Abstract
Shark depredation is a complex social-ecological issue that affects a range of fisheries worldwide. Increasing concern about the impacts of shark depredation, and how it intersects with the broader context of fisheries management, has driven recent research in this area, especially in Australia and the United States. This review synthesises these recent advances and provides strategic guidance for researchers aiming to characterise the occurrence of depredation, identify the shark species responsible, and test deterrent and management approaches to reduce its impacts. Specifically, the review covers the application of social science approaches, as well as advances in video camera and genetic methods for identifying depredating species. The practicalities and considerations for testing magnetic, electrical, and acoustic deterrent devices are discussed in light of recent research. Key concepts for the management of shark depredation are reviewed, with recommendations made to guide future research and policy development. Specific management responses to address shark depredation are lacking, and this review emphasizes that a “silver bullet” approach for mitigating depredation does not yet exist. Rather, future efforts to manage shark depredation must rely on a diverse range of integrated approaches involving those in the fishery (fishers, scientists and fishery managers), social scientists, educators, and other stakeholders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depredation, where a predator (e.g. a shark, cetacean, pinniped, seabird, squid, large teleost) completely or partially consumes an animal caught by fishing gear, is an issue in many fisheries around the world and in recent years has received increasing attention from researchers and fishery managers (Gilman et al. 2007; IOTC 2007; Mitchell et al. 2018a; Tixier et al. 2021). Shark depredation, in particular, has become a focal issue in a range of commercial, small-scale and recreational fishing contexts (Mitchell et al. 2018a). Depredation is a form of human-wildlife conflict (HWC) that has become a highly topical and emotive subject in many regions and generates polarising views due to its intersection with the broader context of fisheries management issues, such as declining fish stocks (Britten et al. 2021), increased recreational fishing participation (Arlinghaus et al. 2021) and the global push towards conserving historically over-harvested and potentially now recovering shark populations (Carlson et al. 2019; Pacoureau et al. 2021). There are a range of negative biological, economic, and social impacts from shark depredation, including, but not limited to, increased mortality of target species, loss or damage of catch and fishing gear and associated revenue, damage to the fishing experience (especially for recreational fishers), increasingly hostile views towards sharks, and retaliatory killing of sharks. Furthermore, higher shark depredation rates can lead to higher catchability risk and concomitant bycatch fishing mortality in some fisheries where the fishing gear used is capable of catching sharks (e.g. longlines, droplines), which is a concern due to the poor conservation status of many shark populations (Dulvy et al. 2014; Pacoureau et al. 2021) and because high shark catch rates reduce operational efficiency in fisheries where sharks are not retained.
Research into shark depredation dates back to the 1950s and remained at low levels until 2000, after which there was a notable increase in published literature (Gilman et al. 2007; Mitchell et al. 2018a). This likely reflects growing awareness of the issue amongst fisheries scientists and increasing calls from stakeholders to address its occurrence and impacts. Much of the early research focused on quantifying depredation rates in commercial longline fisheries (Sivasubramaniam 1964; Hirayama 1976; Mandelman et al. 2008; MacNeil et al. 2009). More recently, the focus has shifted towards recreational fisheries, particularly in Australia and the United States, where there are large recreational fishing communities that have become increasingly vocal about the need to mitigate shark depredation. This may be driven in part by increasing attention given to depredation in regular media (Major 2020; van Hoose 2021) and social media platforms (e.g. Sportsmen Fighting for Marine Balance Facebook page). As a result, there has been a strong focus on investigating shark depredation in recreational, charter (larger ‘for-hire’ vessels with guides, which typically carry > 10 fishers) and commercial fisheries in these countries, with recent studies quantifying depredation rates (Mitchell et al. 2018a; Ryan et al. 2019; Carmody et al. 2021), identifying shark species involved (Drymon et al. 2019; Fotedar et al. 2019; Mitchell et al. 2019; Vardon et al. 2021) and investigating changes in shark behaviour in the context of depredation (Mitchell et al. 2020, 2021). However, studies exploring the human dimensions of depredation conflicts remain relatively scarce.
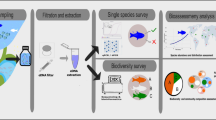
Due to this recent increase in research on depredation and an increasingly strong stakeholder focus on the issue across all fishing sectors, there is a need to review progress to date and identify priority areas for future research. This review will synthesise recent advances in the field of shark depredation research, describe best-practice methods to characterise both the social and biological aspects of depredation, and highlight key areas for future targeted research, framed within the context of how fisheries managers and fishers can apply research to characterise, reduce and manage shark depredation (Fig. 1).
Characterising shark depredation
Depredation occurs within complex systems that include multiple elements across the social and biological sciences. A comprehensive characterisation of the myriad social and biological aspects of depredation is a critical precursor to effectively reducing and managing this human-wildlife conflict.
Social science
Research on the human dimensions of depredation is only just beginning. This review outlines findings of the few studies conducted to date and describes the methods and approaches that could be applied in future research. Research on HWCs in terrestrial environments has revealed that merely understanding the physical conflict between humans and wildlife only addresses a fraction of the problem. Often, HWCs are less driven by the direct conflicts with wildlife (Fraser-Celin et al. 2018). Instead, they stem from differences in human values, beliefs, or attitudes, such as balancing conservation goals with community well-being (e.g. Bagchi and Mishra 2006; Simpfendorfer et al. 2021), which must be identified and understood to adequately address and effectively resolve contentious HWCs (Guerra 2019). Despite the increased frequency of fisher–shark conflicts, shark depredation research has only recently started to draw on such insights from terrestrial HWC studies (Tixier et al. 2021). Yet, understanding what drives human beliefs has helped navigate discord between negative human–shark interactions (e.g. shark bites) and shark conservation efforts (Pepin-Neff and Wynter 2017; Niella et al. 2021), and may prove useful in better navigating the shark depredation conflict. To date, several social science frameworks have been used to explore the conflict (Table 1).
Survey sampling
Survey sampling utilising questionnaires (where data are self-reported by the respondent) or interviews (both structured and semi-structured; where data are recorded by an interviewer) represent the most popular social science strategies to understand shark depredation impacts, including rates of depredation and fishers’ attitudes, perceptions, and behaviours (Gilman et al. 2008; Drymon and Scyphers 2017; Ryan et al. 2019; Mitchell et al. 2018a, b; Casselberry et al. 2022). Survey designs aim to maximise survey participation, using a formal list of structured questions relevant to the survey objectives and appropriate for the contact method. Published studies on fisher–shark interactions have included online questionnaires (e.g. Casselberry et al. 2022), computer assisted telephone interviews (e.g. Ryan et al. 2019), and face-to-face interviews at boat ramps (e.g. Mitchell et al. 2018b) (Table 1). Such approaches can quantify physical phenomena or responses associated with depredation, as well as illuminating less tangible aspects (e.g. attitudes). For example, a state-wide survey on shark depredation across commercial, charter and recreational fishing sectors in Western Australia identified regional depredation hotspots and characterised fishers’ concerns around the issue (Ryan et al. 2019). Additionally, boat ramp surveys of recreational fishers in Western Australia quantified depredation frequency and identified potential drivers of these interactions (Mitchell et al. 2018b). Surveys have been useful to document fishers’ mitigation strategies (Coulson et al. 2022) and understand behavioural responses of fishers to depredation (Casselberry et al. 2022). Though not directly targeting depredation, Drymon and Scyphers (2017) also used online surveys to gauge fisher attitudes, perceptions, and beliefs about shark conservation, finding fishers often view sharks as competitors. Survey sampling approaches can help guide and prioritise future depredation research. However, despite being a useful tool, surveys can have limitations, particularly with respect to minimising survey errors under potentially limited resourcing and lack the ability to explore nuances or new ideas beyond their initial design.
Though less widely applied in shark depredation research, semi-structured interviews, which use open-ended questions to prompt discussion that allows the interviewer to explore responses, are a useful tool to characterise the respondent’s attitudes, behaviours and knowledge, as well as gaining additional insights into their beliefs and values with fewer constraints (Newing et al. 2010). While semi-structured interviews are longer and more in-depth than structured questionnaires and interviews, they can uncover less obvious conflicts, values, perceptions, and opinions surrounding depredation. Semi-structured interviews can be conducted through targeted focus groups to facilitate ongoing questions and review of emerging trends and engage fishers in an ongoing process which can increase the perceived legitimacy of research findings. This review found only three studies that used semi-structured interviews to understand fisher–shark interactions more broadly, both of which included depredation. Gilman et al. (2008) took a global approach to understand and reveal trends in shark interactions with pelagic longline fisheries through interviews with fishers and port officials, finding that responses to shark depredation varied widely depending on whether sharks were viewed as nuisance bycatch or as byproduct. Key strategies that fishers reported using were moving location or switching bait types to avoid interactions (Gilman et al. 2008). Though not specifically targeting the shark depredation conflict, Iwane et al. (2021) conducted interviews with Hawaiian fishers to gauge and effectively define the problems within fisher–shark interactions, revealing that fishers viewed sharks as competitors for target species (through depredation), and more deeply, their livelihoods. Robinson et al. (2022) used semi-structured interviews to collect data on the impacts of shark depredation and how it affected fisher support for the Maldivian Shark Sanctuary established in 2010. Shark depredation was reported to cause high levels of catch and gear loss for reef fishers, although this was much lower for pelagic handline and pelagic pole and line fishers, with the latter even reporting that sharks sometimes play a beneficial role by pushing tuna up to the surface (Robinson et al. 2022). Most fishers (especially those who used to actively target sharks) had a negative view of the establishment of the shark sanctuary, reporting that it led to increases in shark populations and greater depredation (Robinson et al. 2022). As a result, 12% of fishers reported that they kill sharks as a means of reducing depredation, reducing the legitimacy and efficacy of the shark sanctuary (Robinson et al. 2022). These studies are consistent with surveys of recreational fishers in the southeast United States which found that “sharks threaten fishing efforts” (Drymon and Scyphers 2017). Likewise, Casselberry et al. (2022) found through surveys that fishing guides reported sharks to be a main threat to their livelihoods. Beyond these tangible problems, the interviews conducted by Iwane et al. (2021) also illuminated deeper fisher conflicts with management and science, which would likely have been missed through surveys alone. Gaining such valuable insights can help inform broader surveys to better quantify fisher experiences with shark depredation and provide guidance to managers and scientists about how to engage stakeholders successfully.
The limited survey and interview-based research conducted so far suggests that at a surface “dispute” level, the issue can derive from economic or physical losses and practical decisions like site or gear choices. Most of the current strategies being used to mitigate depredation (e.g. moving locations, decreasing soak times, modifying gear setups) are behavioural solutions (Gilman et al. 2008; Tixier et al. 2021), which primarily address this dispute level. However, underlying and deeply rooted conflicts around poor perceptions of management legitimacy and threatened fisher identities add complexity to the fisher–shark conflict, and call for broader interventions beyond these visible effects. Therefore, to effectively understand and address the complexities of shark depredation, these underlying conflicts and perceptions should be further explored and characterised.
