. 1988). Weak tidal patterns influence sediment resuspension and transport in deep-water and deep-sea areas of the western Mediterranean Sea (Palanques et al. 2002; Grinyó et al. 2017), likely affecting the phenology of sessile megafauna (Grinyó et al. 2018). A tidal pattern in the species catchability was evidenced in the North Sea (Bell et al. 2008) and may be the product of animals’ locomotor activation or inhibition upon predators or preys’ odor cues within water flows. Odor cues play a relevant role in the feeding activity and success of deep-sea fauna (Aguzzi et al. 2012; Stuckless et al. 2021) and may contribute to activity rhythm regulation (e.g., Aguzzi et al. 2018). At the same time, a tidal regulation of locomotor activity also occurs in laboratory hydrodynamically controlled environments with no chemical cues (Sbragaglia et al. 2013). This indicates that animals may vary behavioral activity to compensate for energy expenditures in relation to hydrodynamic drag (e.g., Aguzzi et al. 2010). As observed in other deeper dwelling species such as the deep-sea crab Geryon longipes from the same area at 1000 m depth, locomotor patterns can be synchronized to both tidal and inertial currents in the laboratory (Nuñez et al. 2016).
Contrary to the tidal periodicity of the water column depth, current speed and salinity showed inertial rhythms, while temperature was arrhythmic. In a previous analysis comparing locomotor activity in laboratory tested animals of the same depth range and geographic location by Aguzzi et al. (2009a), inertial rhythms were explained based on near seafloor mooring data obtained at 1,000 m depth. Here, with our shallower mooring deployment (380 m depth), we observed a mixture of inertial currents and a tidal signal. However, water speed ranges were different. In Aguzzi et al. (2009b), water mean speed was approximately 0.035 m/s during 30 days (from 15th September to 14th October), while here from the 8th of June to 23rd of September, our averaged values are of 0.06 ± 0.03 m/s. In any case, we still have inertial related periodicity in the displacement behavior of some animals that show no clear burrowing-oriented activity. Possibly, rather than being the sign of synchronization of behavioral rhythms upon this hydrodynamic cue, inertial periodicity in displacements could be the results of a disruption of the biological clock according to a population of coupled oscillators (Strogatz & Stewart 1993; Aguzzi et al. 2011). For unknown conditions, some animals clock oscillators would split in different functional subgroups, hence producing, inertial-like rhythms, equivalent to ultradian periodicity in laboratory studies (Aguzzi et al. 2011).
Our data on water properties are in line with the generally accepted preferences of N. norvegicus (i.e., highly saline waters with small temperature fluctuations; Eriksson et al. 2013). However, there is no sufficient literature describing the influence of salinity on this species’ rhythmic behavior (apart from survival studies showing higher mortality under low salinity regimes). Differently, a negative impact of high salinity on CPUE has been recently identified (Chiarini et al. 2022). It is possible that animals react to salinity fluctuations as an additional chemical cue indicative of the change of tides and hydrodynamic activity, as it has been observed in laboratory conditions of rhythmic behavioral entrainment in other benthic decapods (Naylor 1985).
Sex and size (i.e., CL) did significantly affect burrow emergence rhythms of tracked individuals. The value of the Bayes Factor comparing the sex ratio contingency table was within the “not worthy of mentioning” category (i.e., for the range of 1 to 3.2), according to Kass and Raftery (1995). A potential exception could be the case of inertial rhythms, for which the observed sex-ratio deviates from the observed 1:2 (females:males) of all other behavioral categories and across the entire sample. Similarly, the median CL of individuals displaying inertial periodicity is lower. Females are generally reported to be smaller than males (McQuaid et al. 2006), which was also the case in our study. In any case, these results should be treated with caution, as the number of individuals is low (i.e., N = 3), and should not be considered conclusive evidence suggesting dimorphism in behavioral rhythmic response. Reproductive behavior occurring in spring–summer at our latitude (Aguzzi et al. 2004b, a) could affect reproductively mature individuals of both sexes in different ways, but more field observations are needed.
