Abstract
Coral reef fishes often exhibit specific or restricted depth distributions, but the factors (biotic or abiotic) that influence patterns of depth use are largely unknown. Given inherent biological gradients with depth (i.e. light, nutrients, habitat, temperature), it is expected that fishes may exploit certain depths within their environment to seek out more favourable conditions. This study used baited remote underwater video (BRUV) systems to document variation in the taxonomic and functional (trophic and size) structure of a fish assemblage along a shallow to upper-mesophotic depth gradient (13–71 m) at a submerged, offshore shoal in the northern Great Barrier Reef. BRUVs were deployed during two separate time periods (February and August 2017), to separately examine patterns of depth use. Both the relative abundance and diversity of reef fishes declined with depth, and there were pronounced differences in the taxonomic and functional structure of the fish assemblage across the depth gradient. In shallow habitats (< 30 m), the fish assemblage was dominated by herbivores, detritivores, planktivores and sessile invertivores, whereas the fish assemblage in deeper habitats (> 30 m) was dominated by piscivores and mobile invertivores. Depth and habitat type were also strong predictors for important fisheries species such as coral trout (Plectropomus spp.), emperors (Lethrinus spp.) and trevallies (Carangid spp.). We found limited evidence of temporal changes in depth and habitat use by fishes (including fisheries target species), although recorded temperatures were 4 °C higher in February 2017 compared to August 2017.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In aquatic ecosystems, environmental conditions vary markedly with depth, underpinning distinct differences in the distribution and abundance of many functionally important aquatic taxa across this gradient (Brokovich et al. 2008; MacDonald et al. 2016). Depth, and consequently habitat-use by mobile animals, such as fishes, influences access to resources (Hussey et al. 2015), predation risk (Furey et al. 2013), sociality (Alanara et al. 2001) and reproductive success (Samoilys and Squire 1994). Mobile species may also be able to exploit intrinsic depth-related gradients in environmental conditions to moderate exposure to extreme and/or changing environmental conditions, such as increasing temperature and wave energy (Kahler et al. 2001; Bonagerts et al. 2010; Smith et al. 2016). Importantly, the suitability of habitats can vary spatially (i.e. with depth) and temporally, such that mobile organisms may utilise different habitats diurnally or seasonally (Furey et al. 2013).
The structure of coral reef ecosystems varies markedly with depth, as the architects of these ecosystems (corals and algae) are directly influenced by depth-related changes in light availability (Adey 1998; Zintzen et al. 2012). The differential abundance, composition and growth of habitat-forming organisms in-turn drives depth-related changes in habitat structure and resource availability (Holt 1987; Hixon and Menge 1991; Bell et al. 2009). Accordingly, many coral reef fishes are restricted to particular depth-ranges, with major changes in the structure and function of fish assemblages across moderate depth gradients (Brokovich et al. 2008; Gonzalez-Sanson et al. 2009). Recently, there has been increasing interest in deeper (mesophotic) coral reef ecosystems as potential refuges, particularly for recreationally and commercially exploited fishes that inhabit shallower environments (e.g. Loya et al. 2016; Sih et al. 2017; Rocha et al. 2018). This is because environmental disturbances such as coral bleaching and storm damage generally attenuate relatively quickly with increasing depth (Bridge et al. 2014; Smith et al. 2014). In general, deeper habitats may not be impacted by disturbances to the same extent as shallow habitats (but see Bonagaerts et al. 2013; White et al. 2017), creating a more stable environment for some fishes, especially in the case of thermal anomalies (Brown 1997; Glynn 1996; Neal et al. 2014). Nevertheless, distinct assemblages of fishes inhabit mesophotic reef ecosystems (e.g. > 150 m, Sih et al. 2017), suggesting limited capacity for these systems to act as refuges for shallow-water taxa (Lindfield et al. 2016; Sih et al. 2019; Rocha et al. 2018; Williams et al. 2019). Understanding the capacity for shallow water fishes to utilise deeper habitats requires accurate information on species distributions, abundances and fish-habitat associations along depth gradients.