Content analysis
Content analysis examines published material, such as magazine articles or social media content to explore the discourse surrounding a specific issue, event, or phenomenon. These analyses can range from simple enumeration exercises (e.g. how often a specific word is used), to more complex examinations of the themes, values, and meanings of the content. This methodology has been used to examine dominant narratives and attitudes in the media reporting on shark bites (Muter et al. 2013) and how attitudes towards sharks have changed over decadal time scales (Whatmough et al. 2011; Whitenack et al. 2021). While content analysis is a widely used social research tool, this review did not identify any examples applied to shark depredation. Nevertheless, content analysis could be very informative in understanding fisher experiences, perceptions, and attitudes towards sharks and shark depredation, and help guide scientists and managers in engaging with fishers on the issue. More specifically, content analysis could be used to identify common depredation experiences, perceptions regarding drivers of depredation and the potential solutions, and highlight conflicting narratives and associated values, beliefs, and attitudes between different groups. Therefore, content analysis could provide valuable opportunities for biologists to access fisher knowledge and could help managers in designing engagement activities. Nevertheless, content analyses need to be carefully designed and interpreted. Social scientists need to decide if sampling should be purposive or probabilistic based, to test keyword search strings for ‘recall’ and ‘precision’, and test for inter- and intra-coder reliability (Lacy et al. 2015). Furthermore, researchers may need to consider issues, such as ‘confirmation bias’ and ‘echo chambers’ in interpreting results where personal beliefs and values affect what content is created and how it is disseminated, as evidenced in social media induced polarisation regarding the COVID-19 pandemic (Modgil et al. 2021).
Participatory modelling
Participatory modelling techniques can also be used to study depredation. While no studies have yet applied these approaches to examine depredation, they are included here as a primer to guide future attempts. Among the most common participatory modelling approaches involves fuzzy-cognitive mapping (FCM) to represent individual or group “mental models” of a complex system, such as depredation (Fig. 2). Fuzzy-cognitive mapping has become increasingly popular in fisheries science to represent local ecological knowledge of complex issues, such as food web dynamics (Stier et al. 2017), climate change (McClenachan et al. 2020), and social-ecological interactions (Gray et al. 2020). In essence, FCM involves conducting interviews or focus groups to map out the most important components and causal relationships within a system. A major strength of FCM is the ability to develop models with (1) abstract (e.g. satisfaction) and aggregate (e.g. water quality) variables, (2) relationships that are not known with certainty, (3) feedback loops and cross linkages among model components, and often most importantly, (4) visual representations of scenarios or potential outcomes of policy options (Özesmi and Özesmi 2004; Gray et al. 2014). Compared to other social science approaches, FCM fits in a middle space between qualitative interviews, which are often easier to interpret but provide less detail on complex interactions or trade-offs, and more quantitative models that are often more challenging for stakeholders to interpret (Voinov et al. 2018).
Cognitive map of a US offshore fisher’s mental model of depredation (Prasky et al. unpublished data), illustrating the complex and interconnected nature of depredation. Arrows indicate the direction and influence between concepts and can be positive (+), negative (−), or unknown (?). Arrow thickness indicates the strength of the effect
Biological data collection
Depredation is influenced by a range of factors, including shark behaviour and abundance, spatial and temporal distribution of fishing effort and changes in fishing methods, gear and equipment. However, there remains a lack of empirical data to characterise these issues, and consequently much of the current knowledge is composed of anecdotal accounts and personal opinions. Biological data collection can help to address key questions relating to depredation, such as the impacts of shark depredation on target species, which shark species are involved and how their movement patterns and behaviour influence the occurrence of depredation. Such information can then inform future work to design effective mitigation approaches.
Impacts on target species
Shark depredation can have substantial impacts on the target species of a diverse range of fisheries. In particular, shark depredation can increase the overall level of mortality that occurs, especially in fisheries where fishers are seeking to reach an allowed quota (commercial fishers) or bag limit (charter and recreational fishers). Depredation of target species in catch-and-release recreational fisheries causes mortality for released fish that would otherwise mostly survive (thus undermining the catch-and-release objective), as well as diminishing the recreational fishing experience and negating the benefits of tagging programs.
There are relatively few studies that have collected data on shark depredation rates for teleost target species (Table 2), predominantly in commercial longline fisheries where valuable tuna and billfish species are targeted. The results of these studies are detailed in IOTC (2007), Gilman et al. (2007) and Mitchell et al. (2018a). Carmody et al. (2021) also quantified shark depredation rates for narrow-barred Spanish mackerel (Scomberomorus commerson) in a commercial trolling fishery in Australia. Two studies in northwest Western Australia presented results for shark depredation rates for common recreational target species, including narrow-barred Spanish mackerel, spangled emperor (Lethrinus nebulosus) and coral trout (Plectropomus spp.) (Sumner et al. 2002; Williamson et al. 2006). Shark depredation impacting catch-and-release recreational flyfishing has been investigated with permit (Trachinotus falcatus) in the Florida Keys, USA (Holder et al. 2020) and bonefish (Albula sp.) in the Bahamas (Cooke and Phillip 2004) and French Polynesia (Lennox et al. 2017). Red snapper (Lutjanus campechanus) is another key target species known to be affected by shark depredation in the southeast USA (Streich et al. 2018; Drymon et al. 2019, 2020a).
Although focused mainly on depredation by toothed whales, NOAA Fisheries has developed standardised protocols to collect depredation rate data in an annual fishery-independent survey and account for depredation mortality when conducting stock assessments and setting quotas in the Alaska sablefish fishery (Hanselman et al. 2018). To allow the impacts of shark depredation to be incorporated into stock assessments, future data collection protocols should therefore be implemented to enable quantification of depredation rates at a whole fishery level, and for the main target species in multi-species fisheries, where possible.
Identifying shark species responsible for depredation
Video
Underwater video cameras have been used in several studies to investigate depredation (Mitchell et al. 2019, 2020; Van Den Hoff et al. 2017; Wiley and Pardee 2018; Coulson et al. 2022). This research has predominantly focused on specific fisheries to identify which species are depredating, the amount of catch lost, and the behaviour of depredating species. Research has been carried out under both experimental and observational settings, including controlled baited camera drops and camera deployment during operations on-board fishing vessels. Camera systems range from inexpensive action cameras to line mounted fishing cameras and expensive purpose-built deep-sea video camera setups, with all systems similarly limited in their effectiveness by weather, sea conditions, and high turbidity. These cameras varied in technological performance and capabilities, with differences in size, resolution, depth rating and camera shape.
Small action cameras (e.g. GoPro™) are commonly used to assess depredation. Video footage collected using GoPro™ cameras mounted to crab traps showed that the primary species responsible for depredation of spanner crabs (Ranina ranina) were species not often perceived as predators, such as those from the families Aetobatidae and Rhinidae, and that there are a variety of species that simply interact with bait rather than depredating catches (Wiley and Pardee 2018; Milburn 2021). Mitchell et al. (2020) also used GoPro™ cameras attached to a crossbar and suspended below a float to determine the time of arrival of sharks to a bait suspended below the cameras. In these two studies, the static nature of the crab trap or crossbar generated clear footage and enabled observation of the shark behaviour, interactions and identification of shark species. Drymon et al. (2020a) successfully used GoPro™ cameras to identify shark species depredating fish from descender devices (i.e., weighted return-to-depth tools which are used to improve post-release survival of captured teleosts, Bohaboy et al. 2020). In other research, GoPro™ action cameras have been found suitable for identifying sharks to species level, where sharks were inadvertently observed depredating other sharks and teleosts (O’Shea et al. 2015; Streich et al. 2018). The maximum depth rating of 50 m for the housing of GoPro™ action cameras is a limiting feature of these cameras, although aftermarket housings with deeper maximum depth ratings are available. Future development of underwater housings to extend the depth range and the use of red camera filters to further improve image quality could greatly improve the applicability of GoPro™ cameras in depredation research.
There are several commercially available underwater cameras that are designed to be attached to fishing lines (Table 3). These cameras provide the opportunity to collect important observational information before, during and after depredation events. Mitchell et al. (2019) used WaterWolf™ fishing cameras attached to the lines of charter fishing customers, two meters above the baited hook. While this enabled the behaviour and interaction of various shark and teleost species with bait and hooked fish to be observed and depredation rates to be quantified, the ability to identify the sharks to species level was relatively low (37%). Likewise, GoPro™ cameras on specifically designed fishing line camera mounts were able to capture clear footage of depredation events, but shark species identification was difficult (Coulson et al. 2022). In both cases, the inability to identify shark species is largely due to the chaotic nature of depredation events that cause the fishing line and camera to shake and spin rapidly as well as the subtle morphological differences between species, particularly species of the genus Carcharhinus. Other purpose-built, line-mounted fishing video cameras, such as Spydro™ and GoFish cam™, possess high image quality and LED lighting, which may address some of these issues, but not the difficulty in identifying shark species. In some situations, specialised underwater camera systems may be the only option. For example, specifically designed underwater camera systems outfitted with twin 500 lm lights, able to tolerate very low temperatures and depths of over 1000 m, revealed that southern elephant seals (Mirounga leonina) were depredating and interacting with hooked fish on a longline (Van Den Hoff et al. 2017).
While video cameras have been used to successfully identify depredating species, their application in observing depredation behaviour and calculating depredation rates is still in its infancy. Given the variety of environments where depredation occurs, there is no single video camera system that can be used for all areas of research. When working in shallow depths, GoPro™ action cameras provide clear high-resolution video and allow potential identification of depredating species. When collecting data while line fishing on vessels using rod and reel, droplines or longlines, line mounted underwater cameras appear to be a good option. However, future research in line fisheries should consider trialling high resolution 360-degree underwater action cameras to collect high quality images of depredation. Further research should also trial line mounted fishing cameras while trolling to observe if depredation varies with different fishing methods. In addition, the use of underwater drones (ROVs, AUVs) to view depredation could also be trialled to see if the combination of greater manoeuvrability and a live camera feed is able to allow additional data to be collected.
Genetics
The use of genetic techniques for the identification of predator species responsible for depredation continues to grow, and shows increasing promise, with several research papers successfully using genetic approaches to identify depredating shark species from trace DNA collected off the remains of depredated species (Drymon et al. 2019; Fotedar et al. 2019; Vardon et al. 2021). This process is undertaken by swabbing the bite wound on the fish after depredation (Drymon et al. 2019; Fotedar et al. 2019). Although this method had been widely used across terrestrial environments (Williams et al. 2003; Blejwas et al. 2006; Caniglia et al. 2013; Fabbri et al. 2018), application in marine environments has only been recently undertaken (Drymon et al. 2019; Fotedar et al. 2019; Van Bleijswijk et al. 2014; Vardon et al. 2021). The application has now been trialled on commercial line (e.g. Webb et al. 2022) and net fisheries, as well as in charter and recreational fisheries, but considerable scope for broadening and enhancing the application of genetic approaches in depredation research exist.