The cease of the detection of animals within the monitored area could be the product of their dispersion outside the detection range area. As the MPA gets progressively depleted of individuals, and the releasing areas experience a transitory high density, animals seem to adjust their territoriality with different ranges of wandering (see Vigo et al. 2021 for a spatial representation of those data). This could occur for a current process of individuals’ redistribution as caused by aggressive hierarchic social interactions (Sbragaglia et al. 2013). In fact, animal burrowing capability is influenced by a balance between aggressive territorial interactions and overall local demography (Chapman and Rice 1971; Johnson et al. 2013). Possibly, individuals with no defined detection and wandering rhythmic patterns are those with no burrowing capability due to their subordinate social ranking level. Future studies are needed, to monitor this enigmatic component of the population; e.g., acoustic tracking should be accompanied by a network of video-recording cameras from fixed and docked mobile platforms, branching off the MPA central release area (Aguzzi et al. 2022a). HD images could be used to identify those displacing animals, evidencing the reasons for their burrow digging incapability.
Conclusion
The use of acoustic telemetry to characterize the movements and burrowing behavior of N. norvegicus shows promising applications for ecological monitoring and recovery. Firstly, it allows the validation of UWTV and trawling surveys with an individual tracking technology, indicating that the same animals do emerge constantly over consecutive days (at least for those time series sections of continuous data, we analyzed). In animals with no burrows (for unknown reasons impairing the digging capability), behavioral activity can be measured under the form of a constant wandering on the seabed at a periodicity that matches inertial currents. So, when the burrow is present, it allows the temporal structuring of behavior into different stationary door-keeping locomotor wandering activities according to light cycles. Those observations indicate that trawl or video sampling for stock assessment purposes by ROV and towed sledges should not only count burrows but also animals both in door-keeping and in full emergence over consecutive 24-h cycles, in the same areas. Even if the bias (i.e., under- or overestimation of true abundance) can assumed to be constant, knowing the timing of maximum emergence of populations may assist in setting the best timings for video surveys, where both animals and tunnels should be counted. With repeated, temporally-oriented surveys, the difference between counted burrow systems and animals can be estimated in order to obtain a proportion coefficient to adjust the derived stock estimates. Finally, we need to extend the number of moored hydrophones in order to enlarge the MPA monitored area, to get an insight on animals’ longer range displacements. In the end, we need to better assess if captured and reintroduced animals may contribute to the local stock refurbishing into the MPA or individuals wander across its borders, vanishing the conservation strategy.
References
Aguzzi J, Sardà F (2008) A history of recent advancements on Nephrops norvegicus behavioral and physiological rhythms. Rev Fish Biol Fish 18:235–248. https://doi.org/10.1007/s11160-007-9071-9
Aguzzi J, Sardà F, Abelló P, Company JB, Rotllant G (2003) Diel and seasonal patterns of Nephrops norvegicus (Decapoda: Nephropidae) catchability in the western Mediterranean. Mar Ecol Prog Ser 258:201–211. https://doi.org/10.3354/meps258201
