Abstract
In mammalian research, the implementation of the 3Rs is ubiquitous. However, the adaptation of procedures for experimental work on fish seems less extensive in comparison, even though fish model organisms are common in a broad range of research fields already. To strengthen animal welfare in experimental research, we provide an overview of current research results, including studies on the nociception of fishes. Furthermore, we describe the potential of implementing the 3Rs in fish experimental research. In the context of "Reduction", we show alternative research methods to lethal sampling. Considering "Refinement", we point out possibilities to improve fish handling and indicate that adaptations to the individual species ecology are necessary. Under the aspect of "Replacement", we describe the high potential of cell cultures that can be obtained from fish tissue and give an overview of the already extensive use in ecotoxicology and virology. In addition, we illustrate that cell cultures could also be increasingly used for basic research.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background of animal experiments
Experiments on animals have a long history dating back to the sixth century B.C. (Ahne 2007). For example, Alkmaion (Alkmaion of Kroton, ca. late 6th to early 5th c. B.C.) discovered the brain with its neuronal activity and described it as the central location of cognition and perception, but also the optical nerve. His work provided fundamental knowledge and made him become a pioneer in neuroscience (https://www.dasgehirn.info/entdecken/meilensteine/alkmaion-pionier-der-experimentellen-hirnforschung). Despite Alkmaion’s achievements, Herophilos (c. 330–260 B.C.) is considered the "father of anatomy" (von Stande 1992; Pearce 2013). To better understand the anatomy, also with the aim to develop medical treatment, research was and is conducted not only on dead, but also on living animals and humans. Considerations of the implications that these experimental design pose on animal welfare became a concern only recently, because animals were long considered insensitive organisms (Maehle 2005). Until the public recognized the capabilities of animals to have a sentience similar to humans, animal welfare by our current definition, was not considered and animals were studied without ethical restrictions (in terms of specimen numbers and impact of the experiments on them).
At the end of the eighteenth century, but especially in the nineteenth century, the public became increasingly critical of animal experiments as the suffering of the animals was recognised, and the scientific necessity of these experiments was questioned. In the United Kingdom, the animal protection movement was founded and animal protection laws (Martin's Act, 1822 & "Cruelty to Animals Act (Act CtA)" 1876) were passed. But still, in 1960, approximately 30 million vertebrates were killed in animal experiments for predominantly biomedical research. In 1959, the 3Rs principle was written by William Russel and Rex Burch in the book, ‘The Principles of Humane Experimental Techniques’ (Russel and Burch 1992). The philosophy of the 3Rs (reduce, refine, and replace) is to substantially reduce the number of animal experiments (Reduction), to minimize animal suffering (Refinement), and, where possible, to eliminate animal experiments altogether and develop alternatives (Replacement). Today, the 3Rs are widely recognized as a part of high-quality scientific work ensuring ethical considerations, as well as regulating the use of animals in experimental procedures (Kirk 2018). The 3R principles developed by Russel and Burch were incorporated by the European Union into Directive 86/609/EEC in 1986 (https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A31986L0609). This directive was replaced by the current Directive 2010/63/EU of the European Parliament and of the Council on 22 September 2010, which deals with the protection of animals used for scientific purposes or for breeding (https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32010L0063&qid=1668672282372). The aim of animal experiments has always been knowledge gain in basic research, including possible later applications in humans and other animals (e.g. veterinary medicine). Thus, animal experiments are carried out to obtain a basic understanding of anatomy and physiology. Exemplarily, animal experimental research has presently a strong focus on getting a better understanding on expression of genetic mutations, and pathogenesis, bacterial and viral effects on the infected organism, its organs and immune system.
The high variety of research topics that incorporate animal based research results in a large number of experiments to be carried out on them. Consequently, animals, including fishes, are still commonly used in vast numbers.
Numbers of animals/fishes used within experiments
In 2015, a number of 192 million animals killed in experiments worldwide was estimated (Taylor and Alvarez 2019). Despite the increasing numbers of experiments since the mid-twentieth century, the appreciation for animal life has also increased, making animal health and welfare significant. Nevertheless, the numbers of animals for scientific purposes are on a constant high level and stable within the 27 EU members, UK, and Norway (Table 1, Eur-Lex 2022).
Including the data from Norway in 2018, the repartition of species used for the first time is slightly different compared to earlier years. There is an increase of 13% in fishes in animal experiments (Table 1), but a decrease in mice (− 9%), rats (− 2%) and “other mammals” (− 1%) as well as − 1% in birds (Eur-Lex 2022).
Norways Salmon (Salmo salar) aquaculture started in 1964, and became commercially profitable in the 1970’s. In the 1980’s, net cage production was developed along the entire coastline. While the total production in 1983 (17,298 MT) was still small compared to 2020 (1,388,433 MT), diseases became a challenge already in the early 1980’s (FAO 2022). Consequently, the fish farmer’s Sales organization established a joint project to work on fish welfare and research into fish health (https://en.seafood.no/news-and-media/news-archive/celebrating-50-years-of-modern-aquaculture). Given the large scale commercial production of Atlantic salmon in Norway and the need for disease prevention, the number of fishes in experiments is high, and most trials are conducted on the development of new vaccines (Knudsen et al. 2005). Thereby, the number of fish depends on projects. For example, in 2016, 10.6 million salmon, equalling five times the total number of salmons used for research in Norway during previous years, were used in two large-scale field trials with lice remedies alone (https://www.mattilsynet.no/dyr_og_dyrehold/dyrevelferd/forsoksdyr/bruk_av_dyr_i_forsok_i_2021.47085/binary/Bruk%20av%20dyr%20i%20fors%C3%B8k%20i%202021).
In the whole EU and Norway, in 2019, animals were used for basic research (45%), translational and applied research (27%) regulatory use to satisfy legislative requirements (17%) and 6% for routine production (Eur-Lex 2022). With 24.6% (2,559,532 animals, Table 1), fish are the second most used animal group after mice (52.5%). For fish, the Directive distinguishes zebrafish (Danio rerio) from other fish species. Of these 2,559,532 fishes used in 2019, 20% were zebrafish mostly used for basic research and as a model for genetically disorders. Approximately half of the remaining 2,042,339 fish were salmon (Eur-Lex 2022). Within the group of “other fishes”, the animals were used mostly for the following categories: animal diseases and disorders (709,198 fish), preservation of the species (207,051 fish), protection of the natural environment in the interests of the health or welfare of human beings or animals (205,317 fish), regulatory use (102,802 fish), as well as 126,053 fish for animal welfare experiments (Eur-Lex 2022). However, these numbers only include those fish on which an experiment was carried out and therefore an application for an animal experiment had to be submitted. Fish that were euthanised, e.g., for species surveys, zoological collections or obtaining organ tissue without prior experiment are not included. Although, also these animal numbers must also be recorded by the individual research institutions, and therefore documented by the authorities, no official statistics are publicly available.
Fish: pain and expression
Another aspect of experiments on fish are studies on pain perception. Fish are very dissimilar to humans in their way of expression. For this reason, fish have not been able to gain much “sympathy” in the past compared to, for example, mammals (Thomas 1984; Message, 2019). However, the idea that fish suffer from stress and may well experience pain is widespread (Schreck 1981; Sneddon 2009, 2015; Sloman et al. 2019). The International Association for the Study of Pain (IASP) developed a definition of pain, which is widely accepted by the scientific and medical community. Pain can be defined as "an unpleasant sensory and emotional experience associated with actual or potential tissue damage or resembles it" (IASP 2020). Further described by the IASP, pain is a "personal experience that is influenced to varying degrees by biological, psychological, and social factors. ….. Pain and nociception are different phenomena. Pain cannot be inferred solely from activity in sensory neurons. … inability to communicate does not negate the possibility that a human or nonhuman animal experiences pain" (IASP 2020).
Research in recent years suggests that fish have highly developed cognitive abilities and the necessary physiological capacity for pain reception, and thus, similar to other vertebrate groups, are capable of nociception and pain (Chandroo et al. 2004; Reilly et al. 2008; American Fisheries Society 2014; Bshary and Brown 2014; Sneddon 2019). The formerly even in academia widespread position that fishes do not feel pain or have a consciousness is now only held by a minority of researchers (Riberolles 2020). As early as 2011, Metcalfe and Craig expressed the opinion that the ethical considerations, made for higher vertebrates, should be applied equally to fish (Metcalfe and Craig 2011). In considering pain in animals, reference is often made to human pain, which according to Sneddon (2019) is in many ways a flaw in thinking. Both nociception and the pain processing system in animals may be shaped differently due to ecological and evolutionary history (Sneddon 2017; Sneddon et al. 2018; Birch 2018). In fish, different risks for survival are present resulting from their aquatic habitat, so an ecological adaptation has occurred during evolution. Thus, nociception and the pain system in fish is different from terrestrial vertebrates. However, the peripheral nervous system, which is responsible for the recognition of painful stimuli, is nevertheless homologous in many aspects among vertebrates. Many receptors involved in nociception and pain are correspondingly found in fishes and mammals (Bshary and Brown 2014).
One such example for the evidence for pain perception is the TRP (transient receptor potential) channels, which allow thermal nociception (from noxious cold to high temperatures). Hereby, it should be noted that temperature fluctuations are lower in an aquatic environment than in a terrestrial environment due to the high buffering capacity of water. Because of this property, the risk of heat or cold damage is generally lower in aquatic organisms. Any differences in the nociceptive system of fish may reflect these environmental differences (Saito and Shingai 2006). The different ecological niches within the fish animal group also led to evolutionary adaptation of TRP channels in this case. Thus, electrophysiological studies on rainbow trout (Oncorhynchus mykiss) revealed no cold-responsive nociceptors in the range + 4 °C to −7 °C (Ashley et al. 2007). This could be due to the habitat, since this species lives at temperatures of 0–25 °C. Zebrafish, on the other hand, being native to tropical waters and therefore to higher temperature range of 20–30 °C, respond to cold stimuli. Thermo-TRP channels, which are activated by damaging heat in the respective species, have been demonstrated in various fish species (Ashley et al. 2007; Nordgreen et al. 2009; Majhi et al. 2013; Lopez-Luna et al. 2017). In these studies, it could additionally be shown that the response to noxious heat is diminished by analgesics (Lopez-Luna et al. 2017).
Another example is the physiological role of acid-sensitive ion channels (ASICs), which monitor extracellular pH and are involved in synaptic transmission, mechanosensitivity and nociception. The existence of ASICs has been demonstrated in Hemicordata and Chordata and is thus evolutionarily highly conserved (St. John Smith and Lewin 2009; Omerbašić et al. 2015; Sneddon et al. 2017; Burrell 2017; Lynagh et al. 2018). The similarities of molecular masses as well as amino acid sequences of fish, rats, and humans ASICs are high (Viña et al. 2015). For fish, altered behaviours (e.g., decrease in swimming activity) and altered cerebral action potentials have occurred after injections with acetic acid on the lip and facial skin or in the caudal fin (Batista et al. 2018).