Refuge seeking behaviour (i.e. movements to seek optimal habitat) by coral reef fishes, especially in species targeted by recreational and commercial fisheries, is poorly studied and may be limited due to high levels of site fidelity and strong microhabitat associations (Lindberg et al. 2006). For example, recent studies have demonstrated that some large-bodied coral reef fishes move to cooler, deeper waters when they experience elevated temperatures (Richards et al. 2012; Currey et al. 2015) and shelter under tabular coral structures during times of day when solar irradiance is strongest (Kerry and Bellwood 2015). Furthermore, many fishes move between habitats (on hourly, daily or seasonal scales), thereby experiencing different environmental conditions (e.g. varying ambient temperature) (Kahn et al. 2017; Scott et al. 2019). Because fishes are generally ectothermic, temperature can have direct impacts on foraging (Cartamil and Lowe 2004; Scott et al. 2017), mobility (Azumaya and Ishida 2005; Thums et al. 2013), and digestion (Neverman and Wurtsbaugh 1994). One of the best opportunities for fishes to exploit heterogeneity within their environment may be with depth (Goyer et al. 2014) as there can be marked changes in light and temperature over relatively short distances (Bertolo et al. 2011). Movement among habitats may moderate effects of environmental change on tropical fishes, including important tropical fisheries species, although it is important to consider potential trade-offs associated with using different habitats.
Historically, many depth-related studies of coral reef fishes focused on relatively shallow water habitats < 30 m deep (Cappo et al. 2007; Harvey et al. 2007; McLean et al. 2011), largely due to the logistical constraints associated with surveying depths > 30 m. However, advances in diver-independent survey methods, such as baited remote underwater video (BRUV) systems (e.g. Cappo et al. 2007; Stowar et al. 2008; Langlois et al. 2018) are overcoming traditional challenges to studying fish assemblages in deep water. On the Great Barrier Reef (GBR), and throughout other ecosystems in Australia and around the world, there has been increased focus on the mesophotic zone (Bridge et al. 2011; Sih et al. 2017). Nevertheless, studies investigating both the photic (< 30 m depth) and mesophotic zones (30 – 300 m depth) simultaneously remain relatively limited (but see Stowar et al. 2008; Fitzpatrick et al. 2012; Bond et al. 2018; Currey et al. 2020), especially in the case of submerged shoal habitats. Submerged shoals are unique habitats and differ from emergent coral reefs, as they do not form conspicuous structures, but exist as either discrete or diffuse patches of hard substratum above the surrounding seafloor (Stowar et al. 2008). Submerged shoals throughout the Indo-Pacific region remain relatively unexplored but are thought to harbor many new species (Pyle 2000) as well as provide hotspots for recreational and commercial fisheries (Stowar et al. 2008).
The potential for submerged shoals to act as fisheries hotspots is particularly notable as millions of people worldwide are directly reliant on coral reef fisheries to meet their basic nutritional requirements (Bell et al. 2009; McClanahan et al. 2015; Hicks et al. 2019). However, global coral reef fishery catches are declining (Newton et al. 2007; Cuetos-Bueno and Hook 2015), due to the cumulative impacts of stressors such as anthropogenic climate change, coastal development and overfishing (Pauly et al. 2005; Newton et al. 2007; Graham et al. 2007; Robinson et al. 2019). Fisheries effort is generally concentrated in accessible, near-shore, shallow waters (< 30 m), such that many once productive fishing grounds have been overexploited (Lindfield et al. 2016). This has prompted fishers to adopt new strategies, such as fishing in deeper waters or submerged shoals, to maintain high catch rates (Pauly et al. 2005; Friedman et al. 2011; Lindfield et al. 2014). As such, the utilisation of submerged shoal habitats by reef fishes warrants further investigation.