Aguzzi J, Company JB, Sardà F (2004a) Feeding activity rhythm of Nephrops norvegicus of the western Mediterranean shelf and slope grounds. Mar Biol 144:463–472. https://doi.org/10.1007/s00227-003-1221-6
Aguzzi J, Sardà F, Allué R (2004b) Seasonal dynamics in Nephrops norvegicus (Decapoda: Nephropidae) catches off the Catalan Coasts (Western Mediterranean). Fish Res 69:293–300. https://doi.org/10.1016/j.fishres.2004.04.010
Aguzzi J, Bullock NM, Tosini G (2006) Spontaneous internal desynchronization of locomotor activity and body temperature rhythms from plasma melatonin rhythm in rats exposed to constant dim light. J Circadian Rhythms 4:6. https://doi.org/10.1186/1740-3391-4-6
Aguzzi J, Company JB, Sardà F (2007) The activity rhythm of berried and unberried females of Nephrops norvegicus (Crustacea, decapoda). Crustaceana 80:1121–1134. https://doi.org/10.1163/156854007782008577
Aguzzi J, Bahamon N, Marotta L (2009a) The influence of light availability and predatory behavior of the decapod crustacean Nephrops norvegicus on the activity rhythms of continental margin prey decapods. Mar Ecol 30:366–375. https://doi.org/10.1111/j.1439-0485.2008.00276.x
Aguzzi J, Puig P, Company JB (2009b) Hydrodynamic, non-photic modulation of biorhythms in the Norway lobster, Nephrops norvegicus (L.). Deep Sea Res Part i: Oceanogr Res Pap 56:366–373. https://doi.org/10.1016/j.dsr.2008.10.001
Aguzzi J, Costa C, Furushima Y, Chiesa JJ, Company JB, Menesatti P, Iwase R, Fujiwara Y (2010) Behavioral rhythms of hydrocarbon seep fauna in relation to internal tides. Mar Ecol Prog Ser 418:47–56. https://doi.org/10.3354/meps08835
Aguzzi J, Company JB, Costa C, Menesatti P, Garcia JA, Bahamon N, Puig P, Sardà F (2011) Activity rhythms in the deep-sea: a chronobiological approach. Front Biosci 16:131–150. https://doi.org/10.2741/3680
Aguzzi J, Jamieson AJ, Fujii T, Sbragaglia V, Costa C, Menesatti P, Fujiwara Y (2012) Shifting behaviour of deep-sea buccinid gastropods at natural and simulated food falls. Mar Ecol Prog Ser 458:247–253. https://doi.org/10.3354/meps09758
Aguzzi J, Sbragaglia V, Tecchio S, Navarro J, Company JB (2015) Rhythmic behaviour of marine benthopelagic species and the synchronous dynamics of benthic communities. Deep Sea Res Part I Oceanogr Res Pap 95:1–11. https://doi.org/10.1016/j.dsr.2014.10.003
Aguzzi J, Fanelli E, Ciuffardi T, Schirone A, De Leo FC, Doya C, Kawato M, Miyazaki M, Furushima Y, Costa C, Fujiwara Y (2018) Faunal activity rhythms influencing early community succession of an implanted whale carcass offshore Sagami Bay. Japan Sci Rep 8:11163. https://doi.org/10.1038/s41598-018-29431-5
Aguzzi J, Chatzievangelou D, Marini S, Fanelli E, Danovaro R, Flögel S, Lebris N, Juanes F, De Leo FC, Del Rio J, Thomsen L, Costa C, Riccobene G, Tamburini C, Lefevre D, Gojak C, Poulain PM, Favali P, Griffa A, Purser A, Cline D, Edigington D, Navarro J, Stefanni S, D’Hondt S, Priede IG, Rountree R, Company JB (2019) New high-tech interactive and flexible networks for the future monitoring of deep-sea ecosystems. Environ Sci Technol 53:6616–6631. https://doi.org/10.1021/acs.est.9b00409
Aguzzi J, Chatzievangelou D, Company JB, Thomsen L, Marini S, Bonofiglio F, Juanes F, Rountree R, Berry A, Chumbinho R, Lordan C, Doyle J, del Rio J, Navarro J, De Leo FC, Bahamon N, García JA, Danovaro PR, Francescangeli M, Lopez-Vazquez V, Gaughan P (2020a) The potential of video imagery from worldwide cabled observatory networks to provide information supporting fish-stock and biodiversity assessment. ICES J Mar Sci 77:2396–2410. https://doi.org/10.1093/icesjms/fsaa169
Aguzzi J, Chatzievangelou D, Francescangeli M, Marini S, Bonofiglio F, del Rio J, Danovaro R (2020b) The hierarchic treatment of marine ecological information from spatial networks of benthic platforms. Sensors 20:1751. https://doi.org/10.3390/s20061751