In addition to nociception and pain sensation in fish, it has also been documented that fish are adaptable and social animals that hunt in a coordinated manner (Arnegard and Carlson 2005; Herbert-Read et al. 2016; Oliveira et al. 2017). They play, use tools, and in some cases can even distinguish quantities, allowing fish to notice when the number of conspecifics in the tank is dwindling (Millot et al. 2014; Rischawy et al. 2015; Gómez-Laplaza et al. 2018).
For all these reasons, the implementation of the 3Rs has to be considered a critical goal for fish research, as it is already the case for other vertebrate groups.
Application of the 3Rs principles in fish research
Increasing concerns about wild fish population declines and ocean health, as well as the simultaneous increase in aquaculture production, have led to a rethinking during the recent years. The sustainability as well as the animal welfare of food fish produced in aquaculture has become an issue for both the general public and the scientific community (Clover 2005; Roberts 2007; Balcombe 2017; Message and Greenhough 2019; Stien et al. 2020). However, one of the major controversies in fish conservation, although now enshrined in law (Oberlandesgericht Celle1997; Schmidt 2021), is the question of accepting or rejecting the idea that fish are conscious beings (Braithwaite et al. 2013; Brown 2015; Sneddon 2015). This largely determines the extent to which a researcher or fish keeper contributes to fish welfare (refinement: e.g., through analgesia, animal handling) (Browman et al. 2019). Ethical understanding in relation to fish has therefore become a highly controversial and hotly debated topic, especially in recent years (Chatigny 2019).
In principle, animal welfare uses the behaviour or behavioural deficits of the animals as assessment criteria (Mason and Mench 1997). In addition, in behavioural biology, selection tests are conducted. These tests have limitations as they are generally based on the assumption that an animal knows and would choose what is best for its own welfare (Sloman et al. 2019), which, as maybe also for us humans, may not necessarily be the case. For example, in selection of food, the healthiest and most beneficial is not always chosen, as other factors may influence the selection (Mela 1999; Leng et al. 2017).
Since “communication” and articulation of fish is problematic, a variety of indicators have been proposed for this animal group to assess the level of well-being, such as colour change, decrease or increase of respiratory rate and swimming breathing rate, different swimming behaviour, decreased food intake, loss of condition, slower growth, abnormalities in morphology, injuries or disease outbreaks, and also decrease in reproductive performance (Huntingford et al. 2006; Sneddon et al. 2016). In reality, combined indicators are probably the best way to assess well-being to account for intra- and interindividual differences in specific reactions and responses that exist across animal groups/species (Mason et al. 1993; Huntingford et al. 2006; Sloman et al. 2019).
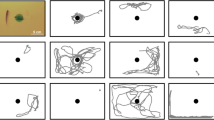
In order to promote animal welfare, further adjustments can be made in research or alternatives can also be used to further advance the 3Rs principles. In Norway, the government has even established a National Consensus–Platform for Replacement, Reduction and Refinement of Animal, with advices on application of the 3Rs related to fish research as main areas of focus (Grimholt et al. 2009). Some examples of the 3Rs in fish research are presented below (Fig. 1).
Summary of possibilities to alternative methods in fish research. Three areas are present “Reduce”, “Refine” and “Replace” of fish in experiments”. The number of fish in research can be reduced by e.g. “recycling” of fish or using water samples. As part of refinement, samples can be taken by mucosal swabbing or gastric lavage, but the proper way to anaethetise a fish must also be considered. From a replacement perspective, cell cultures established from fish can provide a research tool
Three R’s–reduction of fish experiments and lethal sampling in fish
Within the 3R's, reduction is defined as reducing the number of test animals to a necessary level. The rule here is: "As few as possible, as many as necessary". This rule states that it should be clarified in advance how many animals are necessary to obtain for a statistically valid statement. In addition, a literature search should be conducted whether the planned studies have already been carried out by other researchers. Therefore, a well thought-through experimental design, statistical and methodological optimisations need to be planned. In addition, animals can be used more than once for reducing the total number of animals. However, this aspect is only permitted under specific conditions and regulated by the Directive 2010/63/EU (https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32010L0063&qid=1668672282372). Here it is described, that the re-use of animals always depends on the actual level of severity experienced by the animal in a previous procedure, and the health and well-being of the animal, taking into account the lifetime experience of the individual (Eur-Ex 2022).
In total, the number of reuses of animals used for research, testing, routine production and educational purposes remains stable with 2% (Eur-Ex 2022). This average value is also present in fish (Table 2; Eur-Ex 2022). However, there are also animal groups in which the animals are used several times, such as larger mammals, whose reproduction time is greater and fewer offspring are produced (Table 2, Eur-Ex 2022).
Besides the reuse of animals, suitable animal models should be carefully selected based on empirical values. Further, a central registration of the results obtained from animal experiments and good coordination between scientists should prevent similar experiments from being carried out more often than necessary. In addition to these described procedures, further reductions in animal experiments or even euthanisations of fish can be achieved through alternative methods in fish research. In the following, some examples will be described in more detail.
-
1.
Non-invasive/lethal method for examining species diversity and population
During the last decade, the use of eDNA (environmental DNA) analysis has been increased in fish research (Wang et al. 2021) for example to identify individual species, but also to monitor fish populations in terms of abundance and biomass (Rourke et al. 2021). The eDNA can be mitochondrial or nuclear DNA from a variety of sources, such as excreted intestinal, blood, and skin cells, faces, urine, mucus, eggs, or semen, which can be found in water samples, in both cellular and extracellular forms (Balasingham et al. 2018). Rourke et al. concluded, that the advantages of eDNA monitoring are not only a non-lethal sampling, but also lower costs and higher sampling efficiency compared to traditional lethal sampling (Rourke et al. 2021).
-
2.
Non-invasive/lethal method for measuring hormone level in fish
In aquaculture, knowledge of spawning time is of great economic importance, on the one hand, to standardise the spawning time of the fish within different tanks and, on the other hand, to transfer the spawn so that it is not eaten directly by conspecifics and to be able to monitor and treat it specifically. For this purpose, the hormone status of the spawners is determined. In general, fishermen and scientists use blood sampling for hormone analysis. But, especially with larger shoals of fish, few animals are sometimes taken to look directly at the reproductive organs or brains in order to conclude that they are ready to spawn. As alternative to that, steroid and testosterone levels of fish can be determined directly from water samples as a non-lethal and non-invasive method (Scott et al. 2007; Scott et al. 2013; Félix et al. 2013).
Three R’s—refinement of fish husbandry and handling
The aim of refinement is to ensure that the animals are kept in a manner appropriate to their species, which includes abiotic parameters such as temperature, salinity, tank size, but also biotic parameters like social contact with conspecifics. Another important aspect in fish experimental research is also the issue of anaesthesia and euthanasia and general stress reduction during handling.
Husbandry and fish farming
Meanwhile, fish welfare is also an important issue in commercial farming. The welfare of fish depends on many decisions in husbandry practices and long-term production planning. In many countries, the topic of fish welfare is being addressed by NGOs, animal welfare organizations, regulators, policy makers and consumers. For some years now, there have been strong efforts to make aquaculture more sustainable and responsible. For this reason, the Aquaculture Stewardship Council (ASC) was founded, which sets standards for the aquaculture industry so that certified products can be sold. These standards represent sustainability and welfare, and include water quality, responsible feed sourcing, disease prevention, animal welfare, among others (https://www.asc-aqua.org/). To keep fish species-appropriate, husbandry, breeding, and rearing manuals already exist for some economically important species like salmon (RSPCA 2018a, b). These manuals describe the different options of aquaculture systems, as well as the optimal conditions of biotic and abiotic factors, and outline how the health status of the animals should be checked. Due to high number of fish species and their ecological diversity, their requirements differ. In the wild, fish actively relocate their position to areas where the physicochemical properties of the water are more favorable for them. This active relocation is not possible in animal husbandry or laboratory facilities. The physicochemical parameters of the water are set by the keeper and need to be adjusted to the optimal well-being of the fish species and must be determined in advance (Toni et al. 2019). Thus, in aquaculture and fish research, animal testing usually consists of studies on husbandry conditions to obtain an optimal survival rate, fish growth, reproduction and excellent fish meat quality. In this context, the factor of animal welfare has become increasingly important, especially in recent years.
-
1.
Light regime—what should be considered
In order to explain the difficulty and extent of this abiotic adaptation, the factor light shall be considered as an example as it plays a major role in fish husbandry and aquaculture (Assan et al. 2021). In temperate and polar regions, the reproductive cycle, fish growth and activity is influenced not only by temperature but also by the different light intensities, colour, and photoperiod (Boeuf and Le Bail 1999). In general, fishes are either more active in light, less active in darkness, or vice versa (Boeuf and Le Bail 1999). For example, in aquaculture, the bream (Abramis brama) is fed at night (Žiliukienė 2005), while nile tilapia is fed at day-time (Wang et al. 2023) due to their activity. Especially in the larval and juvenile stages, many "light" attempts were made to determine optimal fish survival and growth (Blanco-Vives et al. 2010; Schligler et al. 2021; Wang et al. 2023). For normal development without deformities or good growth, animals require species-specific lighting conditions (intensity and spectrum) with a minimum threshold intensity (Boglione et al. 2013; Sánchez-Vázquez et al. 2019; Blanco-Vives et al. 2010) as light changes with water depth. By this, constant light or darkness, or red lights can lead to increased malformations and mortality (Boglione et al. 2013; Sánchez-Vázquez et al. 2019). Since the blue light dominates with increasing water depth, this colour usually results in best hatching rates and growth performance. Light is also important for localisation of prey or even detection of predators. Furthermore, light is also critical for body pigmentation, which in turn is for camouflage and is related to early development and growth (Grunow et al. 2022). Too intense light, on the other hand, can be stressful or even fatal. Information on lighting conditions was provided in several papers, even if they mostly refer to the circadian rhythm rather than to the light source, the light intensity and the wavelength used (Isorna et al. 2017; Toni et al. 2019). In salmonid aquaculture, the 24/0 light regime is often used for faster growth due to the permanent activity of the animals. Even if various studies show that a 24/0 rhythm does not lead to stress or an increased mortality, but rather to best growth performance in e.g. Atlantic salmon, it does not seem to be true for all salmonids. For brook trout (Salvelinus fontinalis), it could be shown that a more natural light regime seems to be more suitable. Lundova et al. exposed specimen to 48 h of natural photoperiod alternating with 24 h of constant light, which resulted in increased somatic growth in both sexes (Lundova et al. 2021). Another positive effect was the delayed gonadal development and the onset of puberty (Lundova et al. 2021). Additionally, Valenzuela et al. concluded in their study that a 24/0 regime contributes to increased stress production in rainbow trout (Valenzuela et al. 2022). During their evolution, fish have been adapted to their natural habitats, so that these should also be maintained in the husbandry as far as possible regarding to animal welfare.
-
2.