The purpose of this study was to document variation in the structure (relative abundance, biodiversity, and taxonomic and functional structure) of reef fish assemblages across a broad depth range (13–71 m) at Linden Bank, an offshore submerged shoal, subject to some fishing pressure, in the northern GBR. While all fishes were considered, our explicit focus was on fisheries target species. Sampling was conducted using BRUV systems which allowed for consistent sampling across the entire depth range. Moreover, sampling was conducted at two separate time periods (February and August, 2017) to explore temporal variation in the depth distribution of the fish assemblage. Notably, recorded temperatures in February 2017 (28.75–29.63 °C) were substantially higher than August 2017 (24.53–25.28 °C), such that the depth distribution of fishes (including fisheries target species) might be expected to differ between the two sampling periods if they exploit depth-gradients to mediate exposure to sub-optimal environmental conditions. If so, any changes may have ramifications for the structure and function of fish assemblages across spatial scales in different habitats, particularly for important fisheries species that have high ecological and economic value and are more vulnerable to the effects of fishing.
Methodology
Study area
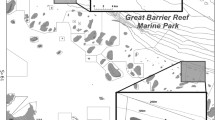
Linden Bank (−16.296900°S, 145.993066°E) is a submerged shoal situated along the outer shelf edge of the Great Barrier Reef (Fig. 1), 57 km offshore and < 1 km from the Australian continental shelf. The bank is 6.9 km long and 4.1 km wide. The top of the bank is in 13–15 m of water and slopes gradually to 71 m (Fig. 1).
Map showing the spatial and temporal deployments of BRUVS at Linden Bank in February 2017 (triangles) and August 2017 (circles) with associated bathymetry. Bathymetric information from Geoscience Australia using the AusSeabed Marine Data Discovery Portal (http://marine.ga.gov.au/#/)
Sampling methods
Baited remote underwater video: We used BRUV systems to sample the fish assemblage structure across the full range of depths (13 – 71 m) at Linden Bank. Each BRUV consisted of a Sony Mini-DV handycam inside a simple underwater housing custom made from PVC pipe and pressure rated to over 100 m. The camera housing was mounted inside a pyramid-shaped galvanised steel frame that protected the camera, maintained its orientation (tilted 10 degrees below horizontal and held approximately 400 mm above the seafloor) and facilitated attachment of a bait arm, ballast weights and rope to the surface. The flexible bait arm made of rigid PVC conduit held a plastic mesh bait bag containing 1 kg of crushed pilchards (Sardinops or Sardinella spp.) at approximately 1 m in front of the camera. BRUV frames were ballasted with steel bars according to the prevailing sea-state and current conditions to ensure stability on the seabed. An 8 mm diameter polypropylene rope with surface floats attached enabled the BRUV to be deployed and later retrieved from the surface (Fig. 2, Stowar et al. 2008).

taken from Stowar et al. 2008)
Depiction of the Baited Remote Underwater Video System setup. Steel ballast bars are attached to pegs on the base according to local sea surface and current conditions to prevent movement in situ (image
BRUV deployment: Following best-practice BRUV sampling protocols available at the time of our study (Cappo et al. 2007; Harasti et al. 2015; Walsh et al. 2017; Cundy et al. 2017; Langlois et al. 2018), each BRUV (Fig. 2) was deployed sequentially along a transect across a distance of ~ 2 km. Within each transect, BRUV deployments were separated by a minimum of 200 m to minimise any potential effects of the bait plume from the preceding unit. This sampling design also allowed BRUV units to be dropped at varying depths according to the topography of Linden Bank (Fig. 1). Each BRUV was soaked for up to 1 h (based on previous studies; Cappo et al. 2007; Harasti et al. 2015; Walsh et al. 2017; Cundy et al. 2017; Langlois et al. 2018) before being retrieved by an hydraulic pot hauler. On each sampling day 24–30 BRUV units were deployed. Sampling was conducted over five days in February 2017 (n = 86) and three days in August 2017 (n = 79) (Fig. 1). All deployments were conducted during daylight hours (0730–1700) and ranged in depth from 13 to 71 m. No BRUV was deployed within one hour of sunset or sunrise. Due to the topography of the site, the most common sampling depth was between 30 and 40 m.