Aguzzi J, López-Romero D, Marini S, Costa C, Berry A, Chumbinho R, Ciuffardi T, Fanelli E, Pieretti N, Del Río J, Stefanni S, Mirimin L, Doyle J, Lordan C, Gaughan P (2020c) Multiparametric monitoring of fish activity rhythms in an Atlantic coastal cabled observatory. J Mar Syst 212:103424. https://doi.org/10.1016/j.jmarsys.2020.103424
Aguzzi J, Bahamon N, Doyle J, Lordan C, Tuck ID, Chiarini M, Martinelli M, Company JB (2021) Burrow emergence rhythms of Nephrops norvegicus by UWTV and surveying biases. Sci Rep 11:5797. https://doi.org/10.1038/s41598-021-85240-3
Aguzzi J, Chatzievangelou D, Robinson NJ, Bahamon N, Berry A, Carreras M, Company JB, Costa C, del Rio FJ, Falahzadeh A, Fifas S, Flögel S, Grinyó J, Jónasson JP, Jonsson P, Lordan C, Lundy M, Marini S, Martinelli M, Masmitja I, Mirimin L, Naseer A, Navarro J, Palomeras N, Picardi G, Silva C, Stefanni S, Vigo M, Vila Y, Weetman A, Doyle J (2022a) Advancing fishery-independent stock assessments for the Norway lobster (Nephrops norvegicus) with new monitoring technologies. Front Mar Sci 9:969071. https://doi.org/10.3389/fmars.2022.969071
Aguzzi J, Violino S, Costa C, Bahamon N, Navarro J, Chatzievangelou D, Robinson J, Doyle J, Martinelli M, Lordan C, Company JB (2022) Established and emerging research trends in Norway lobster. Nephrops Norvegicus Biology 12:225. https://doi.org/10.3390/biology12020225
Albalat A, Collard A, Brucem C, Coates CJ, Fox CJ (2016) Physiological condition, short-term survival, and predator avoidance behavior of discarded Norway Lobsters (Nephrops norvegicus). J Shellfish Res 35:1053–1065. https://doi.org/10.2983/035.035.0428
Aréchiga H, Atkinson RJA, Williams JA (1980) Neurohumoral basis of circadian rhythmicity in Nephrops norvegicus (L). Mar Freshw Behav Physiol 7:185–197. https://doi.org/10.1080/10236248009386980
Aspillaga E, Safi K, Hereu B, Bartumeus F (2019) Modelling the three-dimensional space use of aquatic animals combining topography and Eulerian telemetry data. Methods Ecol Evol 10:1551–1557. https://doi.org/10.1111/2041-210X.13232
Atkinson RJA, Naylor E (1976) An endogenous activity rhythm and the rhythmicity of catches of Nephrops norvegicus (L). J Exp Mar Biol Ecol 25:95–108. https://doi.org/10.1016/0022-0981(76)90079-4
Belgrano A, Novaglio C, Svedäng H, Villasante S, Melián CJ, Blenckner T, Bergström U, Bryhn A, Bergström L, Bartolino V, Sköld M, Tomczak M, Wikström SA, Hansen AS, Linke S, Emmerson R, Morf A, Tönnesson K (2021) Mapping and evaluating marine protected areas and ecosystem services: a transdisciplinary delphi forecasting process framework. Front Ecol Evol 9:652492. https://doi.org/10.3389/fevo.2021.652492
Bell MC, Elson JM, Addison JT, Revill AS, Bevan D (2008) Trawl catch composition in relation to Norway lobster (Nephrops norvegicus L.) abundance on the Farn Deeps grounds. NE England Fish Res 90:128–137. https://doi.org/10.1016/j.fishres.2007.10.003
Bell MC, Redant F, Tuck I (2006) Nephrops species. In: Phillips BF (ed) Lobsters: biology, management, aquaculture and fisheries. Blackwell, Oxford, pp 412–461. https://doi.org/10.1002/9780470995969.ch13
Bicknell AWJ, Godley BJ, Sheehan EV, Votier SC, Witt MJ (2016) Camera technology for monitoring marine biodiversity and human impact. Front Ecol Environ 14:424–432. https://doi.org/10.1002/fee.1322
Chapman CJ, Howard FG (1979) Field observations on the emergence rhythm of the Norway lobster Nephrops norvegicus, using different methods. Mar Biol 51:157–165. https://doi.org/10.1007/BF00555195
Chapman CJ, Rice AL (1971) Some direct observations on the ecology and behaviour of the Norway lobster Nephrops norvegicus. Mar Biology 10:321–329. https://doi.org/10.1007/BF00368092