Anaesthesia and euthanasia—what should be considered
In research and in aquaculture, it is occasionally necessary to anesthetise the animals for general examinations, to implant transponder or also for the slaughter process. Guidelines have been developed for the aquaculture industry to implement this, stating that fish should be killed in a manner that does not cause fear or pain (EFSA 2004; OIE 2018). For the slaughter process, an immediate and irreversible unconsciousness is crucial, so that slaughter personnel have sufficient time to kill the fish (EFSA 2004; OIE 2018). Because a large volume of fish typically must be stunned in the aquaculture industry, the proposed stunning methods that meet the requirement for immediate loss of sentience when properly applied are percussive and electrical stunning (EFSA 2004; OIE 2018). By this, Bowman et al. (2020) have shown that the CO2 fumigation method should not be used for this purpose because the time of unconsciousness is prolonged at low temperatures and the animals generally show less activity at low temperatures and therefore, the degree of unconsciousness is difficult to detect visually. Therefore, this method can lead to errors regarding of the status of sedation (Bowman et al. 2019).
In fish research, the range of commonly used chemicals for anaesthesia is wide: Benzocaine (Ethyl p-Aminobenzoate), Diazepam (Valium), Ethanol (Ethyl Alcohol), Ether (Dimethyl Ether), Eugenol/Isoeugenol (Clove Oil), Isoflurane (1-Chloro-2,2,2-Trifluoroethyldifluoromethyl Ether), Ketamine Hydrochloride, Propofol (2,6-Diisopropylphenol), Quinaldine Sulfate (2-Methylquinoline Sulfate), Tricaine Methane Sulfonate, MS-222 (3-Aminobenzoic Acid Ethyl Ester) (Saint-Erne et al. 2015). To monitor the animals during sedation, it is recommended to check the water temperature and the dissolved oxygen concentration in the water (Saint-Erne et al. 2015). Moreover, it is advised to monitor the fish itself with a pulse oximeter (Saint-Erne et al. 2015). But, how much of the anaesthetics should be added to the water? This is species and age dependent, but also influenced by abiotic parameters, such as temperature (Neiffer and Stamper 2009; Öğretmen et al. 2014). For example, for adult zebrafish most researchers use MS222 at a concentration of 164 mg/L (Collymore et al. 2014), but for the spotted knifejaw (Oplegnathus punctatus) a concentration of just 80 mg/L (Jia et al. 2022) is necessary. These examples show that species react different to anaesthetics and concentration, which in total require assessment for each species used (Matsche et al. 2011; Machnik et al. 2018; Ferreira et al. 2022). The traditional method of visually evaluating unconsciousness by examining movement, swimming position and gill breathing has been shown to be insufficient (Bowman et al. 2019). Since anesthetics, such as MS222, are partly based on muscle relaxants, they can lead to paralysis with full consciousness. In this case, too, the fish would lie paralysed in the water, could not actively swim and no gill breathing would be present. Therefore, Bowman et al. (2019) described that the accurate assessment of changes in consciousness—and thus the exclusion of paralysis—should be done by analysing brain activity using electroencephalography (EEG) and electrocardiography (ECG) in big and small fish (Rendon-Morales et al. 2005; Bowman et al. 2018; Bowman et al. 2019). In principle, consideration should be given to whether the examinations and thus anaesthesia are necessary, as they are detrimental to the animal, and can results in an impairment in memory and cognitive flexibility (Fontana et al. 2021).
Non-invasive/lethal method for measuring stress level in fish
As another example of the possibility of Refinement, we would like to address the examination of stress levels. For determining the stress level (e.g. in climate change studies—temperature increase), it has been successfully demonstrated that cortisol levels can be obtained and analysed directly from skin mucus, faeces or water samples (Fanouraki et al. 2008; Félix et al. 2013; Carbajal et al. 2019; Tilley et al. 2020), so that blood sampling or organ removal can be omitted. This method not only reduces fish testing and euthanisations, but is also easier and faster to perform. Additionally, blood sampling is particularly problematic in smaller fish species (Zemanova 2020) and has been associated with higher mortality rates for American cliff swallows Petrochelidon pyrrhonota (Brown and Brown 2009).
Non-invasive method for tracking individuals
Animal research often requires the tagging of individual animals, as the identification of individuals is often crucial in studies of wild and captive populations (Marshall et al. 2012) to obtain data on migration, behaviour, survival, reproduction, and growth of individual animals. Virtually all tagging methods require capturing the animals, which is stressful for them. Tagging methods commonly used in fish biology include fin clipping and physical tagging with e.g. T-bar anchors and PIT tags, which involve tissue damage. In Norwegian aquaculture, Atlantic salmon, Salmo salar is fitted with an acoustic transmitter for real-time monitoring (Føre et al. 2017). For migration tracking, other tags have been implanted in the fish in the form of radio transmitters, each of which is an invasive intervention (Pincock et al. 2010). In addition, the physical methods of single animal tracking in particular are unsuitable for younger developmental stages due to their small body size (Vollset et al. 2020). Other limitations and disadvantages are the loss of tags, growth reduction, health restrictions, e.g. due to injuries, and also the difficulty of fleeing from predators (Hoyle et al. 2015; Burdick 2011; Stansbury et al. 2015; Zemanova 2020). Thus, tagging can directly and indirectly lead to the death of the animal. Furthermore, it was reported for several decades that fin tagging resulted in reactions demonstrating pain in the fishes (Hansen 1988; Roques et al. 2010; Deakin et al. 2019). Therefore, one aim in aquaculture and fisheries research is the development of non-invasive methods for tracking and identifying fish in aqaculture and wild. It could already be proven with several fish species that individual animal recognition can successfully used via the natural markings, such as scars and pigmentation, which make individuals unique and are also retained for life (Arzoumanian and Norman 2005; Meekan et al. 2006; Martin-Smith 2011; Gonzalez-Ramos 2017; Saberioon et al. 2017; Hook et al. 2019b; Zemanova 2020). Saleh et al. (2022) presented an overview of computer vision and deep learning techniques used in research for identifying fish under water. Even in salmon aquaculture, the identification of individual fish could be shown to be possible via analysing their dotted pigmentation patterns (Cisar et al. 2021). In total, the various computer vision techniques for image processing can be used to study fish swimming movements (Alvaro et al. 2017; Landgraf et al. 2021), classify fish species or individual fish (Zion et al. 2000; Cisar et al. 2021; Saleh et al. 2022), determine size (Petrell et al. 1997; Zion et al. 2000) or even monitor their behaviour (Zhou et al. 2019; Cisar et al. 2021; Saleh et al. 2022). These softwares, available in the web, can be used free of charge or the code is free available and can be adjusted to the necessary “fish” requirement and research question.
Non-invasive/lethal method for examining trophodynamics
To understand ecosystems and develop effective species conservation strategies, knowledge on life history traits, such as reproduction, growth, and survival of animals, is essential. So far, in this field of research large numbers of fish need to be sampled, whether through notified animal testing or euthanisation without a prior animal experiment, even though the final aim is to conserve the species. In order to reduce the number of fish killed, Hammerschlag and Sulikowski (2011) described how non-invasive methods can be used to study fish for some experimental questions. For example, for trophodynamic studies, the intestine is often dissected, and the contents analysed. Alternatives to this lethal method would be gastric lavage or faecal examination followed by DNA analysis. Gastric lavage under anaesthesia has been widely applied and, with a high survival rate successfully used for herbivorous fish as well as apex predatory fish (Barnett et al. 2010; Hammerschlag and Sulikowski 2011; Elston et al. 2015; Braga et al. 2017). These methods could ensure greater knowledge about the ecology and conservation of fishes, especially for populations of small and rare species that are endangered.
Non-invasive/lethal method for measuring DNA
Furthermore, especially in the field of genetics, methods have been greatly improved in recent years. Meanwhile, complex analyses on species identification, hybridization, population structures and genetic diversity can be conducted with non-invasive methods. Both nuclear DNA (nDNA) and mitochondrial DNA (mtDNA) have been even used in elasmobranch species for the study of population structure and behaviour (Domingues et al. 2017; Larson et al. 2017; Hook et al. 2019a). The obtained genetic fingerprint thereby provides a specific DNA pattern of an individual animal. The specimen-specific profile can thus be obtained from body tissue or fluid, and relationships between individuals within one or more populations can thus be identified or distinguished (Hoelzel 1998; Hook et al. 2019a). Questions concerning population dynamics and genetic health can be answered in this way. DNA samples can be obtained directly for further genetic analyses by swabbing the skin or buccal mucosa. This method is not new and has proven successful over many years in numerous diverse fish species (Reid et al. 2012; Taslima et al. 2017; Domingues et al. 2019) and can further help to reduce the number of fish killed.
Three R’s—replacement of in vivo to in vitro experiments
The aim of "replacement" is to establish alternative model systems and to apply them in experiments instead using animals. Depending on the scientific question, it must be examined whether "simpler" organisms such as bacteria or invertebrates can be used, or whether complete cell and tissue cultures as well as computer models can be used. The establishment of cell models has become a rapidly growing field of research since the 1950s. Thus, the first cell line was developed from a cervical carcinoma. The name of this cell line HeLa is due to the name of the "donor" Henrietta Lacks (Sklott 2010).
Ten years later, the first fish cell culture was developed. This cell culture was established from rainbow trout gonads (RTG-2), (Wolf and Quimby 1962). By 1980, 61 fish cell lines from 36 fish species in 17 families had been described (Fent 2001). Thirty years later, 503 cell lines were established from finfish worldwide (Lakra et al. 2011). Now, the cellosaurus database lists 908 cell lines derived from about 200 fish species (https://www.cellosaurus.org/). Currently, a few fish cell lines from various teleost fishes can be purchased from ECACC (European Collection of Authenticated Cell Cultures), ATCC (American type culture collection) and ISZLER (Istituto Zooprofilattico Sperimentale della Lombardia e dell'Emilia Romagna) and two Indian companies (NRFC and NCCS), among others. But, most of the cell lines are unavailable commercially and are only stored in individual research institutions.
Use of fish cells in basic research
The high number of fish currently used in basic research or for the conservation of the species (see Table 2) illustrates the need to establish cell lines of fish species. Cell models can serve as an essential tool for detailed research purposes, such as cell physiological reactions to understand the fish physiology in more detail. In addition, cell lines should also be established for the fish species of the temperate climate zone, because numerous in vivo experiments are being carried out in "climate research" in particular. Thereby, the production of cell lines from IUCN red-listed fish species should be a priority in order to avoid further reducing these populations through animal testing (Kaya et al. 2022). In the last decades, it has been shown that climate change and the associated rising temperatures have a significant impact on fish (e.g. Beamish 1995; Rijnsdorp et al. 2009; Asch et al. 2019). Reactions to temperature fluctuations or other stressors—such as changes in oxygen content or pH—can be simulated under controlled exogenous conditions and thus studied physiologically, especially in the first phase of studies at the cell level, to replace animal experiments (Yebra-Pimentel et al. 2019; Grunow et al. 2021; Schäfer et al. 2021; Goswami et al. 2022).
The use of fish cells in applied research
The high potential of fish cell cultures for applied research has exemplarily been proven in ecotoxicological studies, in fish virology, and for risk assessments, as well as for (fish) pharmacology (Grunow et al. 2011; Fierro-Castro et al. 2013; Mehnert et al. 2013; Hodgson et al. 2018; Chang et al. 2019). Additionally, in the key areas of aquaculture, such as fish health, disease diagnosis, safety and nutritional aspects that challenge, aquaculture production can be examined on in vitro models without the need of in vivo models (Goswami et al. 2022). Besides this, also genetically modified fish cell lines have enormous potential for use in fish health, genetics and biotechnological research. Goswami et al. (2022) described in more detail the establishment of fish cell lines and their application. Therefore, here, just two examples for very successful application in fish research will be given.