Temperature measurements: A temperature logger (Vemco Minilog-II-T) was attached to each BRUV unit to provide accurate and real time temperature profiles for each deployment. Each logger was programmed to record temperature every 10 min. During deployments in February 2017, water temperatures ranged from 28.75 to 29.63 °C (apart from two outlying measurements which recorded temperatures of 26.35 °C and 27.58 °C). Notably, these lower temperatures (< 28 °C) were recorded at the greatest depths sampled (> 60 m), and recorded temperatures were very consistent from 10 to 60 m (Fig. 3). In August 2017, recorded temperatures ranged from 24.53 to 25.28 °C (apart from one outlying measurement which recorded a temperature of 25.98 °C) and varied relatively little (< 1 °C) from 10 to 60 m depth (Fig. 3).

taken from temperature loggers attached to the BRUVS. Each point represents an average temperature measurement from each BRUVS deployment. The shaded ribbon represents the 95% confidence interval across each sampling period
Temperature-depth profiles of Linden Bank during February 2017 (red) and August 2017 (blue)
Habitat classification: For each deployment, habitat was classified (by visual estimation) into one of 6 categories; algal covered sand (ACS), coral reef (CR), low-relief rubble (LRR), open sand (OS), seawhip/algal reef (SWA), or sand next to reef (SNR) based on previous studies using BRUV at similar depths and in similar habitats on the GBR (Stowar et al. 2008).
Fish identification and analysis of video metrics: BRUV footage was analysed by two primary observers, however the chief observer watched all the videos to ensure consistency. Each observer recorded the time of the video on the sea-bed, time of first appearance of each species, and relative abundance of each species calculated as MaxN. MaxN is the maximum number of individuals from a single fish species observed in a single frame of footage during one deployment (Willis and Babcock 2000) and is a common abundance metric used for BRUV studies (e.g. Langlois et al. 2018; Bouchet et al. 2018). MaxN is a conservative estimate of abundance but eliminates the possibility of re-counting fishes swimming in and out of the field of view. To standardise MaxN across all deployments, imagery was analysed for 60 min from the time the BRUV landed on the sea-bed. Relative abundance for each BRUV sample was calculated, by summing MaxN values across species on each BRUV. Fish were identified to the lowest possible taxonomic level, with the assistance of experts, fish identification books and Fishbase™. Every effort was made to identify large, conspicuous fishes in addition to smaller, cryptic species. Visibility was approximated for each deployment, using the 1 m bait arm as a reference. On average, visibility was 10–15 m for all deployments. Nine deployments were excluded from the final analysis due to technical malfunctions including; the camera being out of focus, bad point of view (i.e. camera focussed straight down or straight up), or the video didn’t record. One BRUV was lost all together. For the remaining BRUVs, all fish present within the field of view were counted.
Fisheries species: Queensland’s Commercial Line Fishery is made up of five fisheries including; coral reef finfish fishery (CRFFF), rocky reef finfish fishery, the pelagic fishery, the Gulf of Carpentaria finfish fishery and the deepwater multiple-hook fishery. Because of the location of our study on the GBR, we focused on species from the CRFFF and pelagic fisheries only (see Table S1 for a full list of species). Data on Commercial Line Fishery species were obtained from logbook records via the QFish online portal (QDAF 2020), Queensland Fisheries Management Plan 2003 (QDAF 2003) and existing Queensland Fisheries species groups categorisations from Brown et al. (2020). In the CRFFF, there are 20 primary target species (accounting for approximately 95% of the total harvest) and 125 ‘other species’ that are regularly retained as by-product for domestic markets (Tobin et al. 2013). Notably, for the CRFFF, QFish recognises parrotfishes as Scaridae (i.e., separate from Labridae), and this separation is retained for the fisheries analysis herein. In the pelagic fishery, primary targets are; spanish mackerel, spotted mackerel and trevallies but most scombrids, and carangids are retained (Table S1, QDAF 2020).