Chapman C, Shelton P, Shanks A, Gaten E (2000) Survival and growth of the Norway lobster Nephrops norvegicus in relation to light-induced eye damage. Mar Biol 136:233–241. https://doi.org/10.1007/s002270050681
Chiarini M, Guicciardi S, Angelini S, Tuck ID, Grilli F, Penna P, Domenichetti F, Canduci G, Belardinelli A, Santojanni A, Arneri E, Milone N, Medvešek D, Isajlović I, Vrgoč N, Martinelli M (2022) Accounting for environmental and fishery management factors when standardizing CPUE data from a scientific survey: aa case study for Nephrops norvegicus in the Pomo Pits area (Central Adriatic Sea). PLoS ONE 17:e0270703. https://doi.org/10.1371/journal.pone.0270703
Chiesa JJ, Aguzzi J, García JA, Sardà F, de la Iglesia H (2010) Light intensity determines temporal niche switching of behavioral activity in deep-water Nephrops norvegicus (Crustacea: Decapoda). J Biol Rhythms 25:277–287. https://doi.org/10.1177/0748730410376159
Dobby, H., Doyle, J., Jónasson, J., Jonsson, P., Leocádio, A., Lordan, C., Weetman, A., and Wieland, K. 2021. ICES Survey Protocols – Manual for Nephrops Underwater TV Surveys, coordinated under ICES Working Group on Nephrops Surveys (WGNEPS). ICES Techniques in Marine Environmental Science, 65: 39 https://doi.org/10.17895/ices.pub.8014
Doya C, Chatzievangelou D, Bahamon N, Purser A, De Leo FC, Juniper SK, Thomsen L, Aguzzi J (2017) Seasonal monitoring of deep-sea megabenthos in Barkley Canyon cold seep by internet operated vehicle (IOV). PLoS ONE 12:e0176917. https://doi.org/10.1371/journal.pone.0176917
Dunlap JC, Loros JJ, DeCoursey PJ (2003) Chronobiology: biological timekeeping. Sinauer Associates Inc., Sunderland
Eriksson SP, Hernroth B, Baden SP (2013) Stress biology and immunology in Nephrops norvegicus. Adv Mar Biol 64:149–200. https://doi.org/10.1016/B978-0-12-410466-2.00005-4
Fernández-Chacón A, Buttay L, Moland E, Knutsen H, Olsen EM (2021) Demographic responses to protection from harvesting in a long-lived marine species. Biol Conserv 257:109094. https://doi.org/10.1016/j.biocon.2021.109094
Francescangeli M, Sbragaglia V, del Rio FJ, Trullols E, Antonijuan J, Massana I, Prat J, Nogueras Cervera M, Mihai Toma D, Aguzzi J (2022) Long-term monitoring of diel and seasonal rhythm of Dentex dentex at an artificial reef. Front Mar Sci 9:837216. https://doi.org/10.3389/fmars.2022.837216
Garcia JA, Sbragaglia V, Masip D, Aguzzi J (2019) Long-term video tracking of cohoused aquatic animals: a case study of the daily locomotor activity of the Norway Lobster (Nephrops norvegicus). J vis Exp 146:358515. https://doi.org/10.3791/58515
Grinyó J, Isla E, Peral L, Gili J-M (2017) Composition and temporal variability of particle fluxes in an insular canyon of the northwestern Mediterranean Sea. Prog Oceanogr 159:323–339. https://doi.org/10.1016/j.pocean.2017.11.005
Grinyó J, Viladrich N, Díaz D, Muñoz A, Mallol S, Salazar J, Castillo R, Gili J-M, Gori A (2018) Reproduction, energy storage and metabolic requirements in a mesophotic population of the gorgonian Paramuricea macrospina. PLoS ONE 13:e0203308. https://doi.org/10.1371/journal.pone.0203308
Grinyó J, Francescangeli M, Santín A, Ercilla M, Estrada F, Mecho A, Fanelli E, Costa C, Danovaro R, Company JB, Sobrino I, Valencia J, Aguzzi J (2022) Megafaunal assemblages in deep-sea ecosystems of the Gulf of Cadiz, northeast Atlantic ocean. Deep Sea Res Part i: Oceanogr Res Pap 183:03738. https://doi.org/10.1016/j.dsr.2022.103738
Gunel E, Dickey J (1974) Bayes factors for independence in contingency tables. Biometrika 61:545–557. https://doi.org/10.1093/biomet/61.3.545