-
1.
Fish cell cultures in ecotoxicology
Fish have always served as test organisms for ecotoxicological studies because ecotoxic substances (e.g. esters, aldehydes, alcohols, phenols and ketones) enter the aquatic environment through various pathways. Since many biological systems have been conserved through evolution, the effects on fish can serve as a warning of possible effects on human health. Fish are therefore important as laboratory models for the study of ecotoxicology, also regarding human health (Bols et al. 2005). Efforts in ecotoxicology to dispense with animal experiments have existed since the 1980s (Bols et al. 1985; Ahne and Halder 1990; Babich et al. 1987; Kramer et al. 2009; Tanneberger et al. 2013). The laboratory of Niels Bols has succeeded in establishing various fish cell lines, such as RTL-W1 from the liver of rainbow trout (O. mykiss), and RTgill-W1, with which, in addition to RTG-2, specific toxicity reactions for individual chemicals (e.g.Clemons et al. 1996; Behrens et al. 2001; Bols et al. 2005; Lee et al. 2009) or for toxicity screening of complex environmental samples such as wastewater or sediment extracts can be measured (e.g.Castaño et al. 1994; Brack et al. 2000; Dayeh et al. 2002; Kramer et al. 2009). As early as 1985, Bols et al. showed a significant correlation between the in vitro cytotoxicity of 12 aromatic hydrocarbons in a fish cell line and their in vivo LC50 toxicity values. Other studies on this topic also reported high in vitro-in vivo correlations of relative toxicity ranking (Babich et al. 1987; Kramer et al. 2009; Tanneberger et al. 2013; Scott et al. 2021). Published data from the last decades and from the different laboratories demonstrate that cytotoxicity tests with fish cell lines are a valuable tool for the relative classification of in vivo fish toxicity of individual chemicals and effluents, and can be used as an animal-free alternative to the in vivo lethality test with fish (Rehberger et al. 2018; Fischer et al. 2019). The EAWAG (Swiss Federal Institute of Aquatic Science and Technology, Switzerland) made a major breakthrough in April 2019: the RTgill-W1 cell line assay for predicting acute fish toxicity of chemicals and water samples, was accepted by the International Standards Organisation (ISO) as the first standardised test using fish cell lines: Preparation (submission, drafting and editing following ballot comments) of a guideline: Water quality—determination of acute toxicity of water samples and chemicals to a fish gill cell line (RTgill-W1); (https://www.iso.org/standard/69933.html?browse=tc).
In addition to these successes, however, it was also shown that further analyses are required, especially with regard to climate change. Recent studies indicate the investigation of ecotoxic substances and concentrations in connection with elevated temperatures and show the complex reactions depending on the fish species (Grunow et al. 2021). This study compared the physiological response of cell lines generated from Atlantic sturgeon (Acipenser oxyrinchus) and Maraena Whitefish (Coregonus maraena), both fish species listed on the IUCN-red list, and showed the different reaction depending on temperature and solvent and its concentration (Grunow et al. 2021). With the expected higher water temperatures due to climate change, more emphasis should be placed on fish cell cultures of different species in order to avoid animal experiments.
-
2.
Fish cell cultures in fish virology
Another application of cell cultures is in fish virology. Fish virology is an important field of research due to the very high economic losses caused by fish viruses in aquaculture (Jones et al. 2019). Thus, fish disease outbreaks can have severe economic and social impacts. In Chile, between 2007 and 2009, outbreaks of infectious salmon anaemia virus (ISAV) resulted in losses of approximately US$2 billion and 15,000 jobs in aquaculture (Mardones et al. 2011). In this field of research, too, attempts are being made to switch to in vitro models in order to reduce the high number of experimental animals.
Salmonids are particularly susceptible to diseases in aquaculture due to stock sizes. For example, farmed Atlantic salmon are susceptible to several cardial virus-related diseases, such as Cardiomyopathy Syndrome (CMS, induced by Piscine Myocarditis Virus), Heart and Skeletal Muscle Inflammation (HSMI, induced by Piscine Reovirus), Infectious Salmon Anaemia—ISA (ISAV), and Pancreatic Disease (PD, induced by Salmonid Alphavirus, also known as SPDV—Salmon Pancreas Disease Virus), (McLoughlin and Graham 2007; Haugland et al. 2011; Finstad et al. 2012). SPDV has been detected in salmonids (Atlantic salmon, rainbow trout and brown trout (S. trutta)) for over three decades (Villoing et al. 2000; McLoughlin and Graham 2007) and in some non-salmonid marine species such as common dab (Limanda limanda) for the last decade (Bruno et al. 2014). The mortality rate due to these diseases varies, but extreme growth reduction also causes economic losses in surviving animals. All these cardiotropic infections cause different immunopathological responses of the heart (Yousaf et al. 2013). Some significant findings into host–pathogen connections could be achieved through the use of non-cardiac cell lines, such as Chinook salmon (O. tshawytscha) embryo cell line (CHSE-214), the RTG-P1 cell line (RTG-2 cell line transfected and selected with a trout Mx1 promoter-luciferase construct) or the SHK-1 cell line developed from the head kidney of Atlantic salmon (Gahlawat et al. 2009; Matejusova et al. 2013). Furthermore, also cardiotropic cell models have already been used to study heart-specific viruses in Atlantic salmon (Noguera et al. 2017, 2021). However, since viruses are animal species and cell type specific, the quest for cell type specific cell models is great. Therefore, it is important to develop species- and tissue specific fish cell lines from aquaculture species. In the past, some of these established cell lines like Bluegill fry (BF-2), Chinook salmon embryo (CHSE-214), Epithelioma papulosum cyprinid (EPC), minnow (FHM), rainbow trout gonad (RTG-2) and SAF-1 could be used successfully for study common viruses such as infectious Pancreatic Necrosis (IPN), viral haemorrhagic septicemia virus (VHSV), infectious haematopoietic necrosis virus (IHNV), infectious pancreatic necrosis virus (IPNV), Koi Herpesvirus (KHV) and Channel Catfsh Virus (CCV) (Goswami et al. 2022).
Conclusion
Considerable progress has been made in implementing the 3Rs principles in fish research over the past decades. Many alternatives are already available and are constantly being further developed and researched (Fig. 1). We hope that through this article the fish community becomes more aware of these existing alternatives, whether in reduction, refinement or replacement. Hopefully, the number of in vivo studies and the number of fish euthanised for research without a prior animal experiment will decrease in the future.
Data availability
Not applicable.
References
Ahne W (2007) Tierversuche, im spannungsfeld von praxis und bioethik, 1st edn. Klett-Cotta Verlag J G Cotta’sche Buchhandlung Nachfolger GmbH, Stuttgart, Germany, p 21
Ahne W, Halder M (1990) The use of the R1-fish cell culture for detection of toxicity of waste water according to the German Waste Water Act. Altex 7:17–26
Alvaro R, Rico A, Rabuñal J, Gestal M (2017) Fish tracking with computer vision techniques: an application to vertical slot fishways. In: Multi-core computer vision and image processing for intelligent applications ISBN: 9781522508908, pp 74–105.
American Fisheries Society (2014) Guidelines for the use of fishes in research. available online: https://fisheriesorg/docs/wp/Guidelines-for-Use-of-Fishes.pdf, page 20–21. (Accesses on 17.06.2022)
Arnegard ME, Carlson BA (2005) Electric organ discharge patterns during group hunting by a mormyrid fish. Proc Biol Sci 272:1305–1314. https://doi.org/10.1098/rspB2005.3101
Arzoumanian Z, Holmberg J, Norman B (2005) An astronomical pattern-matching algorithm for computer-aided identification of whale sharks Rhincodon typus. J Appl Ecol 42:999–1011. https://doi.org/10.1111/j.1365-2664.2005.01117.x
ASC-Aqua (Access on 2022, December, 15), https:, , wwWasc-aquAorg, ., 2022 ASC-Aqua (Access on 2022, December, 15), https://wwwasc-aquaorg/
Asch RG, Stock CA, Sarmiento JL (2019) Climate change impacts on mismatches between phytoplankton blooms and fish spawning phenology. Glob Chang Biol 25:2544–2559. https://doi.org/10.1111/gcB14650
Ashley PJ, Sneddon LU, McCrohan CR (2007) Nociception in fish: stimulus–response properties of receptors on the head of trout Oncorhynchus mykiss. Brain Res 1166:47–54. https://doi.org/10.1016/JbrainreS2007.07.011
Assan D, Huang Y, Mustapha UF, Addah MN, Li G, Chen H (2021) Fish feed intake, feeding behavior, and the physiological response of apelin to fasting and refeeding. Front Endocrinol 12, 798903. doi: https://doi.org/10.3389/fendO2021.798903
Babich H, Borenfreund E (1987) Cultured fish cells for the ecotoxicity testing of aquatic pollutants. Toxic Assess 2:119–133. https://doi.org/10.1002/toX2540020202
Balasingham KD, Walter RP, Mandrak NE, Heath DD (2018) Environmental DNA detection of rare and invasive fish species in two Great Lakes tributaries. Mol Ecol 27:112–127. https://doi.org/10.1111/meC14395
Balcombe J (2017) What a fish knows: the inner lives of our underwater cousins. Oneworld Publications, London, UK
Barnett A, Redd KS, Frusher SD, Stevens JD, Semmens JM (2010) Non-lethal method to obtain stomach samples from a large marine predator and the use of DNA analysis to improve dietary information. J Exp Mar Biol Ecol 393:188–192. https://doi.org/10.1016/JjembE2010.07.022
Batist FLA, Lima LMG, Abrante IA, de Araújo JIF, Batista FLA, Abrante IA, Magalhães EA, de Lima DR, Lima M, do Prado BS, et al (2018) Antinociceptive activity of ethanolic extract of Azadirachta indica A Juss (Neem, Meliaceae) fruit through opioid, glutamatergic and acid-sensitive ion pathways in adult zebrafish (Danio rerio). Biomed Pharmacother 108:408–416. https://doi.org/10.1016/JbiophA2018.08.160
Beamish RJ (1995) Climate change and northern fish populations. canadian special publication of fisheries and aquatic sciences121.
Behrens A, Schirmer K, Bols NC, Segner H (2001) Polycyclic aromatic hydrocarbons as inducers of cytochrome P4501A enzyme activity in the rainbow trout liver cell line, RTL-W1, and in primary cultures of rainbow trout hepatocytes. Environ Toxicol Chem 20:632–643. https://doi.org/10.1002/etC5620200324
Birch J (2018) Degrees of sentience? A Sent 21. doi: https://doi.org/10.51291/2377-7478.1353
Blanco-Vives B, Villamizar N, Ramos J, Bayarri MJ, Chereguini O, Sánchez-Vázquez FJ (2010) Effect of daily thermo- and photo-cycles of different light spectrum on the development of Senegal sole (Solea senegalensis) larvae. Aquaculture 306:137–145. https://doi.org/10.1016/JaquaculturE2010.05.034
Blüte J (2018) Strafrechtliches gutachten zur rechtmässigkeit des einstellungsbescheids der staatsanwaltschaft gera vom 14.5.2018 in einem ermittlungsverfahren wegen vergehens nach dem tierschutzgesetz, AZ: 745 Js 41636/17, 2018.