Comparisons among shark depredation studies to date have revealed variability in successful genetic identification of depredating animals. The greatest success (100%) was achieved by Fotedar et al. (2019) using a combination of COPAN FLOQSwabs™ and a QIAGEN QIAamp™ Stool kit. Despite the notable success of these approaches, considerable opportunities remain to optimize the DNA collection, extracting and sequencing methods. Similar research by Drymon et al. (2019) reported a success rate of 61.5% using buccal swabs and the Omega BIO-TEK E-Z 96™ Tissue DNA Kit. Using trained fishers, Webb et al. (2022) reported 90% success rate. Although Fotedar et al. (2019), Drymon et al. (2019) and Webb et al. (2022) reported high success, sample sizes were low (16, 13, and 29, respectively). Research by Vardon et al. (2021) used a larger sample size of 52 but reported a lower success rate (19.2%), using polyurethane foam medium head swabs (Texwipe) and the QIAGEN DNeasy™ Blood and Tissue Kit. Unlike Fotedar et al. (2019) and Drymon et al. (2019), Vardon et al. (2021) used samples that had been collected and frozen by fishers before being defrosted and swabbed. Due to the varying methodologies, a comparative study using standardised testing would be required to determine which swabs and DNA extraction kits are optimal for use.
Variation in the time taken to swab depredated remains is also believed to affect the success rate of identifying depredating species. Vardon et al. (2021) found that depredating shark species can be identified from swabs taken from frozen depredated fish remains; however, high identification success was achieved by both Fotedar et al. (2019) and Drymon et al. (2019), which swabbed freshly depredated samples. The delay in swabbing depredated fish or freezing samples prior to swab sampling likely resulted in lower success as shark DNA would have degraded faster in situ on the fish than it would have if swabs had been collected immediately upon landing the depredated catch. Harms et al. (2015), recommended that swab samples be taken off depredated animals within 1–24 h. Additional research would benefit from investigating the amount of time after swabbing occurs before DNA degradation prevents the identification of depredating species (Drymon et al. 2019; Fotedar et al. 2019; Vardon et al. 2021). Variation also exists in the best way to swab a depredated sample to optimise depredating species identification. While research is limited, both Van Bleijswijk et al. (2014) and Wheat et al. (2016) found that swabs taken around teeth marks on depredated remains resulted in the most successful identification of depredating species. The optimisation of the collection and storage process presents a clear avenue for development where sample collection by non-researchers may be advantageous. If a standard protocol can be developed that allows for the collection of samples by non-researchers, then it would present enhanced capacity for ongoing monitoring of the issue and large-scale data collection through citizen science.
All genetic approaches to date have focused on traditional DNA sequencing methods, where primers are designed based on a presumed group of target taxa. In all instances so far, these are universal shark specific primers, which are designed to target several genera of sharks while not amplifying fish or human DNA. This approach is derived from traditional DNA barcoding applications that focus on conserved mitochondrial (mtDNA) regions, such as COI, ND2, ND4 and CtyB, which provide additional benefits when working with trace DNA due to the multi-copy nature of mtDNA. Several studies have also highlighted the importance of using primers that are specifically designed to amplify DNA of depredating species and block DNA of the species being depredated (Drymon et al. 2019; Fotedar et al. 2019; Van Bleijswijk et al. 2014; Vardon et al. 2021). Research by Drymon et al. (2019) and Fotedar et al. (2019) both used primers to target mitochondrial DNA from the gene region (cytochrome oxidase subunit 1; COI). Although often referred to as the barcoding gene, the COI gene has been found to be too conserved in sharks for the discrimination of closely related species (Ward et al. 2009). To overcome these challenges, Vardon et al. (2021) used primers to amplify the mitochondrial shark DNA NADH dehydrogenase two (ND2) and four (ND4) genes, which was successful in mostly identifying between different Carcharhinus spp., one of the most common genera responsible for depredation events studied to date (Mitchell et al. 2018a). Using both ND2 and ND4 improved species resolution. Thus, it is recommended that both ND2 and ND4 genes should be used in future research to maximise success in determining depredating species.
While traditional approaches using designed primers have been effective in selectively amplifying trace amounts of shark species DNA, this method is limited to the primer target group. Therefore, when the depredating species are not sharks, as has been noted in various studies (Gilman et al. 2008; Mitchell et al. 2018a, b, 2019; Rabearisoa et al. 2018), these single copy barcoding approaches become less effective. DNA metabarcoding presents a clear opportunity for improving methods to identify depredating animals by enabling simultaneous detection of multiple species by using a high-throughput sequencing platform. Metabarcoding approaches are now used routinely as part of environmental DNA (eDNA) studies and have been shown to be particularly effective in detecting the presence or absence of taxa to species level through water samples (Miya 2021). Their application in depredation studies has so far been limited to a single study that collected source DNA from fishing nets to investigate the depredation of a cetacean (De Bruyn et al. 2021). Yet, metabarcoding techniques could be challenging from a citizen-science point of view, since the risk of contamination is much higher and therefore maintaining sterility may not be feasible.
Depredation has been observed in net, longline, other hook and line, seine, trawl, and trap fisheries and as a result considerable opportunity remains for genetic identification in many of these unexplored fisheries. However, there are a unique set of considerations which may also need to be overcome. For example, the large phylogenetic distance among depredating predator and depredated prey species may increase the accuracy of identifying the predator at the species level (particularly where the predator and prey are closely related taxa). Furthermore, the ability to swab residual DNA of different tissue types (e.g. hard exoskeletons of crustaceans or molluscs) remains untested and could be either more or less suitable than swabbing of fish soft tissue. In some circumstances, examining the bite wound on a depredated fish or invertebrate could be used to distinguish between broader groups of depredating taxa (Gilman et al. 2007; IOTC 2007), if time and/or budget is limited for conducting genetic methods.
Reducing shark depredation
The development of deterrent technologies that prevent interactions with humans (bites) (Huveneers et al. 2018) or reduce shark bycatch while not decreasing catch rates of target species (Robbins et al. 2011), provides useful insights into potential technical applications to reduce shark depredation. Past studies have tested deterrents using static baits; however, it must be considered that the stimulus for a shark to depredate a struggling hooked fish is likely to be stronger than feeding on static bait, so the potential effectiveness of deterrents for reducing depredation may be lower than when static bait is used. Various types of shark deterrents have been investigated including magnets (O’Connell et al. 2014a, b), electropositive lanthanide metals (Brill et al. 2009; Kaimmer and Stoner 2008; Robbins et al. 2011), electrical (Howard et al. 2018; Verschueren et al. 2019), acoustic (Chapuis et al. 2019), and chemical (Stroud et al. 2014; Broadhurst and Tolhurst 2021) deterrents. There has also been significant work investigating the effectiveness of personal electrical shark deterrents for ocean users, such as surfers and divers (Gauthier et al. 2020; Huveneers et al. 2018; Marcotte and Lowe 2008; Thiele et al. 2020). Only recently has this technology been adapted in the development of deterrents for use in recreational fisheries, with a single study investigating their effectiveness. While the probability of depredation was not significantly reduced during fishing sessions when the use of three types of deterrent [magnetic (“SharkBanz Fishing—Zeppelin” n.d.), electrical (Ocean Guardian 2019) and acoustic (“SharkStopper” n.d.)], those deterrents were, collectively, effective in reducing the overall proportion of fish depredated by sharks (by more than 60%) and increased the time taken for fish to be depredated after becoming hooked (Department of Primary Industries & Regional Development [DPIRD], Western Australia, unpublished data).
Magnetic, electropositive lanthanide metal and electrical deterrents all work on the same premise of overwhelming the electrosensory system of the shark and thus evoking avoidance behaviours. The electrosensory system has been demonstrated to override other sensory modalities, with sharks documented to ignore the visual and chemical stimuli produced by nearby food items to preferentially bite at electrodes (Kalmijn 1972; Kajiura 2003). Therefore, a mitigation strategy that targets the electrosensory system may provide a mechanism to selectively deter sharks from biting while not affecting teleost fishes. The use of lanthanide metals as shark deterrents has been investigated with mixed results. Some studies demonstrated shark avoidance to the metals, while others did not (reviewed in McCutcheon and Kajiura 2013; O’Connell et al. 2014a, b). There are significant limitations to the lanthanide metals which makes them unsuitable for recreational or commercial application. These metals are expensive and are hazardous to machine (Smith 2013). They are also classified as potentially toxic and should not be stored in air or in moist environments, so are unsuitable for use on a fishing vessel. Finally, they dissolve in water which would necessitate frequent and thus costly replacement (McCutcheon and Kajiura 2013).
Magnets have been investigated as a potential alternative to lanthanide metals. A shark swimming near a magnet will induce an electric field around its body that is potentially detectable by its electrosensory system (Kalmijn 1974). Various types of permanent magnets, including ferrite (Fe2O3), barium ferrite (BaFe12O19), and neodymium ferrite (NdFeB) have had varied success as shark deterrents and are not always effective, particularly when there is competition among sharks during feeding (Robbins et al. 2011; DPIRD unpublished data). Moreover, the effective range of many magnets is very small, requiring a shark to be in close proximity before being deterred (O’Connell et al. 2014a, b; Rigg et al. 2009). Recent work mapping the magnetic field intensity around a commercially available deterrent (“SharkBanz Fishing—Zeppelin”) illustrates that the magnetic field decreases to background levels approximately 30–40 cm from the device (S. Kajiura, unpublished data), largely because of the physical properties of the magnetic field, which decays with distance as an inverse square function (Kalmijn 1974). This produces a limited effective range which necessitates that the device be positioned close to the hooked fish to provide adequate protection (S. Kajiura, unpublished data).
Electrical deterrent devices work by using a battery to drive an electric current between pairs of electrodes, which produces an electric field around the device. Previous studies investigating the effectiveness of personal shark deterrents determined that the Shark Shield Pty Ltd (Ocean Guardian 2019) devices are consistently more effective than other deterrents (Huveneers et al. 2013, 2018). This technology has been applied to create the Shark Shield Pty Ltd Fish01 device which is intended to deter sharks from an area 3 m either side of the device (Ocean Guardian 2019). The Fish01 device was effective in deterring sharks when hooked fish were within the effective range of the device but is limited because the position of the device in the water column is fixed and potentially too far away from the fishing line (DPIRD unpublished data). There is also a risk that fishing lines would get tangled around this device, which may limit its practicality for use on larger vessels where multiple lines are in the water at the same time. Smaller devices that could be deployed directly on recreational fishing gear have been demonstrated to significantly reduce bait consumption (Howard et al. 2018). However, the system would need significant refinement to create a commercially viable product that is applicable for reducing shark depredation in a fishing setting.