Hopkins TS (1985) Physics of the sea. In: Margalef R (ed) Key Environments: Western Mediterranean. Pergamon Press, New York, pp 100–125
Hussey NE, Kessel ST, Aarestrup K, Cooke SJ, Cowley PD, Fisk AT, Whoriskey FG (2015) Aquatic animal telemetry: a panoramic window into the underwater world. Science 348(6240):125. https://doi.org/10.1126/science.1255642
Issifu I, Alava JJ, Lam VW, Sumaila UR (2022) Impact of ocean warming, overfishing and mercury on European fisheries: a risk assessment and policy solution framework. Front Mar Sci 8:770805. https://doi.org/10.3389/fmars.2021.770805
Johnson MP, Lordan C, Power AM (2013) Habitat and ecology of Nephrops norvegicus. Adv Mar Biol 64:27–63. https://doi.org/10.1016/B978-0-12-410466-2.00002-9
Kass RE, Raftery AE (1995) Bayes factors. J Am Stat Assoc 90:773–795. https://doi.org/10.1080/01621459.1995.10476572
Katho E, Sbragaglia V, Aguzzi J, Breithaupt T (2013) Sensory biology and behaviour of Nephrops norvegicus. Adv Mar Biol 64:65–106. https://doi.org/10.1016/B978-0-12-410466-2.00003-0
Kruschke JK (2021) Bayesian analysis reporting guidelines. Nat Hum Behav 5:1282–1291. https://doi.org/10.1038/s41562-021-01177-7
Lin Y, Hsiung J, Piersall R, White C, Lowe CG, Clark CM (2016) A Multi-autonomous underwater vehicle system for autonomous tracking of marine life. J Field Robot 34:757–774. https://doi.org/10.1002/rob.21668
Masmitja I, Navarro J, Gomariz S, Aguzzi J, Kieft B, O’Reilly T, Katija K, Bouvet PJ, Fannjiang C, Vigo M, Puig P, Alcocer A, Vallicrosa G, Palomeras N, Carreras M, Del Rio J, Company JB, (2020), Mobile robotic platforms for the acoustic tracking of deep water demersal fishery resources. Sci Robot 5:eabc3701. https://doi.org/10.1126/scirobotics.abc3701
Matabos M, Aguzzi J, Robert K, Costa C, Menesatti P, Company JB, Juniper SK (2011) Multi-parametric study of behavioural modulation in demersal decapods at the VENUS cabled observatory in Saanich Inlet, British Columbia, Canada. J Exp Mar Biol Ecol 40:189–196. https://doi.org/10.1016/j.jembe.2011.02.041
Matley JK, Klinard NV, Barbosa Martins AP, Aarestrup K, Aspillaga E, Cooke SJ, Cowley PD, Heupel MR, Lowe CG, Lowerre-Barbieri SK, Mitamura H, Moore J-S, Simpfendorfer CA, Stokesbury MJW, Taylor MD, Thorstad EB, Vandergoot CS, Fisk AT (2022) Global trends in aquatic animal tracking with acoustic telemetry. Trends Ecol Evol 37:79–94. https://doi.org/10.1016/j.tree.2021.09.001
McGrayne SB (2011) The Theory That Would Not Die: How Bayes' Rule Cracked the Enigma Code, Hunted Down Russian Submarines, and Emerged Triumphant from Two Centuries of Controversy. Yale University Press, New Haven. https://doi.org/10.12987/9780300175097
McQuaid N, Briggs RP, Roberts D (2006) Estimation of the size of onset of sexual maturity in Nephrops norvegicus (L.). Fish Res 81:26–36. https://doi.org/10.1016/j.fishres.2006.06.003
Moller TH, Naylor E (1980) Environmental influence on locomotor activity in Nephrops norvegicus (Crustacea: Decapoda). J Mar Biolog Assoc U.K. 60:103–113. https://doi.org/10.1017/S0025315400024152
Morey RD, Rouder JN (2022) BayesFactor: Computation of Bayes Factors for Common Designs. R package version 0.9.12–4.4. https://CRAN.R-project.org/package=BayesFactor
Naylor E (1985) Tidally rhythmic behaviour of marine animals. Symp Soc Exp Biol 39:63–93
Newland PL, Neil DM, Chapman CJ (1988) The reactions of the Norway lobster, Nephrops norvegicus (L.), to water currents. Mar Behav Physiol 13:301–313. https://doi.org/10.1080/10236248809378680
Nuñez JD, Sbragaglia V, García JA, Company JB, Aguzzi J (2016) First laboratory insight on the behavioral rhythms of the bathyal crab Geryon longipes. Deep Sea Res Part i: Oceanogr Res Pap 116:165–173. https://doi.org/10.1016/j.dsr.2016.08.007