Boeuf G, Le Bail P-Y (1999) Does light have an influence on fish growth? Aquaculture 177:129–152. https://doi.org/10.1016/S0044-8486(99)00074-5
Boglione C, Gisbert E, Gavaia PE, Witten P, Moren M, Fontagné S, Koumoundouros G (2013) Skeletal anomalies in reared European fish larvae and juveniles. Part 2: main typologies, occurrences and causative factors. Rev Aquac 5:121–167. https://doi.org/10.1111/raQ12016
Bols NC, Boliska SA, Dixon DG, Hodson PV, Kaiser KLE (1985) The use of fish cell cultures as an indication of contaminant toxicity to fish. Aquat Toxicol 6:147–155. https://doi.org/10.1016/0166-445X(85)90013-X
Bols NC, Dayeh VR, Lee LEJ, Schirmer K (2005) Chapter 2: Use of fish cell lines in the toxicology and ecotoxicology of fish Piscine cell lines in environmental toxicology. in biochemistry and molecular biology of fishes, tp mommsen, and tw moon, edS (Elsevier), pP 43–84. doi: https://doi.org/10.1016/S1873-0140(05)80005-0
Bowman J (2018) Development of novel methodology to measure EEG activity non-invasively in fish. University of Gothenburg, Gothenburg, Master of Science
Bowman J, Hjelmstedt P, Gräns A (2019) Non-invasive recording of brain function in rainbow trout: Evaluations of the effects of MS-222 anaesthesia induction. Aquac Res 50:3420–3428. https://doi.org/10.1111/arE14300
Bowman J, van Nuland N, Hjelmstedt P, Berg C, Gräns A (2020) Evaluation of the reliability of indicators of consciousness during CO2 stunning of rainbow trout and the effects of temperature. Aquac Res 51:5194–5202. https://doi.org/10.1111/arE14857
Brack W, Segner H, Möder M, Schürmann G (2000) Fixed-effect-level toxicity equivalents - a suitable parameter for assessing ethoxyresorufin-O-deethylase induction potency in complex environmental samples. Environ Toxicol Chem 19:2493–2501. https://doi.org/10.1002/etC5620191015
Braga RR, Ribeiro VM, Bornatowski H, Abilhoa V, Vitule JRS (2017) Gastric lavage for dietary studies of small fishes: Efficiency, survival and applicability. Acta Ichthyol Piscat 47:97–100. https://doi.org/10.3750/AIEP/02079
Braithwaite VA, Huntingford F, van den Bos R (2013) Variation in emotion and cognition among fishes. J Agric Environ Ethics 26:7–23. https://doi.org/10.1007/s10806-011-9355-x
Browman HI, Cooke SJ, Cowx IG, Derbyshire SWG, Kasumyan A, Key B, Rose JD, Schwab A, Skiftesvik AB, Stevens ED, aL, (2019) Welfare of aquatic animals: where things are, where they are going, and what it means for research, aquaculture, recreational angling, and commercial fishing. ICES J Mar Sci 76:82–92
Brown C (2015) Fish intelligence, sentience and ethics. Anim Cognit 18:1–17. https://doi.org/10.1007/s10071-014-0761-0
Brown MB, Brown CR (2009) Blood sampling reduces annual survival in cliff swallows (Petrochelidon pyrrhonota). Auk 126:853–861. https://doi.org/10.1525/auk.2009.09048
Bruno D, Noguera P, Black J, Murray W, Macqueen D, Matjusová I (2014) Identification of a wild reservoir of salmonid alphavirus in common dab Limanda limanda, with emphasis on virus culture and sequencing. Aquacult Environ Interact 5:89–98. https://doi.org/10.3354/aei00097
Bshary R, Brown C (2014) Fish cognition. Curr Biol 24:947–950
Burdick SM (2011) Tag loss and short-term mortality associated with passive integrated transponder tagging of juvenile Lost River suckers. N Am J Fish Manag 31. doi: https://doi.org/10.1080/02755947.2011.641067
Burrell BD (2017) Comparative biology of pain: What invertebrates can tell us about how nociception works. J Neurophysiol 117:1461–1473. https://doi.org/10.1152/jN00600.2016
Carbajal A, Reyes-López FE, Tallo-Parra O, Lopez-Bejar M, Tort L (2019) Comparative assessment of cortisol in plasma, skin mucus and scales as a measure of the hypothalamic-pituitary-interrenal axis activity in fish. Aquaculture 506:410–416. https://doi.org/10.1016/JaquaculturE2019.04.005
Castaño A, Vega M, Blazquez T, Tarazona JV (1994) Biological alternatives to chemical identification for the ecotoxicological assessment of industrial effluents: The RTG-2 in vitro cytotoxicity test. Environ Toxicol Chem 13:1607–1611. https://doi.org/10.1002/etC5620131009
Oberlandesgericht celle (oberlandesgericht celle, celle, Germany), AZ 23 Ss 50/97, 1997.
Database cellosaurus (Access on 2022, October, 24), Available online: https://wwwcellosaurusorg/
Chandroo KP, Duncan IJH, Moccia RD (2004) Can fish suffer?: perspectives on sentience, pain, fear and stress. Appl Anim Behav Sci 86:225–250. https://doi.org/10.1016/JapplaniM2004.02.004
Chang ED, Hogstrand C, Miller TH, Owen SF, Bury NR (2019) The use of molecular descriptors to model pharmaceutical uptake by a fish primary gill cell culture epithelium. Environ Sci Technol 53:1576–1584. https://doi.org/10.1021/acSesT8b04394
Chatigny F (2019) The controversy on fish pain: a veterinarian’s perspective. J Appl Anim Welf Sci 22:400–410. https://doi.org/10.1080/10888705.2018.1530596
Cisar P, Bekkozhayeva D, Movchan O, Saberioon M, Schraml R (2021) Computer vision based individual fish identification using skin dot pattern. Sci Rep 11:16904. https://doi.org/10.1038/s41598-021-96476-4
Clemons J, Lee L, Myers C, Dixon D, Bols N (1996) Cytochrome P4501A1 induction by polychlorinated biphenyls (PCBs) in liver cell lines from rat and trout and the derivation of toxic equivalency factors. Can J Fish Aquat Sci 53:1177–1185. https://doi.org/10.1139/F96-039
Clover C (2005) How overfishing is changing the world and what we eat. Ebury Press, London, UK
Collymore C, Tolwani A, Lieggi C, Rasmussen S (2014) Efficacy and safety of 5 anesthetics in adult zebrafish (Danio rerio). J Am Assoc Lab Anim Sci 53:198–203. PMCID: PMC3966278
Act CtA (1876), (Access on 2022, March 17) Cruelty to Animals Act. Legislation.gov.uk. Available online: https://wwwlegislationgovuk/ukpga/1876/77/pdfs/ukpga_18760077_eNpdf
Dayeh VR, Schirmer K, Bols NC (2002) Applying whole-water samples directly to fish cell cultures in order to evaluate the toxicity of industrial effluent. Water Res 36:3727–3738. https://doi.org/10.1016/s0043-1354(02)00078-7
Deakin AG, Spencer JW, Cossins AR, Young IS, Sneddon LU (2019) Welfare challenges influence the complexity of movement: fractal analysis of behaviour in zebrafish. Fishes 4. doi: https://doi.org/10.3390/fishes4010008
Directive 2010/63/EU of the European parliament and of the council (Access on 2022, November, 11) https://eur-lexeuropaeu/legal-content/EN/TXT/?uri=CELEX%3A32010L0063&qid=1668672282372
Directive 86/609/EEC the European union available online: xxxx Directive 86/609/EEC the European UnioN Available online: https://eur-lexeuropaeu/legal-content/EN/ALL/?uri=celex%3A31986L0609 (accessed on 17.11.2022)
Domingues RR, Hilsdorf AWS, Gadig OBF (2017) The importance of considering genetic diversity in shark and ray conservation policies. Conserv Genet 19:501–525. https://doi.org/10.1007/s10592-017-1038-3
Domingues RR, Garrone-Neto D, Hilsdorf AWS, Gadig OBF (2019) Use of mucus as a non-invasive sampling method for DNA barcoding of stingrays and skates (batoid elasmobranchs). J Fish Biol 94:512–516. https://doi.org/10.1111/jfB13919
EFSA (2004) Opinion of the Scientific Panel on Animal Health and Welfare on a request from the Commission related to welfare aspects of the main systems of stunning and killing the main commercial species of animals. EFSA J 45:1–29. https://doi.org/10.2903/JefsA2004.45
Elston C, von Brandis RG, Cowley PD (2015) Gastric lavage as a non-lethal method for stingray (Myliobatiformes) diet sampling. Afr J Mar Sci 37:415–419. https://doi.org/10.2989/1814232X2015.1076519
Eur-Lex, European Commission 2022 (Access on 2022, November 17) Available online: https://ec.europa.eu/environment/chemicals/lab_animals/pdf/SWD2019_Part_A_and_B.pdf
Fanouraki E, Papandroulakis N, Ellis T, Mylonas C, Scott A, Pavlidis M (2008) Water cortisol is a reliable indicator of stress in European sea bass, Dicentrarchus labrax. Behaviour 145:1267–1281
FAO (2022) Fishery and aquaculture statistics global aquaculture production 1950–2020 (FishStatJ) In: FAO fisheries and aquaculture division online. rome updated 2022. wwWfaOorg/fishery/statistics/software/fishstatj/en
Félix AS, Faustino AI, Cabral EM, Oliveira RF (2013) Noninvasive measurement of steroid hormones in zebrafish holding-water. Zebrafish 10:110–115. https://doi.org/10.1089/zeB2012.0792
Fent K (2001) Fish cell lines as versatile tools in ecotoxicology: assessment of cytotoxicity, cytochrome P4501A induction potential and estrogenic activity of chemicals and environmental samples. Toxicol in Vitro 15:477–488. https://doi.org/10.1016/s0887-2333(01)00053-4
Ferreira JM, Jorge S, Félix L, Morello GM, Olsson IAS, Valentim AM (2022) Behavioural aversion and cortisol level assessment when adult zebrafish are exposed to different anaesthetics. Biology. https://doi.org/10.3390/biology11101433
Fierro-Castro C, Barrioluengo L, López-Fierro P, Razquin BE, Villena AJ (2013) Fish cell cultures as in vitro models of inflammatory responses elicited by immunostimulants expression of regulatory genes of the innate immune response. Fish Shellfish Immunol 35:979–987. https://doi.org/10.1016/JfsI2013.07.015
Finstad OW, Falk K, Løvoll M, Evensen O, Rimstad E (2012) Immunohistochemical detection of piscine reovirus (PRV) in hearts of Atlantic salmon coincide with the course of heart and skeletal muscle inflammation (HSMI). Vet Res 43:27. https://doi.org/10.1186/1297-9716-43-27
Fischer M, Belanger SE, Berckmans P, Bernhard MJ, Bláha L, Coman Schmid DE, Dyer SD, Haupt T, Hermens JLM, Hultman MT, aL, (2019) Repeatability and reproducibility of the RTgill-W1 cell line assay for predicting fish acute toxicity. Toxicol Sci 169:353–364. https://doi.org/10.1093/toxsci/kfz057