The use of acoustics as a shark deterrent has been investigated, particularly the use of orca (Orcinus orca) calls. While these have been shown to be an effective shark deterrent (Chapuis et al. 2019; Myrberg et al. 1978), sharks also exhibit evasive behaviours when exposed to artificially generated sounds that are rapidly increased or suddenly transmitted, even at a low amplitude (Banner 1972; Collin 2012; Hart and Collin 2015; Myrberg 2001). However, an important consideration in the application of acoustic deterrents is that sharks become habituated to attractive and repulsive noise (Myrberg et al. 1978, 1969), as well as the impact that transmitted sounds may have on other marine fauna, such as cetaceans and target fish species (Wartzok et al. 2003).
Another proposed avenue for reducing depredation is to employ shark-specific necromones. Sharks have been reported to be deterred by the odour of necromones from decaying shark tissue and have avoided the area for up to 10 min (Stroud et al. 2014). Applying necromone dispersing canisters to fishing gear might deter sharks from the gear. However, the impact on target teleost fishes would need to be more thoroughly explored. One study found that teleost fishes remained in the presence of a shark necromone whereas two shark species were deterred, which suggests that the response was specific to sharks (Stroud et al. 2014). Yet, in another study, the authors found that the odour of shark flesh from a mixture of species produced no reduction in shark catch on longlines (Broadhurst and Tolhurst 2021), which challenges their utility. Responses of sharks to necromones are likely to be complex and possibly species-specific, so further research is required to increase understanding of whether they can be viable as deterrents.
Protocols for testing deterrent devices
The development of shark deterrent devices is still in its infancy with several companies developing deterrents for this purpose. It is therefore inevitable that there will be a need to undertake independent testing of these devices. Establishing scientific evidence that deterrents are successful can be difficult and time consuming, yet critically important for independently validating product claims from device manufacturers. Recent methods to test the efficacy of personal shark-bite deterrents for surfers have been established (e.g. Huveneers et al. 2018); similarly, it would be advantageous to develop a set of protocols that could act as a guide in testing the effectiveness of current and future shark depredation deterrent devices.
When determining the effectiveness of shark deterrent devices, the sampling design should consider the appropriate sample size required to detect whether deterrents are effective in reducing the probability of shark depredation by determining (1) the level of statistical power required, (2) the current base-level of shark depredation (i.e., without shark deterrents), (3) the level of decrease in depredation that is required for a deterrent to be considered “effective”, and (4) the change in effectiveness over time since the initial exposure (i.e. whether habituation occurs in the sharks). When planning and carrying out field sampling it is necessary to consider: (1) proximity of sampling sites to one another, (2) adequate numbers of catchable fish and sharks are present, (3) duration of fishing session to enable fish to be caught and sharks to be encountered, (4) randomisation of the order in which a control and each deterrent device are tested while fishing, (5) maintaining the same number of fishing lines in the water throughout the sampling, and (6) maintaining consistency in the fishing equipment/hardware (i.e., number of hooks, hook size, line class) used throughout the sampling (Table 4). Yet despite best efforts, some variables are beyond control (e.g. the size and species of fish hooked), exemplifying the inherent challenges with attempting manipulative experiments in a field setting.
Future testing
Most research in the depredation field has focused on the loss of fish that are hooked and being reeled in by the angler. However, some fish are returned to the water while attached to fishing gear (e.g. descender devices) and are thus still subject to depredation. The only study that has investigated this aspect indicated that fish are far less likely to be depredated on descender devices (Drymon et al. 2020a), but note these findings are likely geographically variable. In addition, without directly seeing what animal is depredating a fish, most fishers will suspect that sharks are responsible. However, some teleost species that grow to large sizes (> 1 m), i.e., Epinephelus malabaricus, E. tukula, E. itajara, Hyporthodus nigritus, Sphyraena barracuda, Seriola dumerili, have also been reported to depredate teleosts (Streich et al. 2018; Shideler et al. 2015; DPIRD unpublished data) and may not be affected by deterrent devices aimed to mitigate shark depredation. Understanding what proportion of catch is depredated by teleosts will be an important consideration, particularly in those regions where some of these species are protected, such as goliath grouper (Epinephelus itajara) in Florida (Shideler et al. 2015).
Managing shark depredation
The sections above provide a review of the methods and results of research used to characterise and mitigate shark depredation. An improved understanding of this issue is critical for fishery managers to develop suitable policy responses and respond to stakeholder concerns. To date, we are not aware of any specific management responses to address shark depredation. Despite this, there are several courses of action available to managers and fishers. While the most commonly suggested mitigation approaches are technical (i.e., shark deterrent devices, see “Reducing shark depredation” section) and geared towards reducing or eliminating the physical act of depredation, additional approaches for managing depredation exist, including reducing shark numbers, changing fisher behaviours, and educating stakeholders. We explore the limitations and challenges of these approaches below.
Reducing shark numbers
One solution to depredation advocated by some fishers is to reduce shark numbers by opening or increasing commercial fishing for sharks or even culling (Kagi 2016). Both approaches assume that depredation rates are proportionally related to shark abundance rather than changes in shark behaviour. If this is true, then to achieve appreciable reductions in shark depredation it would be necessary to reduce shark populations to very low levels compared to current levels, which is not allowed in countries with legal mandates to end overfishing (e.g. US and Australia). In many situations, fishers have claimed that increasing shark populations following the protection or management of sharks are responsible for increasing depredation rates. The assumption about a relationship between shark abundance and shark depredation rates remains to be tested, but underscores why fishery managers require scientific studies to underpin decision-making since it can have significant implications for shark populations (some of which are already depleted and threatened with extinction) and the wider marine ecosystem (Heithaus et al. 2008). Unlike other solutions, this approach would require significant involvement from fishery managers and would likely require some form of regulation (e.g. to allow sharks to be caught and killed).
There are many challenges to this type of approach. Firstly, knowing which species of sharks are responsible for depredation would be necessary to focus fishing efforts on these species. However, even if the species are known, selectively fishing for these species is difficult given the low level of species-selectivity normally achieved in shark fisheries (e.g. Smart et al. 2020), so species not responsible for depredation may be unnecessarily depleted. Secondly, sharks identified as depredating species may currently be subject to fishing and thus managed already. In cases where management is maintaining a population at a sustainable level, added catches would affect sustainability and the long-term economic value of the fishery. Thirdly, some shark species are already overfished and of conservation concern because of their elevated extinction risk (Dulvy et al. 2014). Fourthly, for shark species where there is no information on population status or modelling to predict the consequences of increased catches, the challenges for managers are even greater. Finally, managing shark catch to reduce depredation would have a cost to the management agencies involved. There are also other costs to the ecosystem through the loss of important sources of predation that could result in unintended changes in these systems.
An example from Western Australia provides a useful case study to explore the feasibility of reducing shark numbers as a management tactic for shark depredation. There, the dusky shark (Carcharhinus obscurus), a known depredating species (Fotedar et al. 2019), is already subject to managed levels of commercial fishing (Braccini et al. 2018) and has a life history that makes it susceptible to overfishing, including late onset maturity (> 20 years), relatively low fecundity (2–18 pups) and infrequent reproductive periodicity (2–3 year reproductive cycle) (Simpfendorfer et al. 2002; Dudley et al. 2005; Natanson et al. 2014). While the dusky shark is only one of the shark species identified to depredate fish in this region (Fotedar et al. 2019), increasing shark fishing to reduce depredation would likely lead to overfishing of dusky sharks that are still recovering from historic overfishing after years of careful management (Woodhams et al. 2021). Reducing dusky shark abundance at local scales where depredation is an issue is also not viable because the population moves over large distances (> 1000 km) along the Western Australian coast (Braccini et al. 2017; Bartes et al. 2021). To communicate these concepts, a fact sheet on shark depredation in Western Australia was developed to share details on the history of commercial shark fishing, current status of the key shark stocks, and results from recent depredation research, to educate fishers and potentially encourage changes in their fishing behaviour (Anon 2021).
For managers considering reopening commercial shark fisheries to reduce shark populations and shark depredation, there are also important considerations around marketability of the sharks caught, as there is typically low value for shark meat (Ferretti et al. 2020) and large sharks have high concentrations of mercury and other contaminants (polychlorinated biphenyls, PCBs) in their flesh (Pethybridge et al. 2010; Gilbert et al. 2015; Tiktak et al. 2020). Overall, the practicalities and costs of reopening and managing commercial shark fisheries at a sustainable level to reduce depredation are complex. Many of these complexities are not apparent to all stakeholders; therefore, it is important that efforts are made to provide clear messaging around the history of management of shark stocks and limitations of reopening commercial shark fisheries or culling sharks to reduce shark depredation in many jurisdictions where the wider public support (‘social license’) for such approaches does not exist.
Reducing shark populations to address depredation issues poses such significant challenges to fishery managers that they are unlikely to be widely used. However, this may be unpopular with some stakeholders who consider this as the only solution. The danger in that situation is that fishers take matters into their own hands and start killing sharks (Carlson et al. 2019; Casselberry et al. 2022), which would have the potential to reverse positive trends in shark conservation. While pressure from fishers to increase the catch of (or even cull) sharks can be significant, there is likely to be equally strong pressure from conservation groups not to increase shark catches given their conservation status (Dulvy et al. 2014; Pacoureau et al. 2021). This is where the learnings from terrestrial HWC can be used to help design strategies to help address the needs of all stakeholders in the debate and reduce the human–human conflict that arises from different values and beliefs about sharks (Simpfendorfer et al. 2021).
Changing fisher behaviour
Reducing shark depredation via changes to fisher behaviour aligns closely with the technological approach. This requires that fisher behaviours that effectively reduce shark depredation can be identified via appropriately structured research. Managers may play a role in advocating for the adoption of behaviours once research is available, but top-down regulation is unlikely. Unlike technological solutions, behavioural change would not have a direct economic cost, but may have indirect economic costs (e.g. if changes in fishing location were required more often than previously, increasing fuel costs). Behaviour change may also result in indirect-economic costs, such as reduced enjoyment and reduced catches.