Statistical analysis
Relationships between the response variables (relative fish abundance and species richness) and environmental characteristics (depth [continuous: mean centred], temperature [continuous: mean centred], and habitat type [categorical with 6 levels] were assessed using generalised linear models (GLMs) separately for the two sampling periods [February and August 2017]). Due to the high-degree of co-linearity between depth and temperature we constructed models based on two subsets of the predictor variables (subset a: depth and habitat type; subset b: temperature and habitat type) for each response variable. Full models with all interactions were initially fitted in each case, and then simplified. The most parsimonious model for each response variable was selected based on the corrected Akaike Information Criterion (AICc). In all cases the predictor variable ‘subset a’ (i.e. when temperature was not included) resulted in the most parsimonious model (Table 1). A negative binomial error distribution with a log-link was used to account for the non-normal and overdispersed nature of the count data in all cases. Tukey’s adjusted pairwise comparisons were utilised to examine within category differences. Model fit and assumptions were examined using residual plots, all of which were satisfactory. To explore how spatial variation in BRUV deployments between sampling periods (i.e. February and August) may have shaped the patterns in the data, we assessed whether the deployment depths (response variable) differed between February and August (fixed categorical factors) using a generalised linear model (GLM) fitted with a Gamma distribution and log-link (Table S1). We also tested whether the frequency of each habitat sampled differed between sampling periods using a chi-square test.
Composition of the fish assemblage (based on MaxN relative abundance data) was also examined taxonomically and functionally. Taxonomically, fishes were grouped by family (45 families) due to the high presence of zeroes at the genus and species resolution. Functionally, species were categorised into one of 7 trophic groups (planktivorous, herbivorous, detritivorous, invertivorous [sessile], invertivorous [mobile], piscivorous, and omnivorous) and then further distinguished based on body size (< or > 10 cm maximum total length), following Hemingson and Bellwood (2018). Data from each sampling period were analysed separately, as above. In all cases, the analyses were based on Wisconsin double standardised, fourth root-transformed data sets, and Bray Curtis similarity matrices. Initially, the relative importance of depth and habitat type in explaining the variation in the multivariate fish assemblage datasets (taxonomic and functional) were explored using a BIOENV routine. Relationships between the multivariate fish assemblage datasets (taxonomic and functional) and the two relevant environmental parameters (depth and habitat type) were then formally tested using distance-based permutational multivariate analysis of variance (adonis). The results of these analyses were visualised using distance-based redundancy analysis constrained by the environmental variables.
Additionally, we wanted to specifically explore if there was any variability in the relative abundance of key groups of commercially targeted fishes: (Plectropomus [coral trout], carangids, scombrids, lethrinids, serranids, lutjanids, scarids, labrids, siganids and acanthurids and key species groups (carcharhinids, Table S1) across depth and habitat types. Fisheries species groups were classified into i) Top 95%—defined as the species and species groups that make up 95% of catch (by weight) for the coral reef finfish and pelagic fisheries (following categorisations from Brown et al. 2018) and ii) All Fisheries Species, consisting of all species caught in these two fisheries. Although Caesionids are considered a target species for the pelagic fishery (under classification ii), this group was excluded from the fisheries analyses because their high abundance overwhelmingly shaped the patterns documented and masked the contribution of other fisheries targets. In addition, despite the fact parrotfishes (formerly Scaridae) have now been subsumed into Labridae, Scaridae and Labridae were analysed separately in the fisheries analyses following separate groupings in QFish. Data for each sampling period were analysed separately. For all fisheries analyses, a Bray Curtis similarity matrix, based on fourth root transformed data was initially formulated for each subset of data. The relative importance of depth and habitat type in explaining the variation in the fisheries assemblage datasets (Top 95% and All Fisheries Species) was initially explored using a BIOENV routine. Following this, relationships between the two fisheries assemblage datasets and the two relevant environmental parameters (depth and habitat type) were formally tested using distance-based permutational multivariate analysis of variance (adonis). The results of these analysis were visualised using distance-based redundancy analysis constrained by the environmental variables. All statistical analyses was performed in the software R (R Core Team 2019), using the MuMIn (Barton 2020), tidyverse (Wickham et al. 2019), emmeans (Lenth 2020) and vegan (Oksanen et al. 2019) packages.