Fontana BD, Alnassar N, Parker MO (2021) Tricaine Methanesulfonate (MS222) Has short-term effects on young adult zebrafish (Danio rerio) working memory and cognitive flexibility, but not on aging fish. Front Behav Neurosci 15, 686102. doi: https://doi.org/10.3389/fnbeH2021.686102
Føre M, Frank K, Dempster T, Alfredsen JA, Høy E (2017) Biomonitoring using tagged sentinel fish and acoustic telemetry in commercial salmon aquaculture: A feasibility study. Aquac Eng 78(B):163–172. doi: https://doi.org/10.1016/JaquaenG2017.07.004
Gahlawat SK, Ellis AE, Collet B (2009) Expression of interferon and interferon- induced genes in Atlantic salmon Salmo salar cell lines SHK-1 and TO following infection with Salmon AlphaVirus SAV. Fish Shellfish Immunol 26:672–675. https://doi.org/10.1016/JfsI2009.02.021
Gómez-Laplaza LM, Díaz-Sotelo E, Gerlai R (2018) Quantity discrimination in angelfish, Pterophyllum scalare: a novel approach with food as the discriminant. Anim Behav 142:19–30. https://doi.org/10.1007/s10071-017-1104-8
Gonzalez-Ramos MS, Santos-Moreno A, Rosas-Alquicira EF, Fuentes-Mascorro G (2017) Validation of photo-identification as a mark–recapture method in the spotted eagle ray Aetobatus narinari. J Fish Biol 90:1021–1030. https://doi.org/10.1111/jfb.13215
Goswami M, Yashwanth BS, Trudeau V, Lakra WS (2022) Role and relevance of fish cell lines in advanced in vitro research. Mol Biol Rep 49:2393–2411. https://doi.org/10.1007/s11033-021-06997-4
Grimholt U, Johansen R, Smith AJ (2009) A review of the need and possible uses for genetically standardized Atlantic salmon (Salmo salar) in research. Lab Anim 43:121–126. https://doi.org/10.1258/lA2008.008013
Grunow B, Wenzel J, Terlau H, Langner S, Gebert M, Kruse C (2011) In vitro developed spontaneously contracting cardiomyocytes from rainbow trout as a model system for human heart research. Cell Physiol Biochem 27:1–12. https://doi.org/10.1159/000325212
Grunow B, Franz GP, Tönißen K (2021) In Vitro Fish Models for the Analysis of Ecotoxins and Temperature Increase in the Context of Global Warming. Toxics 9:286. https://doi.org/10.3390/toxics9110286
Grunow B, Reismann T, Moritz T (2022) Pre-hatching ontogenetic changes of morphological characters of small-spotted catshark (Scyliorhinus canicula). Fishes 7:100. https://doi.org/10.3390/fishes7030100
Hammerschlag N, Sulikowski J (2011) Killing for conservation: The need for alternatives to lethal sampling of apex predatory sharks. Endanger Species Res 14:135–140. https://doi.org/10.3354/esr00354
Hansen LP (1988) Effects of Carlin tagging and fin clipping on survival of Atlantic salmon (Salmo salar L) released as smolts. Aquac 70:391–394. https://doi.org/10.1016/0044-8486(88)90122-6
Haugland O, Mikalsen AB, Nilsen P, Lindmo K, Thu BJ, Eliassen TM, Roos N, Rode M, Evensen O (2011) Cardiomyopathy syndrome of Atlantic salmon (Salmo salar L) is caused by a double-stranded RNA virus of the Totiviridae family. J Virol 85:5275–5286. https://doi.org/10.1128/jvI02154-10
Herbert-Read JE, Romanczuk P, Krause S, Strömbom D, Couillaud P, Domenici P, Kurvers RHJM, Marras S, Steffensen JF, Wilson ADM, et al (2016) Proto-cooperation: group hunting sailfish improve hunting success by alternating attacks on grouping prey. Proc Royal Soc B 283. doi: https://doi.org/10.1098/rspB2016.1671
Hodgson P, Ireland J, Grunow B (2018) Fish, the better model in human heart research? Zebrafish Heart aggregates as a 3D spontaneously cardiomyogenic in vitro model system. Prog Biophys Mol Biol 138:132–141. https://doi.org/10.1016/JpbiomolbiO2018.04.009
Hoelzel AR (1998) Genetic structure of cetacean populations in sympatry, parapatry, and mixed assemblages: implications for conservation policy. J Hered 89:451–458. https://doi.org/10.1093/jhered/89.5.451
Hook SA (2019) The Application of genetics and proteomics for the conservation of sharks and their relatives. The University of Manchester, Manchester, Doctor of Philosophy
Hook SA, McMurray C, Ripley DM, Allen N, Moritz T, Grunow B, Shiels HA (2019) Recognition software successfully aids the identification of individual small-spotted catsharks Scyliorhinus canicula during their first year of life. J Fish Biol 95:1465–1470. https://doi.org/10.1111/jfB14166
Hoyle SD, Leroy BM, Nicol SJ, Hampton WJ (2015) Covariates of release mortality and tag loss in large-scale tuna tagging experiments. Fish Res 163:106–118. https://doi.org/10.1016/JfishreS2014.02.023
Huntingford FA, Adams C, Braithwaite VA, Kadri S, Pottinger TG, Sandøe P, Turnbull JF (2006) Current issues in fish welfare. J of Fish Biol 68:332–372. https://doi.org/10.1111/J0022-1112.2006.001046.x
IASP (2020) International Association for the Study of Pain. Available online: https://wwwiasp-painorg/PublicationsNews/NewsDetaiLaspx?ItemNumber=10475&navItemNumber=643 (Accessed on 16.06.2021)
ISO-Norm (2019) 2115. https://wwwisoorg/standard/69933.html?browse=tC (Access on 2022, December 12)
Isorna E, de Pedro N, Valenciano AI, Alonso-Gómez ÁL, Delgado MJ (2017) Interplay between the endocrine and circadian systems in fishes. J Endocrinol 232:141–159. https://doi.org/10.1530/joe-16-0330
Jia Y, Xie T, Gao Y, Qin H, Guan C (2022) Anesthetics efficacy and physiological response of MS222 and clove oil in spotted knifejaw Oplegnathus punctatus. Aquaculture Reports 25, 101201. https://doi.org/10.1016/JaqreP2022.101201
John Smith ST, E, Lewin GR, (2009) Nociceptors: a phylogenetic view. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 195:1089–1106. https://doi.org/10.1007/s00359-009-0482-z
Jones AE, Munro LA, Green DM, Morgan KL, Murray AG, Norman R, Ryder D, Salama NKG, Taylor NGH, Thrush MA, aL, (2019) The contact structure of Great Britain’s salmon and trout aquaculture industry. Epidemics 28:100342–100342. https://doi.org/10.1016/JepideM2019.05.001
Kaya Y, Tönißen K, Verleih M, Rebl H, Grunow B (2022) Establishment of an in vitro model from the vulnerable fish species Coregonus maraena (maraena whitefish): Optimization of growth conditions and characterization of the cell line. Cell Biol Int. https://doi.org/10.1002/cbiN11956
Kirk RGW (2018) Recovering the principles of humane experimental technique: The 3Rs and the human essence of animal research. ST&HV 43:622–648. https://doi.org/10.1177/0162243917726579
Knudsen G, Johansen R, Smith AJ (2005), (access: 2022, November 23) An analysis of the fish used in research in Norway in 2003: Numbers, species and use. Oslo: Laboratory Animal Unit, Norwegian School of Veterinary Science http://oslovet.veths.no/analysisfish2003.pdf
Kramer NI, Hermens JL, Schirmer K (2009) The influence of modes of action and physicochemical properties of chemicals on the correlation between in vitro and acute fish toxicity data. Toxicol in Vitro 23:1372–1379. https://doi.org/10.1016/JtiV2009.07.029
Lakra WS, Swaminathan TR, Joy KP (2011) Development, characterization, conservation and storage of fish cell lines: a review. Fish Physiol Biochem 37:1–20. https://doi.org/10.1007/s10695-010-9411-x
Landgraf T, Gebhardt G, Bierbach D, Romanczuk P, Musiolek L, Hafner V, Krause J (2021) Animal-in-the-loop: using interactive robotic conspecifics to study social behavior in animal groups. Annu Rev Control Robot Auton Syst 4:487–507. https://doi.org/10.1146/annurev-control-061920-103228
Larson SE, Daly-Engel TS, Phillips NM (2017) Review of current conservation genet analyses of northeast pacific sharks. Adv Mar Biol 77:79–110. https://doi.org/10.1016/bSamB2017.06.005
Lee LE, Dayeh VR, Schirmer K, Bols NC (2009) Applications and potential uses of fish gill cell lines: examples with RTgill-W1. In Vitro Cellular and Developmental Biololgy – Animal 45:127–134. doi: https://doi.org/10.1007/s11626-008-9173-2
Leng G, Adan RAH, Belot M, Brunstrom JM, de Graaf K, Dickson SL, Hare T, Maier S, Menzies J, Preissl H, Reisch LA, Rogers PJ, Smeets PAM (2017) The determinants of food choice. Proc Comp Nutr Soc 76(3):316–327. https://doi.org/10.1017/S002966511600286X
Lopez-Luna J, Al-Jubouri Q, Al-Nuaimy W, Sneddon LU (2017) Impact of analgesic drugs on the behavioural responses of larval zebrafish to potentially noxious temperatures. Appl Anim Behav Sci 188:97–105. https://doi.org/10.1016/JAPPLANIM2017.01.002
Lundova K, Matousek J, Stejskal V (2021) The Effect of Non-Circadian Photoperiod on Growth and Puberty Onset of Brook Trout Salvelinus fontinalis, Mitchill. Animals 11. doi: https://doi.org/10.3390/ani11030692
Lynagh T, Mikhaleva Y, Colding JM, Glover JC, Pless SA (2018) Acid-sensing ion channels emerged over 600 Mya and are conserved throughout the deuterostomes. Proc Natl Acad Sci USA 115:8430. https://doi.org/10.1073/pnaS1806614115
Machnik P, Schirmer E, Glück L, Schuster S (2018) Recordings in an integrating central neuron provide a quick way for identifying appropriate anaesthetic use in fish. Sci Rep 8:17541. https://doi.org/10.1038/s41598-018-36130-8
Maehle AH (2005) Tierexperimente. In: Gerabek, WE, Haage, BD, Keil, G, Wegner, W Enzyklopädie Medizingeschichte, 1st ed, De Gruyter Verlag, Berlin, Germany, ISBN: 978–3–11–097694–6
Majhi RK, Kumar A, Yadav M, Swain N, Kumari S, Saha A, Pradhan A, Saha GL, S, Samanta L, et al (2013) Thermosensitive ion channel TRPV1 is endogenously expressed in the sperm of a fresh water teleost fish (Labeo rohita) and regulates sperm motility. Channels (austin) 7:483–492. https://doi.org/10.4161/chaN25793
Mardones FO, Perez AM, Valdes-Donoso P, Carpenter TE (2011) Farm-level reproduction number during an epidemic of infectious salmon anemia virus in southern Chile in 2007–2009. Prev Vet Med 102:175–184. https://doi.org/10.1016/JprevetmeD2011.07.005