Fishers have reported testing a wide range of modifications to fishing methods to try and mitigate depredation (Mitchell et al. 2021; Coulson et al. 2022). These can be related to the fishing location, including rotating fishing areas in a systematic way so as not to visit the same location too frequently, which will reduce the chances of sharks associating vessels with food at that location, or moving on from a given fishing location after a short time (e.g. after a few fish have been caught successfully or once sharks are sighted). Identifying locations where sharks may be less likely to occur, such as certain depth ranges, can also help to manage shark depredation (Mitchell et al. 2021). Fishing methods can also be modified to retrieve hooked fish more quickly (i.e., using electric reels or heavy class line), or by using jigs and lures instead of bait to reduce odour cues and reducing or eliminating fish waste discards can also help to prevent sharks from associating fishing vessels with an easily accessible source of food (Mitchell et al. 2021; Coulson et al. 2022). In some areas, diversifying target species may help to mitigate shark depredation because fishers have anecdotally reported that sharks can be more likely to depredate certain fish species, such as tuna (Thunnus spp.) and yellowtail kingfish (Seriola lalandi), compared to demersal species (Mitchell et al. 2021). However, the reasons behind these apparent preferences of sharks are not yet well understood so would need to be investigated and quantified with further research. Additionally, the success of this approach would depend on the shark species present in the area and the fishing methods used. To increase understanding of potential modifications to fisher behaviour and to identify which methods have been successful, survey approaches such as those described previously could be useful. For example, in their review of marine predator depredation, Tixier et al. (2021) found that behavioural responses and gear modifications were the most effective strategies for reducing depredation, while Coulson et al. (2022) found that charter and recreational fishers simply move spots or stop fishing to minimise shark interactions. Gilman et al. (2007) found that altering longline soak times and hook depth are strategies that fishers have used to reduce depredation in some circumstances. Rather than modify gear or move locations, Casselberry et al. (2022) noted that recreational fishers who experienced depredation in the United States were more likely to target and harvest sharks. Mitchell et al. (2021) conducted a survey of recreational fishers at Lord Howe Island, Australia, to collect information on fisher practices, which was then converted into a list of best practice guidelines for fishers to refer to, particularly for visiting recreational fishers who had limited experience of the local fishery. Information on best practice fishing methods can be communicated to fishers using targeted education approaches, to ensure wider uptake. This approach could be particularly beneficial to recreational fishers who have not experienced shark depredation in the past.
Engaging and educating stakeholders
Engaging and educating fishers and other stakeholders can provide deeper insights into the shark depredation conflict and help direct more innovative management options. Finding suitable compromises that account for societal conservation values and potentially conflicting fisher perspectives will likely prove challenging for managing shark depredation, akin to other human–shark conflicts (Gibbs and Warren 2015). Thus, working to uncover common values between stakeholders may help guide research and management strategies that align with multiple stakeholder priorities. Surveys have shown promise for quantifying depredation and gauging fisher attitudes (e.g. Drymon and Scyphers 2017; Ryan et al. 2019) and potential responses (e.g. Casselberry et al. 2022; Coulson et al. 2022) towards sharks. Such information can help reveal the extent that depredation may impact fisheries to further inform stock assessments and fisheries management. An additional role for social science research could be in identifying and communicating areas and times of high shark depredation so that fishers can avoid these areas and times (e.g. Carmody et al. 2021; Mitchell et al. 2018a, b). For example, findings from Holder et al. (2020) prompted a stakeholder driven movement to enact a time-area closure in the Florida Keys to protect spawning permit aggregations from depredation.
Engaging fishers more effectively in shark depredation research is key to better understanding the extent of the conflict, but effectively communicating research findings back to fishers is also crucial. While studies have shown that research institutions can be viewed as more trustworthy information sources (MacKeracher et al. 2018), fisher distrust of scientists has been recognised around the world, and often stems from communication barriers between stakeholders and scientists (Dedual et al. 2013). However, distrust can also form from unmet expectations (Hartley and Robertson 2008), lack of transparency throughout the research process (Iwane et al. 2021), or inconsistencies between fisher experiences and scientific results (Chambers and Carothers 2017). A more prominent role for science communicators may assist in overcoming such obstacles relative to depredation. Li et al. (2010) conducted a fisheries communication study in Queensland, Australia and found that recreational fishers had an acute interest in fisheries science, so more effective science communication could help bridge this gap. Studies have also shown that fishers use a range of information sources (e.g. websites, conventional media, online forums, social networks), and that the penetration of information and trust in the messaging can vary widely between these sources (Li et al. 2016). As such, effective communication and expectation management may vary between different communities and fisheries groups, and researchers and managers need to identify their specific audiences and the best means to effectively communicate and engage with them. Effective engagement of fishers throughout the research process can also be a powerful means to build trust in the process and the legitimacy of research findings. Mease et al. (2018) provide extensive guidance on effective stakeholder engagement to build trust and manage expectations including starting early, communicating often, being inclusive and transparent, and humanising management and managers. Taking the effort to deliver well planned, timely, and meaningful engagement throughout the research process is widely recognised in fisheries management, with numerous case studies that provide guidance on effective engagement processes and techniques (e.g. Iwane et al. 2021).
A commonality within the current body of research that has investigated the effectiveness of depredation mitigation approaches is that none are a “silver bullet” for preventing depredation. The currently limited availability of affordable and effective shark deterrent devices for fishing will require fishers to be willing to consistently modify their behaviour to adapt to changing fishing conditions to mitigate depredation. While this is already occurring in some regions (e.g. Lennox et al. 2017), in most areas where depredation is prevalent, additional work is required to encourage fishers to be proactive in their approach to mitigation while fishing (Janc et al. 2021). For fishers to make informed changes to their fishing behaviours to minimise shark depredation, it will require effective communication between scientists, policy makers and stakeholders (Dedual et al. 2013; Fairclough et al. 2014; Runde 2019).
Discussion
Despite recent interest and advances in shark depredation research, we are still at a relatively early stage of characterising the extent of depredation and its impacts on fisheries, the shark species involved, identifying technical methods for deterring depredation, and managing depredation. This review has highlighted some of the recent progress made in understanding and quantifying depredation, particularly in the United States and Australia, as well as identifying future research gaps to be addressed. As part of this process, it is vital to understand how shark depredation fits into the broader context of fisheries management and shark conservation.
The issue represents an extremely challenging space for fishery managers, decision-makers, and politicians. Many stakeholders believe shark depredation is increasing due to increasing shark populations, particularly after the closure of some shark fisheries in the US and Australia. However, there is minimal long-term scientific data to investigate changes in abundance of sharks and there is no clear evidence to link changes in relative abundance of shark to increases in depredation rates. Studies from Australia (Braccini et al. 2020) and the US (Peterson et al. 2017) have shown relatively stable trends in shark populations over the last 10–15 years with only modest recovery in some species such as sandbar shark (Carcharhinus plumbeus). Yet, there are widespread anecdotal reports that shark populations have increased as fishers report seeing many more sharks around their vessels than in the past. While it is possible that some local populations of sharks have increased, particularly species with more productive life history traits and relatively high site fidelity (Pardo et al. 2016), it is also possible that reports of ‘greater’ shark abundances reflect a shifting baseline (sensu Pauly 1995). Dusky sharks in Western Australia and sandbar sharks in the US are two populations that exemplify this concept. Both stocks were historically overfished yet are now rebuilding under current management measures (Braccini et al. 2020; Peterson et al. 2017). Although fishers may be experiencing recent increases for these two species, populations are likely still lower than before they were overfished. Surveys offer a promising tool for documenting these generational changes in fishers’ perceptions of historic and modern shark populations (e.g. Powers et al. 2013). Further confounding these population-level trends is a need to better understand how shark species distributions are responding to climate change, such as potential range extensions of tropical species into areas where they may not have historically been observed (Last et al. 2011; Bartes and Braccini 2021).
It is also important to disentangle reported changes in relative abundance from the potential influence of changes in shark behaviour when investigating reports of increased depredation. In some areas there has been an increase in recreational fishing activity, such as in remote parts of Western Australia in recent years due to the COVID-19 pandemic (Ryan et al. 2021), so this increasing presence of vessels may have driven habituation of previously naïve sharks and development of associative behaviours where they associate the sounds of boat engines with the availability of food in the form of hooked and/or released fish. This scenario has also been suggested for sandbar sharks in areas of the northern Gulf of Mexico (Drymon et al. 2020b). In these instances, there would likely be a trend where fishers encounter more sharks and therefore perceive that their populations have increased. Changes in fishing practices may also exacerbate these trends. For example, in some areas where commercial trawling used to occur but has now been drastically reduced due to management measures, sharks that used to follow trawlers and feed on discarded fish would no longer have access to this food source, so may switch to following recreational fishing vessels instead.
It is necessary to consider how to manage stakeholders impacted by shark depredation scenarios, as well as the sharks. In several other HWC scenarios, the focus has shifted from one focused solely on the predator responsible, such as where the predominant management approach is to cull or catch and relocate the predator (Reynolds and Tapper 1996), to one focused more on managing human behaviour and the impacts on stakeholders, e.g. by subsidising changes in behaviour that support coexistence with predators (Ravenelle and Nyhus 2017). From a shark depredation context, a key process will be to learn more about how fishing methods can be modified to reduce the occurrence of shark depredation and then communicate this information effectively to fishers. Indeed, since fishing began, fishers have constantly experimented and adapted their fishing techniques to achieve higher catch rates, such as by testing different bait and lure types, targeting different phases of the lunar and tidal cycles and developing new technology, such as echosounders and electric reels. Therefore, modifying fishing methods to overcome the challenge presented by shark depredation is another scenario where adaptation can generate improvements in fishing.
Regardless of the methods used to mitigate depredation, it is highly unlikely that this HWC will be eliminated completely (Lennox et al. 2018). Indeed, if efforts to rebuild historically depleted shark populations are successful, depredation may potentially increase (Carlson et al. 2019). Moving forward, it will be necessary to set realistic goals and manage expectations towards reducing depredation to a tolerable level. Shark depredation sits within the context of a range of other human–shark interactions (Simpfendorfer et al. 2021). The range of functional roles of sharks and the multidimensional aspects of their conflicts with humans (ecological, social, economic) make mitigation of conflict difficult since solving one issue may introduce additional unintended consequences related to another level or type of conflict.
In the future, we can apply lessons learned from similarly contentious HWCs in the terrestrial realm. Recent meta-analyses indicate that reactionary predator removal programs in terrestrial ecosystems are rarely successful in reducing livestock depredation by apex predators (Eklund et al. 2017; Bruns et al. 2020) and in some cases, have even increased depredation (Treves et al. 2016; Smith and Appleby 2018). Efforts to continue to characterise shark depredation should lean on these insights as we move toward developing effective mitigation and management strategies. Ultimately, given the polarising nature of shark depredation, developing effective messaging and education for the diverse range of stakeholders affected is critical for minimising future conflicts.