Results
A total of 7381 (sum of MaxN) individual fishes, sharks and rays were identified, representing 356 species from 45 families. Species richness varied from 1 to 54 species per deployment. Depth and habitat type explained a substantial amount of the variance associated with relative abundance (February; R2 = 0.62, August R2 = 0.65) and richness (February; R2 = 0.73 and August; R2 = 0.74) of the fish assemblage (Tables 1 and S2). Relative abundance for February deployments was 50 individuals per BRUV and in August 38 individuals per BRUV across all habitat types (Fig. 4a, b. Species richness, i.e. number of species observed, did not vary between February and August, irrespective of the spatial variation in BRUV deployments across sampling periods (Table S2). The areal extent of sampling was higher in February (38.16 km2) compared to August (13.6km2), but there was no difference in the range of habitat types or depths surveyed (X2 = 3.749, df = 5, p = 0.586, Table S3). Across both sampling periods, relative fish abundance was strongly influenced by depth, but there was a significant interaction between depth and habitat type (Tables 1 and S2). In coral reef habitats, relative fish abundance increased with depth down to 30 m (the maximum depth for coral reef habitat in this study) and in algal-covered sand habitats fish abundance remained relatively stable across depths. In low-relief rubble habitats, relative fish abundance increased with depth in February, but decreased with depth in August (Fig. 4a, b). Across all other habitats there was a marked decrease in relative abundance with depth (Fig. 4a, b). In contrast to relative abundance, fish species richness declined more sharply with depth in August compared with February across the majority of habitat types (Fig. 4c, d), although in low-relief rubble habitats, species richness increased with depth in February, but decreased with depth in August. These overall declines in species richness also differed among habitats as species richness was substantially higher on more complex reef associated habitats compared to lower-complexity, more open habitats (Fig. 4c, d; Tables S2 & S4).
Relationships between relative fish abundance (MaxN hour−1) (a and b) and species richness (n species hour−1) (c and d) and habitat type categories across depth, and sampling period (February 2017 [a and c], August 2017 [b and d]). Lines show the mean predicted fits from generalised linear models, while shaded ribbons indicate the 95% confidence intervals. For ease of interpretation data points are not shown, however, to see these refer to Fig. S1
The fish assemblage varied markedly across the depth gradient (13–71 m) at Linden Bank. The most speciose families were Labridae (57 spp), Pomacentridae (34 spp), Acanthuridae (30 spp), and Serranidae (21 spp). Labridae and Caesionidae were the most abundant families observed throughout the shallower depth range between 13 and 29 m, comprising approximately 30% and 20% of the shallow water fish assemblage respectively (Fig. 5a). Similarly, planktivores and herbivores were the most abundant trophic groups in this shallow water depth range (Fig. 5b). By contrast, mobile invertivores and piscivores such as lethrinids and carangids were relatively more abundant at depths below 50 m (Figs. 5a and b), and these trophic groups accounted for approximately 68% and 25% of the relative abundance of the fish assemblage > 50 m respectively (Fig. 5b).
Overall, variation in the taxonomic composition of reef fishes was explained by depth and habitat type (Fig. 6, Table S5). In both sampling periods, differences across depths were driven by iconic coral reef taxa (e.g. Chaetodontidae, and Pomacanthidae) that were relatively more abundant in shallow water areas and coral reef, seawhip/algal reef and low-relief rubble habitats (Fig. 6). In contrast, in February and August, as depth increased the fish assemblage became dominated by higher relative abundances of carangids, lethrinids and mullids, particularly in the various sand habitats (Fig. 6).