Marshall AD, Pierce SJ (2012) The use and abuse of photographic identification in sharks and rays. J Fish Biol 80:1361–1379. https://doi.org/10.1111/J1095-8649.2012.03244.x
Martin-Smith KM (2011) Photo-identification of individual weedy seadragons Phyllopteryx taeniolatus and its application in estimating population dynamics. J Fish Biol 78:1757–1768. https://doi.org/10.1111/j.1095-8649.2011.02966.x
Mason GJ, Mench J (1997) Using behaviour to assess animal welfare. In: Appleby M, Hughes BO (eds) Animal Welfare. CAB International, Wallingford, USA, pp 127–141
Mason G, Mendl M (1993) Why is there no simple way of measuring animal welfare. Anim Welf 2:301–319
Matejusova I, Lester K, Li Z, Bravo J, Bland F, Collet B (2013) Comparison of complete polyprotein sequences of two isolates of salmon alphavirus (SAV) type I and their behaviour in a salmonid cell line. Arch Virol 158:2143–2146. https://doi.org/10.1007/s00705-013-1689-4
Matsche M (2011) Evaluation of tricaine methanesulfonate (MS-222) as a surgical anesthetic for Atlantic Sturgeon Acipenser oxyrinchus oxyrinchus. J Appl Ichthyol 27:600–610. https://doi.org/10.1111/J1439-0426.2011.01714.x
McLoughlin MF, Graham DA (2007) Alphavirus infections in salmonids – a review. J Fish Dis 30:511–531. https://doi.org/10.1111/J1365-2761.2007.00848.x
Meekan MG, Bradshaw CJA, Press M, McLean C, Richards A, Quasnichka S, Taylor JG (2006) Population size and structure of whale sharks Rhincodon typus at Ningaloo Reef, Western Australia. Mar Ecol-Prog Ser 319:275–285
Mehnert JM, Brandenburger M, Grunow B (2013) Electrophysiological characterization of spontaneously contracting cell aggregates obtained from rainbow trout larvae with multielectrode arrays. Cell Physiol Biochem 32:1374–1385. https://doi.org/10.1016/JfsI2014.03.027
Mela DJ (1999) Food choice and intake: the human factor. Proc Nutr Soc 58(3):513–521. https://doi.org/10.1017/s0029665199000683
Message R, Greenhough B (2019) “But It’s Just a Fish”: Understanding the Challenges of Applying the 3Rs in Laboratory Aquariums in the UK Animals 9, 1075. doi: https://doi.org/10.3390/ani9121075
Metcalfe JD, Craig JF (2011) Ethical justification for the use and treatment of fishes in research: an update. J Fish Biol 78:393–394. https://doi.org/10.1111/J1095-8649.2010.02900.x
Millot S, Nilsson J, Fosseidengen JE, Bégout M-L, Fernö A, Braithwaite VA, Kristiansen TS (2014) Innovative behaviour in fish: Atlantic cod can learn to use an external tag to manipulate a self-feeder. Anim Cognit 17:779–785. https://doi.org/10.1007/s10071-013-0710-3
Neiffer DL, Stamper MA (2009) Fish sedation, analgesia, anesthesia, and euthanasia: considerations, methods, and types of drugs. Ilar J 50:343–360. https://doi.org/10.1093/ilaR50.4.343
Noguera P, Klinger M, Örün H, Grunow B, del-Pozo J, (2021) Ultrastructural insights into the replication cycle of salmon pancreas disease virus (SPDV) using salmon cardiac primary cultures (SCPCs). J Fish Dis 44:2031–2041. https://doi.org/10.1111/jfD13518
Noguera PA, Grunow B, Klinger M, Lester K, Collet B, Del-Pozo J (2017) Atlantic salmon cardiac primary cultures: An in vitro model to study viral host pathogen interactions and pathogenesis. PLoS One 12, e0181058. doi: https://doi.org/10.1371/journaLponE0181058
Nordgreen J, Garner JP, Janczak AM, Ranheim B, Muir WM, Horsberg TE (2009) Thermonociception in fish: Effects of two different doses of morphine on thermal threshold and post-test behaviour in goldfish (Carassius auratus). Appl Anim Behav Sci 119:101–107. https://doi.org/10.1016/JAPPLANIM2009.03.015
Norwegian Seafood Council Available online:xxxx Norwegian Seafood Council Available online: https://enseafoodno/news-and-media/news-archive/celebrating-50-years-of-modern-aquaculture (accessed on: 23.11.2022)
Öğretmen F, Gölbaşi S, Inanan BE, Kizak V, Kayim M (2014) Use of clove oil and eugenol to anesthetize fingerling shabut barbus grypus. N Am J Aquac 76:9–13. https://doi.org/10.1080/15222055.2013.824942
OIE (2018) Welfare aspects of stunning and killing of farmed fish for human consumption. OIE Aquatic Animal Health Code 7:1–4
Oliveira TA, Idalencio R, Kalichak F, Dos Santos Rosa JG, Koakoski G, de Abreu MS, Giacomini ACV, Gusso D, Rosemberg DB, Barreto RE et al (2017) Stress responses to conspecific visual cues of predation risk in zebrafish. PeerJ 5:e3739–e3739. https://doi.org/10.7717/peerJ3739
Omerbašić D, Schuhmacher LN, Bernal Sierra YA, Smith ES, Lewin GR (2015) ASICs and mammalian mechanoreceptor function. Neuropharmacology 94:80–86. https://doi.org/10.1016/JneuropharM2014.12.007
Pearce JMS (2013) The Neuroanatomy of Herophilus. Eur Neurol 69:292–295. https://doi.org/10.1159/000346232
Petrell RJ, Shi X, Ward RK, Naiberg A, Savage CR (1997) Determining fish size and swimming speed in cages and tanks using simple video techniques. Aquac Eng 16:63–84. https://doi.org/10.1016/S0144-8609(96)01014-X
Pincock D, Welch D, McKinley S, and Jackson G (2010) Chapter 6: acoustic telemetry for studying migration movements of small fish in rivers and the ocean ‒ current capabilities and future possibilities In: PNAMP special publication: tagging, telemetry, and marking measures for monitoring fish populations:105–117
Rehberger K, Kropf C, Segner H (2018) In vitro or not in vitro: a short journey through a long history. Environ Sci Eur 30:23. https://doi.org/10.1186/s12302-018-0151-3
Reid SM, Kidd A, Wilson CC (2012) Validation of buccal swabs for noninvasive DNA sampling of small-bodied imperiled fishes.J Appl Ichthyol 28:290–292. doi: https://doi.org/10.1111/j.1439-0426.2011.01889.x
Reilly SC, Quinn JP, Cossins AR, Sneddon LU (2008) Behavioural analysis of a nociceptive event in fish: Comparisons between three species demonstrate specific responses. Appl Anim Behav Sci 114:248–259
Rendon-Morales E, Prance RJ, Prance H, Aviles-Espinosa R (2005) Non-invasive electrocardiogram detection of in vivo zebrafish embryos using electric potential sensors. Appl Phys Lett 107: 193701. doi: https://doi.org/10.1063/1.4935249
Riberolles G (2020), (Access on 2022, June 16) Douleur des poissons: va-t-on continuer à noyer le poisson? La fondation droit animal, éthics & science. Available online: https://wwwfondation-droit-animalorg/104-douleur-des-poissons-va-t-on-continuer-a-noyer-le-poisson/
Rijnsdorp A, Peck M, Engelhard G, Möllmann C, Pinnegar J (2009) Resolving the effect of climate change on fish populations. ICES J Mar Sci 66:1570–1583
Rischawy I, Blum M, Schuster S (2015) Competition drives sophisticated hunting skills of archerfish in the wild. Curr Biol 25:R595–R597. https://doi.org/10.1016/JcuB2015.06.005
Roberts C (2007) The unnatural history of the sea island press: Washington. DC, USA
Roques JA, Abbink W, Geurds F, van de Vis H, Flik G (2010) Tailfin clipping, a painful procedure: Studies on Nile tilapia and common carp. Physiol Behav 101:533–540. https://doi.org/10.1016/JphysbeH2010.08.001
Rourke ML, Fowler AM, Hughe JM, Broadhurst MK, DiBattista JD, Fielder S, Wilkes Walburn J, Furlan EM (2021) Environmental DNA (eDNA) as a tool for assessing fish biomass: A review of approaches and future considerations for resource surveys. Environ DNA 4:9–33. https://doi.org/10.1002/edn3.185
RSPCA (2018a) Royal Society for the Prevention of Cruelty to Animals Welfare standards for farmed rainbow trout RSPCA, Horsham, 54 p https://science.rspca.org.uk/sciencegroup/farmanimals/standards/trout (accessed on 28.04.2021)
RSPCA (2018b) Royal Society for the Prevention of Cruelty to Animals Welfare standards for farmed Atlantic salmon RSPCA welfare standards for Farmed Atlantic Salmon, RSPCA, Horsham, 100 p https://science.rspca.org.uk/sciencegroup/farmanimals/standards/salmon (accessed on 28.04.2021)
Russel WMS, Burch RL (1992) The Principles of Human Experimental Techniques, Universities Federation for Animal Welfare: Wheathampstead, Hertfordshire, England
Saberioon M, Gholizadeh A, Cisar P, Pautsina A, Urban J (2017) Application of machine vision systems in aquaculture with emphasis on fish: state-of-the-art and key issues. Rev Aquac 9:369–387. https://doi.org/10.1111/raQ12143
Saint-Erne N, DVM, CertAqV (2015) Anesthesiology in Fish. World Small Animal Veterinary Association World Congress Proceedings, Phoenix, AZ, USA
Saito S, Shingai R (2006) Evolution of thermo TRP ion channel homologs in vertebrates. Physiol Genom 27:219–230. https://doi.org/10.1152/physiolgenomicS00322.2005
Saleh A, Sheaves M, Rahimi Azghadi M (2022) Computer vision and deep learning for fish classification in underwater habitats: A survey. Fish Fish 23:977–999. https://doi.org/10.1111/faF12666
Sánchez-Vázquez FJ, López-Olmeda JF, Vera LM, Migaud H, López-Patiño MA, Míguez JM (2019) Environmental cycles, melatonin, and circadian control of stress response in fish. Front Endocrinol (lausanne) 10:279. https://doi.org/10.3389/fendO2019.00279
Schäfer N, Kaya Y, Rebl H, Stüeken M, Rebl A, Nguinkal JA, Franz GP, Brunner RM, Goldammer T, Grunow B, Verleih M (2021) Insights into early ontogenesis: characterization of stress and development key genes of pikeperch (Sander lucioperca) in vivo and in vitro. Fish Physiol Biochem 47:515–532. https://doi.org/10.1007/s10695-021-00929-6
Schligler J, Cortese D, Beldade R, Swearer SE, Mills SC (2021) Long-term exposure to artificial light at night in the wild decreases survival and growth of a coral reef fish. Proc Biol Sci 288:20210454. https://doi.org/10.1098/rspB2021.0454
Schmidt F (2021) Misshandlungen von Tieren als Straftat In: Martínez (HrsG), Göttinger Onlinebeiträge zum Agrarrecht NR 01/21, S 1–29.