References
Anon (2021) Shark depredation, Fisheries science update—November 2021. Department of Primary Industries and Regional Development. Western Australia. http://www.fish.wa.gov.au/Documents/fisheries_research_updates/fisheries_science_update_shark_depredation.pdf
Arlinghaus R, Aas Ø, Alós J, Arismendi I, Bower S et al (2021) Global participation in and public attitudes toward recreational fishing: international perspectives and developments. Rev Fish Sci Aquac 29:58–95
Bagchi S, Mishra C (2006) Living with large carnivores: Predation on livestock by the snow leopard (Uncia uncia). J Zool 268(3):268. https://doi.org/10.1111/j.1469-7998.2005.00030.x
Banner A (1972) Use of sound in predation by young lemon sharks, Negaprion brevirostris (Poey). Bull Mar Sci 22:251–283
Bartes S, Braccini M (2021) Potential expansion in the spatial distribution of subtropical and temperate west Australian sharks. J Fish Biol 99(4):1503–1506
Bartes S, Simpfendorfer C, Walker TI, King C, Loneragan N, Braccini M (2021) Conventional tagging of sharks in Western Australia: the main commercial species exhibit contrasting movement patterns. Mar Freshw Res 72(11):1643
Beerkircher LR, Brown CJ, Lee DW (2002) SEFSC Pelagic observer program data summary for 1992–2000. National Oceanic and Atmospheric Administration, Miami
Blejwas K, Williams C, Shin G, McCullough D, Jaeger M (2006) Salivary DNA evidence convicts breeding male coyotes of killing sheep. J Wildl Manag 70:1087–1093. https://doi.org/10.2193/0022-541x(2006)70[1087:sdecbm]2.0.co;2
Bohaboy EC, Guttridge TL, Hammerschlag N, Van Zinnicq Bergmann MP, Patterson WF III (2020) Application of three-dimensional acoustic telemetry to assess the effects of rapid recompression on reef fish discard mortality. ICES J Mar Sci 77(1):83–96
Braccini M, Rensing K, Langlois T, McAuley R (2017) Acoustic monitoring reveals the broad-scale movements of commercially important sharks. Mar Ecol Prog Ser 577:121–129
Braccini M, Blay N, Hesp A, Molony B (2018) Resource assessment report temperate demersal elasmobranch resource of Western Australia. Fisheries Research Report No. 294, Department of Primary Industries and Regional Development, Western Australia
Braccini M, Molony B, Blay N (2020) Patterns in abundance and size of sharks in northwestern Australia: cause for optimism. ICES J Mar Sci 77:72–82. https://doi.org/10.1093/icesjms/fsz187
Brill R, Bushnell P, Smith L, Speaks C, Sundaram R et al (2009) The repulsive and feeding-deterrent effects of electropositive metals on juvenile sandbar sharks (Carcharhinus plumbeus). Fish Bull 107:298–307
Britten GL, Duarte CM, Worm B (2021) Recovery of assessed global fish stocks remains uncertain. Proc Natl. Acad Sci USA 118(31)
Broadhurst MK, Tolhurst DJ (2021) Null effects of decomposing shark tissue on baited-hook catches of elasmobranchs. Reg Stud Mar Sci 46:101898. https://doi.org/10.1016/j.rsma.2021.101898
Bruns A, Waltert M, Khorozyan I (2020) The effectiveness of livestock protection measures against wolves (Canis lupus) and implications for their co-existence with humans. Glob Ecol Conserv 21:e00868
Caniglia R, Fabbri E, Mastrogiuseppe L, Randi E (2013) Who is who? Identification of livestock predators using forensic genetic approaches. Forensic Sci Int Genet 7:397–404. https://doi.org/10.1016/j.fsigen.2012.11.001
Carlson JK, Heupel MR, Young CN, Cramp JE, Simpfendorfer CA (2019) Are we ready for elasmobranch conservation success? Environ Conserv 46:264–266
Carmody H, Langlois T, Mitchell J, Navarro M, Bosch N et al (2021) Shark depredation in a commercial trolling fishery in sub-tropical Australia. Mar Ecol Prog Ser 676:19–35. https://doi.org/10.3354/meps13847
Casselberry GA, Markowitz EM, Alves K, Dello J, Skomal B et al (2022) When fishing bites: understanding angler responses to shark depredation. Fish Res 246:106174. https://doi.org/10.1016/j.fishres.2021.106174
Chambers C, Carothers C (2017) Thirty years after privatization: A survey of Icelandic small-boat fishermen. Mar Policy 80:69–80. https://doi.org/10.1016/j.marpol.2016.02.026
Chapuis L, Collin SP, Yopak KE, McCauley RD, Kempster RM et al (2019) The effect of underwater sounds on shark behaviour. Sci Rep 9:1–11. https://doi.org/10.1038/s41598-019-43078-w
Clark J, Roberts J, Mees C (2007) Depredation of fish caught on tuna longlines in the BIOT area. In: IOTC (ed) Workshop on the depredation in the tuna longline fisheries in the Indian Ocean, Victoria
Collin SP (2012) The neuroecology of cartilaginous fishes: Sensory strategies for survival. Brain Behav Evol 80:80–96. https://doi.org/10.1159/000339870
Cooke SJ, Philipp DP (2004) Behavior and mortality of caught-and-released bonefish (Albula spp.) in Bahamian waters and implications for a sustainable recreational fishery. Biol Conserv 118:599–607. https://doi.org/10.1016/j.biocon.2003.10.009
Coulson PG, Ryan KL, Jackson G (2022) Are charter and private-boat recreational fishers learning to live with shark depredation? Mar Policy 141:105096. https://doi.org/10.1016/j.marpol.2022.105096
Coulson PG, Jarvis N, Jackson G (2022) New techniques for underwater video photography of line fishing and their application in shark depredation studies. Asian Fish Sci 35:282–287
de Bruyn M, Barbato M, Broadhurst MK (2021) Metabarcoding gillnets to assess unaccounted catch depredation or escape. Environ DNA 4:157–166. https://doi.org/10.1002/edn3.234
Dedual M, Sague Pla O, Arlinghaus R, Clarke A, Ferter K et al (2013) Communication between scientists, fishery managers and recreational fishers: lessons learned from a comparative analysis of international case studies. Fish Manag Ecol 20:234–246. https://doi.org/10.1111/fme.12001
Drymon M, Scyphers S (2017) Attitudes and perceptions influence recreational angler support for shark conservation and fisheries sustainability. Mar Policy 81:153–159. https://doi.org/10.1016/j.marpol.2017.03.001
Drymon JM, Cooper PT, Powers SP, Miller MM, Magnuson S et al (2019) Genetic identification of species responsible for depredation in commercial and recreational fisheries. N Am J Fish Manag 39:524–534
Drymon JM, Jefferson AE, Louallen-Hightower C, Powers SP (2020a) Descender devices or treat tethers: does barotrauma mitigation increase opportunities for depredation? Fisheries 45:377–379. https://doi.org/10.1002/fsh.10476
Drymon JM, Dedman S, Froeschke JT, Seubert E, Jefferson AE et al (2020b) Defining sex-specific habitat suitability for a northern Gulf of Mexico shark assemblage. Front Mar Sci 7:35. https://doi.org/10.3389/fmars.2020.00035
Dulvy NK, Fowler SL, Musick JA, Cavanagh RD, Kyne PM et al (2014) Extinction risk and conservation of the world’s sharks and rays. Elife 3:e00590. https://doi.org/10.7554/eLife.00590
Eklund A, López-Bao JV, Tourani M, Chapron G, Frank J (2017) Limited evidence on the effectiveness of interventions to reduce livestock predation by large carnivores. Sci Rep 7(1):1–9
Fabbri E, Velli E, D’Amico F, Galaverni M, Mastrogiuseppe L et al (2018) From predation to management: monitoring wolf distribution and understanding depredation patterns from attacks on livestock Elena. Hystrix Ital J Mammal 29:101–110. https://doi.org/10.4404/hystrix
Fairclough DV, Brown JI, Carlish BJ, Crisafulli BM, Keay IS (2014) Breathing life into fisheries stock assessments with citizen science. Sci Rep 4:1–10. https://doi.org/10.1038/srep07249
Ferretti F, Jacoby DM, Pfleger MO, White TD, Dent F, Micheli F, Rosenberg AA, Crowder LB, Block BA (2020) Shark fin trade bans and sustainable shark fisheries. Conserv Lett 13:e12708
Fotedar S, Lukehurst S, Jackson G, Snow M (2019) Molecular tools for identification of shark species involved in depredation incidents in Western Australian fisheries. PLoS ONE 14:e0210500
Fraser-Celin VL, Hovorka AJ, Silver JJ (2018) Human conflict over wildlife: exploring social constructions of African wild dogs (Lycaon pictus) in Botswana. Hum Dimens Wildl 23(4):341–358. https://doi.org/10.1080/10871209.2018.1443528
Gauthier ARG, Chateauminois E, Hoarau MG, Gadenne J, Hoarau E et al (2020) Variable response to electric shark deterrents in bull sharks, Carcharhinus leucas. Sci Rep 10:1–13. https://doi.org/10.1038/s41598-020-74799-y
Gibbs L, Warren A (2015) Transforming shark hazard policy: Learning from ocean-users and shark encounter in Western Australia. Mar Policy 58:116–124. https://doi.org/10.1016/j.marpol.2015.04.014
Gilbert JM, Baduel C, Li Y, Reichelt-Brushett AJ, Butcher PA (2015) Bioaccumulation of PCBs in liver tissue of dusky Carcharhinus obscurus, sandbar C. plumbeus and white Carcharodon carcharias sharks from south-eastern Australian waters. Mar Pollut Bull 101(2):908–913
Gilman E, Clarke S, Brothers N, Alfaro-Shugueto J, Mandelman J et al (2007) Shark depredation and unwanted bycatch in pelagic longline fisheries: industry practices and attitudes, and shark avoidance strategies. Honolulu
Gilman E, Clarke S, Brothers N, Alfaro-Shigueto J, Mandelman J et al (2008) Shark interactions in pelagic longline fisheries. Mar Policy 32:1–18. https://doi.org/10.1016/j.marpol.2007.05.001
Gray SA, Zanre E, Gray SR (2014) Fuzzy cognitive maps as representations of mental models and group beliefs. Fuzzy cognitive maps for applied sciences and engineering. Springer, Berlin, pp 29–48
Gray S, Aminpour P, Reza C, Scyphers S, Grabowski J et al (2020) Harnessing the collective intelligence of stakeholders for conservation. Front Ecol Environ 18(8):465–472
Guerra AS (2019) Wolves of the sea: managing human-wildlife conflict in an increasingly tense ocean. Mar Policy 99:369–373. https://doi.org/10.1016/j.marpol.2018.11.002
Hanselman DH, Pyper BJ, Peterson MJ (2018) Sperm whale depredation on longline surveys and implications for the assessment of Alaska sablefish. Fish Res 200:75–83
Harms V, Nowak C, Carl S, Muñoz-Fuentes V (2015) Experimental evaluation of genetic predator identification from saliva traces on wildlife kills. J Mammal 96:138–143. https://doi.org/10.1093/jmammal/gyu014
Hart NS, Collin SP (2015) Sharks senses and shark repellents. Integr Zool 10:38–64. https://doi.org/10.1111/1749-4877.12095