Top: Capscale ordination for the a February 2017 and b August 2017 fish assemblage based on family level taxonomy and constrained by the environmental variables: habitat type category and depth (coloured polygons are to aid interpretation and do not represent significant groupings). Bottom: vectors showing the reef fish families that contributed substantially to the patterns observed in the ordination in c February 2017 and d August 2017, and the direction of depth increases in multidimensional space
In addition to taxonomic differences, there were clear differences in the composition of broad trophic groups across depth, and habitat type (Fig. 7, Table S5). Again, depth and habitat type were significantly correlated with the taxonomic composition of the fish assemblage in February and August 2017 (Table S5). In both sampling periods, the shallow-water fish assemblage, particularly in the coral reef, seawhip/algal reef and low-relief rubble habitats, was dominated by sessile invertivores, with herbivores/planktivores and detritivores also characterising these areas (Fig. 7). As depth increased, the fish assemblage became dominated by large piscivores and predators of mobile invertebrates, in the various sandy habitats (Fig. 7). However, it is important to note that while the relative standardised abundance of some taxa and carnivorous functional groups increased with depth, the total abundance and species richness of fish generally declined with increasing depth (Fig. 4).
Top: Capscale ordination for the a February 2017 and b August 2017 fish assemblage based on broad trophic groups constrained by the environmental variables: habitat type category and depth (coloured polygons are to aid interpretation and do not represent significant groupings). Bottom: vectors showing the reef fish families that contributed substantially to the patterns observed in the ordination in c February 2017 and d August 2017, and the direction of depth increases in multidimensional space
122 species of fishes considered to be fisheries targets of the Queensland Line Fishery were observed on Linden Bank. All species can be found in Table S1, but those included in the top 95% of Queensland catch data by weight were; Plectropomus leopardus, Plectropomus laevis, Plectropomus areolatus, Variola albimarginata, Variola louti, Variola spp., Lethrinus nebulosus, Lutjanus sebae, Caranx sexfasciatus, Caranx melampygus, Alectis indica, Carangoides spp., Scomberomerous spp., Lutjanus spp., Choerodon spp., Epinephelus spp., and Siganus spp. (Table S1). Key shark species were also observed including; Carcharhinus amblyrhynchos, Carcharhinus melanopterus, Galeocerdo cuvier, Triaenodon obesus, Sphyrna mokkaran. These important fishery species were observed across all depths from 13 to 71 m and accounted for ~ 27% (1991 out of 7381) of all fishes observed. Overall, the relative abundance of all fisheries targeted species was fairly constant with depth across both sampling periods (Fig. 8k). However, for individual fisheries groups (i.e. Plectropomus spp., Labrids, and Serranids) we documented higher relative abundances in shallower areas (< 40 m), and fewer individuals at > 40 m depth (Fig. 8c, e, g). This decrease in relative abundance with depth appeared to be consistent between February and August. The relative abundance of scarids in August also followed a similar decreasing pattern with depth, however in February, a high abundance of Scarus spp. were observed at depths below 60 m (Fig. 8j). Alternatively, lethrinids and carangids, were the only fisheries group whose relative abundance increased slightly with depth, a pattern that was consistent between February and August 2017 (Fig. 8a, b).
The relative abundance (MaxN hour−1) of key fish groups (a–j) and all commercially targeted fish species (k) in February 2017 and August 2017 across the depth gradient examined. Coloured lines show the fit of a LOESS smoother, the shaded ribbons delineate the 95% confidence intervals and the coloured points are the raw data points
Finally, for both the Top 95% (Fig S2) and All Fisheries Species (Fig. 9), multivariate analyses described similar patterns to the taxonomic and trophic composition of the entire fish assemblage. Again, depth and habitat type were influential predictors, significantly correlating with the overall taxonomic composition of all fisheries target species in February and August (Table S6). In all cases, Carangidae typified deeper areas (< 40 m), especially sandy habitats (Fig. 9, Fig S2). Lethrinidae also characterised these deeper areas in the subset of data composed of all fisheries target species. In contrast, and irrespective of sampling period, the shallower habitats were characterised by Serranidae and Labridae for both the Top 95% and All Fisheries species data. In addition, the substantial contribution of Plectropomus spp. to the shallow water assemblage in the ‘top 95% of fisheries target’ subset is particularly notable (Fig. 8c & S2, Table S6).