O’Hara RB, Kotze DJ (2010) Do not log-transform count data. Methods Ecol Evol 1:118–122. https://doi.org/10.1111/j.2041-210X.2010.00021.x
Oakley SG (1979) Diurnal and seasonal changes in the timing of peak catches of Nephrops norvegicus reflecting changes in behavior. In: Naylor E and Hartnoll RG (eds) Cyclical phenomena in marine plants and animals. Pergamon Press, Oxford, pp 367–373. https://doi.org/10.1016/B978-0-08-023217-1.50054-0
Palanques A, Puig P, Guillén J, Jiménez J, Gracia V, Sánchez-Arcilla A, Madsen O (2002) Near-bottom suspended sediment fluxes on the microtidal low-energy Ebro continental shelf (NW Mediterranean). Cont Shelf Res 22:285–303. https://doi.org/10.1016/S0278-4343(01)00058-9
Puig P, Palanques A, Guillén J, Garcı́a-Ladona E, (2000) Deep slope currents and suspended particle fluxes in and around the Foix submarine canyon (NW Mediterranean). Deep Sea Res Part i: Oceanogr Res Pap 47:343–366. https://doi.org/10.1016/S0967-0637(99)00062-X
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ Accessed 22 November 2022
Richardson G (1996) The effect of light-induced eye damage on the behaviour of Nephrops norvegicus. University of Leicester and ProQuest Dissertations Publishing, Leicester (UK).
Ruf T (1999) The lomb-scargle periodogram in biological rhythm research: analysis of incomplete and unequally spaced time-series. Biol Rhythm Res 30:178–201. https://doi.org/10.1076/brhm.30.2.178.1422
Sardà F, Aguzzi J (2012) A review of burrow counting as an alternative to other typical methods of assessment of Norway lobster populations. Rev Fish Biol Fish 22:409–422. https://doi.org/10.1007/s11160-011-9242-6
Sbragaglia V, Aguzzi J, García JA, Sarriá D, Gommariz S, Costa C, Menesatti P, Vilaró M, Manuel A, Sardà F (2013) An automated multi-flume actograph for the study of behavioral rhythms of burrowing organisms. J Exp Mar Biol Ecol 446:177–185. https://doi.org/10.1016/j.jembe.2013.05.018
Sbragaglia V, García JA, Chiesa JJ, Aguzzi J (2015) Effect of simulated tidal currents on the burrow emergence rhythms of the Norway lobster (Nephrops norvegicus). Mar Biol 162:2007–2016. https://doi.org/10.1007/s00227-015-2726-5
Sbragaglia V, Leiva D, Arias A, García JA, Aguzzi J, Breithaupt T (2017) Fighting over burrows: the emergence of dominance hierarchies in the Norway lobster (Nephrops norvegicus). J Exp Biol 220:4624–4633. https://doi.org/10.1242/jeb.165969
Sbragaglia V, Nuñez JD, Dominoni D, Coco S, Fanelli E, Azzurro E, Marini S, Nogueras M, Ponti M, del Rio FJ, Aguzzi J (2019) Annual rhythms of temporal niche partitioning in the Sparidae family are correlated to different environmental variables. Sci Rep 9:1708. https://doi.org/10.1038/s41598-018-37954-0
Sbragaglia V, Aguzzi J, García JA, Chiesa JJ, Angelini C, Sardà F (2014) Dusk but not dawn burrow emergence rhythms of Nephrops norvegicus (Crustacea Decapoda). Sci Mar 77:641–647 https://doi.org/10.3989/scimar.03902.28C
Shelton PMJ, Gaten E, Chapman CJ (1985) Light and retinal damage in Nephrops norvegicus (L.) (Crustacea). Proc Royal Soc Lond B 226:217–236. https://doi.org/10.1098/rspb.1985.0092
STECF (2020) Monitoring the performance of the common fisheries policy (STECF-Adhoc-20-01). Publications Office of the European Union, Luxembourg. https://doi.org/10.2760/230469
Strogatz SH, Stewart I (1993) Coupled oscillators and biological synchronization. Sci Am 269:102–109. https://doi.org/10.1038/scientificamerican1293-102
Stuckless B, Hamel J-F, Aguzzi J, Mercier A (2021) Foraging strategies in four deep-sea benthic species. J Exp Mar Biol Ecol 542–543:151607. https://doi.org/10.1016/j.jembe.2021.151607
Tuck ID, Parsons DM, Hartill BW, Chiswell SM (2015) Scampi (Metanephrops challengeri) emergence patterns and catchability. ICES J Mar Sci 72:i199–i210. https://doi.org/10.1093/icesjms/fsu244