Schreck CB (1981) Stress and compensation in teleostean fishes: response to social and physical factors. In: Pickering AD (ed) Stress and Fish, 1st edn. Academic Press, New York, USA, pp 295–321
Scott A, Ellis T (2007) Measurement of fish steroid in water – a review. Gen Comp Endocrinol 153:392–400. https://doi.org/10.1016/JygceN2006.11.006
Scott A, Hirschenhauser K, Bender N, Oliveira R, Earley R, Sebire M, Ellis T, Pavlidis M, Hubbard P, Huertas M et al (2013) Non-invasive measurement of steroids in fish-holding water: Important considerations when applying the procedure to behaviour studies. Behaviour 145:1307–1328
Scott J, Belden JB, Minghetti M (2021) Applications of the RTgill-W1 cell line for acute whole-effluent toxicity testing. In Vitro–in vivo correlation and optimization of exposure conditions. Environ Toxicol Chem 40:1050–1061. https://doi.org/10.1002/etC4947
Die Unsterblichkeit der Henrietta Lacks. Irisiana Verlag, MünchenISBN: 978-3-424-15075-9.
Sloman KA, Bouyoucos IA, Brooks EJ, Sneddon LU (2019) Ethical considerations in fish research. J Fish Biol 94:556–577. https://doi.org/10.1111/jfB13946
Sneddon LU (2009) Pain perception in fish: indicators and endpoints. ILAR J 50:338–342. https://doi.org/10.1093/ilaR50.4.338
Sneddon LU (2015) Pain in aquatic animals. J Exp Biol 218:967–976. https://doi.org/10.1242/jeB088823
Sneddon LU (2017) Comparative physiology of nociception and pain. Physiology 33:63–73. https://doi.org/10.1152/physioL00022.2017
Sneddon LU, Braithwaite VA, Gentle MJ (2003) Do fishes have nociceptors? Evidence for the evolution of a vertebrate sensory system. Proc Biol Sci 270:1115–1121. https://doi.org/10.1098/rspB2003.2349
Sneddon LU, Wolfenden D, Thomson, (2016) Fish Physiology. Elsevier Inc, Amsterdam, The Netherlands, pp 463–539
Sneddon LU, Wolfenden DCC, Leach MC, Valentim AM, Steenbergen PJ, Bardine N, Broom DM, Brown C (2018) Ample evidence for fish sentience and pain. Anim Sentience 3:17. https://doi.org/10.51291/2377-7478.1375
Sneddon LU (2019) Evolution of nociception and pain: evidence from fish models. Philos Trans R Soc Lond, B, Biol Sci 374. doi: https://doi.org/10.1098/rstB2019.0290
von Staden H (1992) The discovery of the body: human dissection and its cultural contexts in ancient Greece. YJBM, 65:223–241. PMCID: PMC2589595
Stansbury A, Götz T, Deecke V, and Janik V (2015) Grey seals use anthropogenic signals from acoustic tags to locate fish: Evidence from a simulated foraging task. Proc Royal Soc B 282. doi: https://doi.org/10.1098/rspB2014.1595
NorecopA Statistikk over dyr brukt i forskning (2022, November 23) Available online: 2022 NorecopA Statistikk over dyr brukt i forskning (2022, November 23) Available online: https://wwwmattilsynetno/dyr_og_dyrehold/dyrevelferd/forsoksdyr/bruk_av_dyr_i_forsok_i_2021.47085/binary/Bruk%20av%20dyr%20i%20fors%C3%B8k%20i%202021
Stien LH, Bracke M, Noble C, Kristiansen TS (2020) Assessing Fish Welfare in Aquaculture In: Kristiansen, TS, Fernö, A, Pavlidis, M, van de Vis, H (eds) The Welfare of Fish Animal Welfare, 1st ed, Springer, Cham, Berlin/Heidelberg, Germany
Tanneberger K, Knöbel M, Busser FJM, Sinnige TL, Hermens JLM, Schirmer K (2013) Predicting fish acute toxicity using a fish gill cell line-based toxicity assay. Environ Sci Technol 47:1110–1119. https://doi.org/10.1021/es303505z
Taslima K, Taggart JB, Wehner S, McAndrew BJ, Penman DJ (2017) Suitability of DNA sampled from Nile tilapia skin mucus swabs as a template for ddRAD-based studies. Conserv Genet Resour 9:39–42. https://doi.org/10.1007/s12686-016-0614-z
Taylor K, Alvarez LR (2019) An estimate of the number of animals used for scientific purposes worldwide in 2015. Altern Lab Anim 47:196–213. https://doi.org/10.1177/0261192919899853
Thomas K (1984) Man and the Natural World: Changing Attitudes in England, New. Penguin, London, UK, pp 1500–1800
Tilley CA, Carreño Gutierrez H, Sebire M, Obasaju O, Reichmann F, Katsiadaki I, Barber I, Norton WHJ (2020) Skin swabbing is a refined technique to collect DNA from model fish species. Sci Rep 10:18212. https://doi.org/10.1038/s41598-020-75304-1
Toni M, Manciocco A, Angiulli E, Alleva E, Cioni C, Malavasi S (2019) Review: Assessing fish welfare in research and aquaculture, with a focus on European directives. Animal 13:161–170. https://doi.org/10.1017/s1751731118000940
Valenzuela A, Rodríguez I, Schulz B, Cortés R, Acosta J, Campos V, Escobar-Aguirre S (2022) Effects of Continuous Light (LD24:0) Modulate the Expression of Lysozyme, Mucin and Peripheral Blood Cells in Rainbow Trout. Fishes 7. doi: https://doi.org/10.3390/fishes7010028
Villoing S, Béarzotti M, Chilmonczyk S, Castric J, Brémont M (2000) Rainbow trout sleeping disease virus is an atypical alphavirus. J Virol 74:173–183. https://doi.org/10.1128/jvI74.1.173-183.2000
Viña E, Parisi V, Sánchez-Ramos C, Cabo R, Guerrera MC, Quirós LM, Germanà A, Vega JA, García-Suárez O (2015) Acid-sensing ion channels (ASICs) 2 and 4.2 are expressed in the retina of the adult zebrafish. Cell Tissue Res 360:223–231. https://doi.org/10.1007/s00441-014-2084-5
Vollset KW, Lennox RJ, Thorstad EB, Auer S, Bär K, Larsen MH, Mahlum S, Näslund J, Stryhn H, Dohoo I (2020) Systematic review and meta-analysis of PIT tagging effects on mortality and growth of juvenile salmonids. Rev Fish Biol Fish 30:553–568. https://doi.org/10.1007/s11160-020-09611-1
Wang K, Li K, Liu L, Tanase C, Mols R, van der Meer M (2023) Effects of light intensity and photoperiod on the growth and stress response of juvenile Nile tilapia (Oreochromis niloticus) in a recirculating aquaculture system. Aquaculture and Fisheries 8:85–90. https://doi.org/10.1016/JaaF2020.03.001
Wang S, Yan Z, Hänfling B, Zheng X, Wang P, Fan J, Li J (2021) Methodology of fish eDNA and its applications in ecology and environment. Sci Total Environ 755, 142622. doi: https://doi.org/10.1016/JscitotenV2020.142622
Wolf K, Quimby MC (1962) Established eurythermic line of fish cells in vitro. Science 135:1065–1066. https://doi.org/10.1126/sciencE135.3508.1065
Wolf C (Access on 2022, April 21) Anatom der ersten Stunde. Das Gehirn. Available online: https://www.dasgehirn.info/entdecken/meilensteine/alkmaion-pionier-der-experimentellen-hirnforschung
Yebra-Pimentel ES, Gebert M, Jansen HJ, Jong-Raadsen SA, Dirks RPH (2019) Deep transcriptome analysis of the heat shock response in an Atlantic sturgeon (Acipenser oxyrinchus) cell line. Fish Shellfish Immunol 88:508–517. https://doi.org/10.1016/JfsI2019.03.014
Yousaf MN, Koppang EO, Skjødt K, Hordvik I, Zou J, Secombes C, Powell MD (2013) Comparative cardiac pathological changes of Atlantic salmon (Salmo salar L) affected with heart and skeletal muscle inflammation (HSMI), cardiomyopathy syndrome (CMS) and pancreas disease (PD). Vet Immunol Immunopathol 151:49–62. https://doi.org/10.1016/JvetimM2012.10.004
Zemanova M (2020) Towards more compassionate wildlife research through the 3Rs principles: Moving from invasive to non-invasive methods. Wildlife Biol. https://doi.org/10.2981/wlB00607
Zhou Y, Yu H, Wu J, Cui Z, Zhang F (2019) Fish Behavior Analysis Based on Computer Vision: A Survey. In: Mao R, Wang H, Xie X, Lu Z (eds) Data Science ICPCSEE 2019. communications in computer and information science, vol 1059. Springer, Singapore doi: https://doi.org/10.1007/978-981-15-0121-0_10
Žiliukienė V (2005) The diet of Abramis brama (L) larvae reared in illuminated cages. J Appl Ichthyol 21:406–409. https://doi.org/10.1111/J1439-0426.2005.00591.x
Zion B, Shklyar A, Karplus I (2000) In-vivo fish sorting by computer vision. Aquac Eng 22:165–179. https://doi.org/10.1016/S0144-8609(99)00037-0
Acknowledgements
We thank George P. Franz from the FBN for the illustration. In addition, we would like to thank Dr. David Bruno from the University of Aberdeen for reviewing the article and discussing fish pathology. We would also like to thank all our friends and colleagues who gave us the most diverse insights into fish research and made this article possible. This article contains results of Bianka Grunow's habilitation thesis 'Die Anwendung der 3R-Grundsätze (Reduction, Refinement und Replacement von Tierversuchen) in der Fischforschung und insbesondere der Aquakultur', which was submitted to the University of Greifswald in 2021.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research was funded by the Research Institute for Farm Animal Biology (FBN).
Author information
Authors and Affiliations
Contributions
“Conceptualization, BG, writing—original draft preparation, BG, writing—review and editing, SMS, BG. All authors have read and agreed to the published version of the manuscript.”
Corresponding author
Ethics declarations
Competeting interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grunow, B., Strauch, S.M. Status assessment and opportunities for improving fish welfare in animal experimental research according to the 3R-Guidelines. Rev Fish Biol Fisheries 33, 1075–1093 (2023). https://doi.org/10.1007/s11160-023-09781-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11160-023-09781-8