Hartley T, Robertson R (2008) The practice and theory of cooperative fisheries research. Hum Ecol Rev 13(2):161–171
Heithaus MR, Frid A, Wirsing AJ, Worm B (2008) Predicting ecological consequences of marine top predator declines. Trends Ecol Evol 23(4):202–210
Hirayama N (1976) Study on predation damages to hooked tuna by sharks in longline fishery. J Tokyo Univ Fish 62:125–136
Holder PE, Griffin LP, Adams AJ, Danylchuk AJ, Cooke SJ, Brownscombe JW (2020) Stress, predators, and survival: exploring permit (Trachinotus falcatus) catch-and-release fishing mortality in the Florida Keys. J Exp Mar Biol Ecol 524:151289. https://doi.org/10.1016/j.jembe.2019.151289
Howard S, Brill R, Hepburn C, Rock J, Pol M (2018) Microprocessor-based prototype bycatch reduction device reduces bait consumption by spiny dogfish and sandbar shark. ICES J Mar Sci 75:2235–2244. https://doi.org/10.1093/icesjms/fsy098
Huveneers C, Rogers PJ, Semmens JM, Beckmann C, Kock AA et al (2013) Effects of an electric field on white sharks: in situ testing of an electric deterrent. PLoS ONE 8:e62730. https://doi.org/10.1371/journal.pone.0062730
Huveneers C, Whitmarsh S, Thiele M, Meyer L, Fox A et al (2018) Effectiveness of five personal shark-bite deterrents for surfers. PeerJ 2018:1–22. https://doi.org/10.7717/peerj.5554
IOTC (2007) Workshop on the depredation in the tuna longline fisheries in the Indian Ocean. Victoria
Iwane MA, Leong KM, Vaughan M, Oleson KLL (2021) When a shark is more than a shark: a sociopolitical problem-solving approach to fisher–shark interactions. Front Conserv Sci 2:669105 https://doi.org/10.3389/fcosc.2021.669105
Janc A, Guinet C, Pinaud D, Richard G, Monestiez P et al (2021) Fishing behaviours and fisher effect in decision-making processes when facing depredation by marine predators. Fish Manag Ecol 28:528–541. https://doi.org/10.1111/fme.12503
Kaimmer S, Stoner AW (2008) Field investigation of rare-earth metal as a deterrent to spiny dogfish in the Pacific halibut fishery. Fish Res 94:43–47. https://doi.org/10.1016/j.fishres.2008.06.015
Kagi J (2016) Shark bite-offs prompt calls for increase of commercial shark fishing on WA coast. https://www.abc.net.au/news/2016-02-25/shark-bite-offs-increasing-in-wa-rick-mazza/7197918
Kajiura SM (2003) Electroreception in neonatal bonnethead sharks, Sphyrna tiburo. Mar Biol 143:603–611
Kalmijn AJ (1972) Bioelectric fields in sea water and the function of the ampullae of Lorenzini in elasmobranch fishes. Scripps Institute of Oceanography Reference Series Contr. no. 72–83, 1–21
Kalmijn AJ (1974) The detection of electric fields from inanimate and animate sources other than electric organs. In: Fessard A (ed) Handbook of sensory physiology. Electroreceptors and other specialized receptors in lower vertebrates, vol 3. Springer, Berlin, pp 147–200, 333 pp
Kobayashi H, Yamaguchi Y (1978) The hooked rate of longline caught fish and shark damage. Bull Fac Fish Mie Univ 5:117–128
Labinjoh L (2014) Rates of shark depredation of line-caught fish on the Protea Banks, KwaZulu-Natal. MSc Thesis, University of Cape Town
Lacy S, Watson BR, Riffe D, Lovejoy J (2015) Issues and best practices in content analysis. J Mass Commun Q 92(4):791–811
Last PR, White WT, Gledhill DC, Hobday AJ, Brown R, Edgar GJ, Pecl G (2011) Long-term shifts in abundance and distribution of a temperate fish fauna: a response to climate change and fishing practice. Glob Ecol Biogeogr 20:58–72
Lennox RJ, Filous A, Danylchuk SC, Cooke SJ, Brownscombe JW et al (2017) Factors influencing postrelease predation for a catch-and-release tropical flats fishery with a high predator burden. N Am J Fish Manag 37:1045–1053. https://doi.org/10.1080/02755947.2017.1336136
Lennox RJ, Gallagher AJ, Ritchie EG, Cooke SJ (2018) Evaluating the efficacy of predator removal in a conflict-prone world. Biol Conserv 224:277–289. https://doi.org/10.1016/j.biocon.2018.05.003
Li O, Sutton SG, Tynan L (2010) Communicating scientific information to recreational fishers. Hum Dimens Wildl 15(2):106–118
Li O, Gray SA, Sutton SG (2016) Mapping recreational fishers’ informal learning of scientific information using a fuzzy cognitive mapping approach to mental modelling. Fish Manag Ecol 23(3–4):315–329
MacKeracher T, Diedrich A, Gurney GG, Marshall N (2018) Who trusts whom in the Great Barrier Reef? Exploring trust and communication in natural resource management. Environ Sci Policy 88:24–31
MacNeil MA, Carlson JK, Beerkircher LR (2009) Shark depredation rates in pelagic longline fisheries: a case study from the Northwest Atlantic. ICES J Mar Sci 66(4):708–719
Mandelman JW, Cooper PW, Werner TB, Lagueux KM (2008) Shark bycatch and depredation in the US Atlantic pelagic longline fishery. Rev Fish Biol Fish 18(4):427–442
Malara D, Battaglia P, Consoli P, Arcadi E, Longo F, Stipa M, Pagano L, Greco S, Andaloro F, Romeo T (2021) When opportunistic predators interact with swordfish harpoon fishing activities: shark depredation over catches in the Strait of Messina (central Mediterranean Sea). Eur Zoological J 88:226–236. https://doi.org/10.1080/24750263.2021.1879284
Marcotte MM, Lowe CG (2008) Behavioral responses of two species of sharks to pulsed, direct current electrical fields: testing a potential shark deterrent. Mar Technol Soc J 42:53–61. https://doi.org/10.4031/002533208786829133
Major T (2020). Shark depredation reports rise as app developer, experts push for more research. ABC Rural. https://www.abc.net.au/news/rural/2020-12-13/citizen-scientist-searches-for-shark-depredation-data/12967626. Accessed 14 Feb 2021
McClenachan L, Scyphers S, Grabowski JH (2020) Views from the dock: warming waters, adaptation, and the future of Maine’s lobster fishery. Ambio 49(1):144–155
McCutcheon SM, Kajiura SM (2013) Electrochemical properties of lanthanide metals in relation to their application as shark repellents. Fish Res 147:47–54
Mease LA, Erickson A, Hicks C (2018) Engagement takes a (fishing) village to manage a resource: principles and practice of effective stakeholder engagement. J Environ Manag 212:248–257
Milburn J (2021) Cryptic mortality and depredation of spanner crabs (Ranina ranina) off the east coast of Australia, Honours thesis, University of the Sunshine Coast
Mitchell JD, McLean DL, Collin SP, Langlois TJ (2018a) Shark depredation in commercial and recreational fisheries. Rev Fish Biol Fish 28:715–748. https://doi.org/10.1007/s11160-018-9528-z
Mitchell JD, McLean DL, Collin SP, Jackson G, Taylor S (2018b) Quantifying shark depredation in a recreational fishery in the Ningaloo Marine Park and Exmouth Gulf, Western Australia. Mar Ecol Prog Ser 587:141–157. https://doi.org/10.3354/meps12412
Mitchell JD, McLean DL, Collin SP, Langlois TJ (2019) Shark depredation and behavioural interactions with fishing gear in a recreational fishery in Western Australia. Mar Ecol Prog Ser 616:107–122. https://doi.org/10.3354/meps12954
Mitchell JD, Schifiliti M, Birt MJ, Bond T, McLean DL et al (2020) A novel experimental approach to investigate the potential for behavioural change in sharks in the context of depredation. J Exp Mar Bio Ecol 530:151440 https://doi.org/10.1016/j.jembe.2020.151440
Mitchell JD, Camilieri-Asch V, Jaine FR, Peddemors VM, Langlois TJ (2021) Galapagos shark movement patterns and interactions with fishing vessels in the marine parks surrounding Lord Howe Island. Final report to Parks Australia. Canberra
Miya M (2021) Environmental DNA metabarcoding: a novel method for biodiversity monitoring of marine fish communities. Ann Rev of Mar Sci 14:161–185 https://doi.org/10.1146/annurev-marine-041421-082251
Modgil S, Singh RK, Gupta S, Dennehy D (2021) A confirmation bias view on social media induced polarisation during Covid-19. Inf Syst Front. https://doi.org/10.1007/s10796-021-10222-9
Muter BA, Gore ML, Gledhill KS, Lamont C, Huveneers C (2013) Australian and U.S. news media portrayal of sharks and their conservation. Conserv Biol 27(1):187–196
Myrberg AA (2001) The acoustical biology of elasmobranchs. Environ Biol Fishes 60:31–46. https://doi.org/10.1023/A:1007647021634
Myrberg AA, Banner A, Richard JD (1969) Shark attraction using a video-acoustic system. Mar Biol 2:264–276. https://doi.org/10.1007/BF00351149
Myrberg AA, Gordon CR, Klimley AP (1978) Rapid withdrawal from a sound source by open-ocean sharks. J Acoust Soc Am 64:1289–1297
Newing H et al (2010) Conducting research in conservation: social science methods and practice. Taylor and Francis Group, London https://ebookcentral.proquest.com/lib/jcu/detail.action?docID=957172
Niella Y, Peddemors VM, Green M, Smoothey AF, Harcourt R (2021) A “wicked problem” Reconciling human–shark conflict, shark bite mitigation, and threatened species. Front Conserv Sci. https://doi.org/10.3389/fcosc.2021.720741
Ocean Guardian (2019) Power module for Boat01 and Fish01—user manual [Internet]. https://cdn.shopify.com/s/files/1/0052/3862/0195/files/Ocean_Guardian_BOAT1_FISH01_User_Manual_A5_v1_3_20191113_low_res.pdf?4767
O’Connell CP, Guttridge TL, Gruber SH, Brooks J, Finger JS et al (2014a) Behavioral modification of visually deprived lemon sharks (Negaprion brevirostris) towards magnetic fields. J Exp Mar Biol Ecol 453:131–137. https://doi.org/10.1016/j.jembe.2014.01.009
O’Connell CP, Stroud EM, He P (2014b) The emerging field of electrosensory and semiochemical shark repellents: Mechanisms of detection, overview of past studies, and future directions. Ocean Coast Manag 97:2–11
O’Shea OR, Mandelman J, Talwar B, Brooks EJ (2015) Novel observations of an opportunistic predation event by four apex predatory sharks. Mar Freshw Behav Physiol 48:374–380. https://doi.org/10.1080/10236244.2015.1054097
Özesmi U, Özesmi SL (2004) Ecological models based on people’s knowledge: a multi-step fuzzy cognitive mapping approach. Ecol Model 176(1–2):43–64
Pacoureau N, Rigby CL, Kyne PM, Sherley RB, Winker H et al (2021) Half a century of global decline in oceanic sharks and rays. Nature 589:567–571
Pardo SA, Kindsvater HK, Reynolds JD, Dulvy NK (2016) Maximum intrinsic rate of population increase in sharks, rays, and chimaeras: the importance of survival to maturity. Can J Fish Aquat Sci 73(8):1159–1163