Vigo M, Navarro J, Masmitja I, Aguzzi J, García JA, Rotllant G, Bahamón N, Company JB (2021) Spatial ecology of Norway lobster Nephrops norvegicus in Mediterranean deep-water environments: implications for designing no-take marine reserves. Mar Ecol Prog Ser 674:173–188. https://doi.org/10.3354/meps13799
Vigo M, Navarro J, Aguzzi J, Bahamón N, García JA, Rotllant G, Recasens L, Company JB (2023) ROV-based monitoring of passive ecological recovery in a deep-sea no-take fishery reserve. Sci Tot Env in Press. https://doi.org/10.1016/j.scitotenv.2023.163339
Wilmers CC, Nickel B, Bryce CM, Smith JA, Wheat RE, Yovovich V (2015) The golden age of bio-logging: how animal-borne sensors are advancing the frontiers of ecology. Ecology 96:1741–1753. https://doi.org/10.1890/14-1401.1
Acknowledgements
We greatly appreciate the assistance of everyone involved in the oceanographic cruises, especially the crews of the RVs ‘García del Cid’ and ‘Sarmiento de Gamboa’. Also, thanks to Dr. P. Puig for providing the CTD and acoustic releasers, to Dr. G. Rotllant and Dr. J.A. Garcia for their help during the tagging procedure. This work was developed within the framework of the Research Unit Tecnoterra (ICM-CSIC/UPC) and the following project activities funded by the Ministerio de Ciencia, Innovación y Universidades: RESNEP (CTM2017-82991-C2- 1-R), RESBIO (TEC2017-87861-R), and PLOME (PLEC2021-007525/AEI/10.13039/501100011033). DC acknowledges funding from the European Commission (LIFE20 NAT/ES/001270 LIFE ECOREST) and the Juan de la Cierva Formación Postdoctoral Fellowship (Ref: FJC2021-047734-I; financed by MCIN/AEI/10.13039/501100011033 and European Union NextGenerationEU/PRTR funds). IM received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie, grant agreement No 893089. ASM was supported by a PhD-fellowship (ref. PRE2021-099558) under the BITER project (PID2020-114732RBC-31, Ministerio de Ciencia e Innovación, Spanish Government). We also profited from the ‘Severo Ochoa Centre of Excellence’ accreditation granted by the Spanish Government to the Institute of Marine Sciences (ICM-CSIC) (CEX2019-000928-S).
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. Ministerio de Ciencia e Innovación,RESNEP (CTM2017-82991-C2- 1-R),RESBIO (TEC2017-87861-R),PLOME (PLEC2021-007525/AEI/10.13039/501100011033),PhD-fellowship5642. (ref. PRE2021-099558) under the BITER project (PID2020-114732RBC-31),Antoni Sánchez-Márquez,Severo Ochoa Centre of Excellence (CEX2019-000928-S),LIFE programme,LIFE20 NAT/ES/001270 LIFE ECOREST,DAMIANOS CHATZIEVANGELOU,H2020 Marie Skłodowska-Curie Actions,893089,Ivan Masmitja
Author information
Authors and Affiliations
Contributions
AJ, VM, BN, NJ, and CJB conceptualized the work. AJ, VM, BN, SMA, and CBJ tagged the animals, deployed and retrieved the monitoring equipment. MI set the functioning of acoustic tracking equipment and with VM processed the tracking data. JA and DC performed all statistic elaborations. JPJ and NJR provided background on behavioral ecology and other tracking studies for this and other species. All authors participated in the discussion of results and all equally contributed to the wiring of the texts.
Corresponding author
Ethics declarations
Conflict of interests
Authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aguzzi, J., Vigo, M., Bahamon, N. et al. Burrow emergence rhythms of deep-water Mediterranean Norway lobsters (Nephrops norvegicus) revealed by acoustic telemetry. Rev Fish Biol Fisheries 33, 1465–1482 (2023). https://doi.org/10.1007/s11160-023-09787-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11160-023-09787-2