Pauly D (1995) Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol Evol 10(10):430
Pepin-Neff C, Wynter T (2017) Shark bites and shark conservation: an analysis of human attitudes following shark bite incidents in two locations in Australia. Conserv Lett. 11:e12407. https://doi.org/10.1111/conl.12407
Peterson CD, Belcher CN, Bethea DM, Driggers WB, Frazier BS et al (2017) Preliminary recovery of coastal sharks in the south-east United States. Fish Fish 18:845–859
Pethybridge H, Cossa D, Butler EC (2010) Mercury in 16 demersal sharks from southeast Australia: biotic and abiotic sources of variation and consumer health implications. Mar Environ Res 69(1):18–26
Poisson F, Marjolet C, Mete K, Vanpouille M (2007) Interactions of cetaceans and sharks with the Reunion Island swordfish longline fishery in the Indian Ocean between 1997 and 2000. In: IOTC (ed) Workshop on the depredation in the tuna longline fisheries in the Indian Ocean, Victoria
Powers SP, Fodrie FJ, Scyphers SB, Drymon JM, Shipp RL, Stunz GW (2013) Gulf-wide decreases in the size of large coastal sharks documented by generations of fishermen. Mar Coast Fish 5:93–102
Rabearisoa N, Sabarros PS, Romanov EV, Lucas V, Bach P (2018) Toothed whale and shark depredation indicators: a case study from the Reunion Island and Seychelles pelagic longline fisheries. PLoS ONE 13:1–26. https://doi.org/10.1371/journal.pone.0202037
Rafferty AR, Brazer EO Jr, Reina RD (2012) Depredation by harbor seal and spiny dogfish in a Georges Bank gillnet fishery. Fish Manag Ecol 19:264–272. https://doi.org/10.1111/j.1365-2400.2011.00837.x
Ravenelle J, Nyhus PJ (2017) Global patterns and trends in human–wildlife conflict compensation. Conserv Biol 31:1247–1256
Reynolds JC, Tapper SC (1996) Control of mammalian predators in game management and conservation. Mammal Rev 26:127–155
Rigg DP, Peverell SC, Hearndon M, Seymour JE (2009) Do elasmobranch reactions to magnetic fields in water show promise for bycatch mitigation? Mar Freshw Res 60:942–948. https://doi.org/10.1071/MF08180
Robbins WD, Peddemors VM, Kennelly SJ (2011) Assessment of permanent magnets and electropositive metals to reduce the line-based capture of Galapagos sharks, Carcharhinus galapagensis. Fish Res 109:100–106. https://doi.org/10.1016/j.fishres.2011.01.023
Robinson D, Newman SP, Whittingham MJ, Francksen RM, Adam MS, Stead SM (2022) Fisher–shark interactions: a loss of support for the Maldives shark sanctuary from reef fishers whose livelihoods are affected by shark depredation. Conserv Lett. https://doi.org/10.1111/conl.12912
Runde BJ (2019) Stakeholder engagement is the path to successful management. Fisheries 44:209–211. https://doi.org/10.1002/fsh.10270
Ryan KL, Taylor SM, McAuley R, Jackson G, Molony BW (2019) Quantifying shark depredation events while commercial, charter and recreational fishing in Western Australia. Mar Policy 109:103674. https://doi.org/10.1016/j.marpol.2019.103674
Ryan KL, Desfosses CJ, Denham AM, Taylor SM, Jackson G (2021) Initial insights on the impact of COVID-19 on boat-based recreational fishing in Western Australia. Mar Policy 132:104646
SharkBanz Fishing—Zeppelin, n.d. URL https://www.sharkbanz.com/products/zeppelin. Accessed 14 Oct 2021.
SharkStopper, n.d. URL http://www.sharkstopper.com/. accessed 14 Oct 2021
Simpfendorfer CA, McAuley RB, Chidlow J, Unsworth P (2002) Validated age and growth of the dusky shark, Carcharhinus obscurus, from Western Australian waters. Mar Freshw Res 53:567–573
Simpfendorfer CA, Heupel MR, Kendal D (2021) Complex human–shark conflicts confound conservation action. Front Conserv Sci 2:692767. https://doi.org/10.3389/fcosc.2021.692767
Sivasubramaniam K (1964) Predation of tuna longline catches in the Indian Ocean, by killer-whales and sharks. Bull Fish Res Stn Ceylon 17:221–236
Shideler GS, Carter DW, Liese C, Serafy JE (2015) Lifting the goliath grouper harvest ban: angler perspectives and willingness to pay. Fish Res 161:15–65
Smart JJ, White WT, Baje L, Chin A, D’Alberto BM, Grant MI, Mukherji S, Simpfendorfer CA (2020) Can multi-species shark longline fisheries be managed sustainably using size limits? Theoretically, yes. Realistically, no. J Appl Ecol 57:1847–1860
Smith KT (2013) Electrogenic metals for elasmobranch bycatch reduction. MS thesis, Florida Atlantic University, 43p
Smith BP, Appleby RG (2018) Promoting human-dingo co-existence in Australia: moving towards more innovative methods of protecting livestock rather than killing dingoes (Canis dingo). Wildl Res 45:1–15. https://doi.org/10.1071/WR16161
Stier AC, Samhouri JF, Gray S, Martone RG, Mach ME et al (2017) Integrating expert perceptions into food web conservation and management. Conserv Lett 10(1):67–76
Streich MK, Ajemian MJ, Wetz JJ, Stunz GW (2018) Habitat-specific performance of vertical line gear in the western Gulf of Mexico: a comparison between artificial and natural habitats using a paired video approach. Fish Res 204:16–25. https://doi.org/10.1016/j.fishres.2018.01.018
Stroud EM, O’Connell CP, Rice PH, Snow N, Barnes et al (2014) Existence of a shark necromone derived from putrefied shark tissue. Ocean Coast Manag 97:50–57
Sumner NR, Williamson PC, Malseed BE (2002) A 12-month survey of recreational fishing in the Gascoyne bioregion of Western Australia during 1998–99. Fisheries Research Report no. 139. Government of Western Australia, Department of Fisheries, Perth
Tiktak GP, Butcher D, Lawrence PJ, Norrey J, Bradley L et al (2020) Are concentrations of pollutants in sharks, rays and skates (Elasmobranchii) a cause for concern? A systematic review. Mar Pollut Bull 160:111701
Thiele M, Mourier J, Papastamatiou Y, Ballesta L, Chateauminois E et al (2020) Response of blacktip reef sharks Carcharhinus melanopterus to shark bite mitigation products. Sci Rep 10:1–12. https://doi.org/10.1038/s41598-020-60062-x
Tixier P, Lea MA, Hindell MA, Welsford D, Mazé C et al (2021) When large marine predators feed on fisheries catches: global patterns of the depredation conflict and directions for coexistence. Fish Fish 22:31–53. https://doi.org/10.1111/faf.12504
Treves A, Krofel M, McManus J (2016) Predator control should not be a shot in the dark. Front Ecol Environ 14(7):380–388
Van Bleijswijk JDL, Begeman L, Witte HJ, IJsseldijk LL, Brasseur SMJM et al (2014) Detection of grey seal Halichoerus grypus DNA in attack wounds on stranded harbour porpoises Phocoena phocoena. Mar Ecol Prog Ser 513:277–281. https://doi.org/10.3354/meps11004
Van Den Hoff J, Kilpatrick R, Welsford D (2017) Southern elephant seals (Mirounga leonina Linn.) depredate toothfish longlines in the midnight zone. PLoS ONE 12:1–13. https://doi.org/10.1371/journal.pone.0172396
van Hoose N (2021) Sandbars surge. Florida Sportsman
Vardon JL, Williams SM, Bucher DJ, Morgan JAT (2021) Identifying shark species responsible for fisheries depredation off Southeast Queensland, Australia. Mol Biol Rep 48:4961–4965. https://doi.org/10.1007/s11033-021-06460-4
Verschueren B, Lenoir H, Soetaert M, Polet H (2019) Revealing the by-catch reducing potential of pulse trawls in the brown shrimp (Crangon crangon) fishery. Fish Res 211:191–203. https://doi.org/10.1016/j.fishres.2018.11.011
Voinov A, Jenni K, Gray S, Kolagani N, Glynn PD et al (2018) Tools and methods in participatory modeling: Selecting the right tool for the job. Environ Model Softw 109:232–255
Ward RD, Hanner R, Hebert PD (2009) The campaign to DNA barcode all fishes. FISH-BOL J Fish Biol 74(2):329–356
Wartzok D, Popper AN, Gordon J, Merrill J (2003) Factors affecting the responses of marine mammals to acoustic disturbance. Mar Technol Soc J 37:6–15. https://doi.org/10.4031/002533203787537041
Webb MK, Kraft DW, Hampp MN, Meyer CG (2022) Kit-based sampling by trained fishers yields successful DNA identification of depredating shark species in the Marianas. Mar Coast Fish 14:e10204. https://doi.org/10.1002/mcf2.10204
Whatmough S, Van Putten I, Chin A (2011) From hunters to nature observers: a record of 53 years of diver attitudes towards sharks and rays and marine protected areas. Mar Freshw Res 62(6):755–763
Wheat RE, Allen JM, Miller SDL, Wilmers CC, Levi T (2016) Environmental DNA from residual saliva for efficient noninvasive genetic monitoring of brown bears (Ursus arctos). PLoS ONE 11:1–18. https://doi.org/10.1371/journal.pone.0165259
Whitenack LB, Mickley BL, Saltzman J, Kajiura SM, Macdonald CC, Shiffman DS (2021) Sharks, Lies, and Videotape: a content analysis of 32 years of Shark Week documentaries. bioRxiv. https://doi.org/10.1101/2021.08.18.456878
Wiley J, Pardee C (2018) Post release mortality in the Hawaiian Kona crab fishery. Poseidon Fisheries Research. http://poseidonfisheriesresearch.org/wp-content/uploads/2018/11/Wiley-and-Pardee-2018_Post-Release-Mortality-in-the-Hawaiian-Kona-Crab-Fishery.pdf
Williams CL, Blejwas K, Johnston JJ, Jaeger MM (2003) A coyote in sheep’s clothing: predator identification from saliva. Wildl Soc Bull 31:926–932. https://digitalcommons.unl.edu/icwdm_usdanwrc/288
Williamson PC, Sumner NR, Malseed BE (2006) A 12-month survey of recreational fishing in the Pilbara region of Western Australia during 1999–2000. Government of Western Australia. Department of Fisheries, Perth
Woodhams J, Braccini M, Peddemors V, Rogers P, Usher M (2021) Dusky Whaler. https://fish.gov.au/report/304-Dusky-Whaler-2020
Acknowledgements
JMD and SBS would like to acknowledge funding from the National Oceanic and Atmospheric Administration's RESTORE Science Program under award 2937818 to Mississippi State University. We thank Mandy Karnauskas for insightful comments that improved this manuscript. The authors thank Danielle McAree and Alena Anderson for creating Fig. 1. SMK thanks the Colgan Foundation for their continued support.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mitchell, J.D., Drymon, J.M., Vardon, J. et al. Shark depredation: future directions in research and management. Rev Fish Biol Fisheries 33, 475–499 (2023). https://doi.org/10.1007/s11160-022-09732-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11160-022-09732-9