Top: Capscale ordination for the a February 2017 and b August 2017 assemblages of all fisheries target species on the Great Barrier based on family level taxonomy and constrained by the environmental variables: habitat type category and depth (coloured polygons are to aid interpretation and do not represent significant groupings) and bottom: vectors showing the reef fish families that contributed substantially to the patterns observed in the ordination in c February 2017 and d August 2017, and the direction of depth increases in multidimensional space
Discussion
This study revealed marked changes in the relative abundance, species richness and composition of reef fishes with depth (13–71 m) on a submerged offshore shoal (Linden Bank) on the Great Barrier Reef, Australia. Overall, the relative abundance and species richness of fishes generally declined with depth, however, this was dependent on habitat. These results support those from previous studies on the GBR, and elsewhere (e.g. Thresher and Colin 1986; Pearson and Stevens 2015; Lindfield et al. 2016; Asher et al. 2017), which have documented strong depth zonation patterns in fish assemblages, with distinct assemblages generally found below 40 m. These patterns of zonation are inextricably linked with both biotic (i.e. competition, predation and nutritional resource availability) and abiotic (i.e. substate, temperature, light) factors that vary with depth (Dustan 1979; Lesser et al. 2009; Kanhg et al. 2010).
While our study was based on a single location, the patterns of reef fish distributions documented are markedly similar to those observed in other tropical (e.g. Red Sea, Marshall Islands) (Brokovich et al. 2008; Thresher & Colin 1986) and sub-tropical reef systems (e.g. North West Hawaiian Islands) (Asher et al. 2017). For example, as in our study, depth is generally found to be a strong predictor of reef fish taxonomic structure (Cooper et al. 2019; Asher et al. 2020). We found that shallow (13–30 m) areas were characterised by relatively higher abundances of iconic coral reef fish taxa (e.g. Acanthuridae, Chaetodontidae, Labridae, Pomacanthidae, Pomacentridae), whereas the fish assemblage at greater depths (i.e. below 30 m), was dominated by species from Balistidae, Carangidae, Lethrinidae and Mullidae (see also Lindfield et al. 2016; Sih et al. 2017; Asher et al. 2017). Furthermore, shallow (< 30 m) areas were typically characterised by herbivores, detritivores, sessile invertivores and planktivores, while deeper (> 30 m) areas were typified by larger predatory fishes that fed on fishes and mobile invertebrates (see also Asher et al. 2017; Cooper et al. 2019). Striking similarities in the results of this study (at Linden Bank) with other comparable reef habitats (e.g. Asher et al. 2017) suggests that depth gradients in the taxonomic and functional structure of reef fish assemblages is highly consistent across locations.
Depth-related variation in the structure of the fish assemblage at Linden Bank was largely attributable to changes in the dominant habitat type. In general, habitat structure changes with depth due to decreasing light levels and variation in temperature and pressure (Done 1983; McGehee 1994). On Linden Bank, coral dominated reefs were the most prevalent habitat in shallow areas (< 32 m) and the associated fish assemblage tended to be comprised of smaller-bodied reef fishes such as planktivores and corallivores that often require benthic habitat complexity for shelter (Srinivasan 2003). Lower complexity, open sand or algal meadows and seawhip gardens dominated habitats in the upper-mesophotic zone (50–70 m) and were typified by mobile piscivores, and invertivores, as in previous studies (Asher et al. 2017; Williams et al. 2019). It is therefore likely that the distinct nature of the shallow water fish assemblage at Linden Bank, is influenced by habitat availability, rather than directly by depth per se.
Aside from habitat availability, higher primary productivity and a broader range of nutritional resources in shallow reef environments will underpin a wider range of niches, promoting co-existence of a higher number of fish species (Pratchett 2005; Wilson et al. 2008). For example, the higher relative abundances of herbivorous/detritivorous fishes observed in shallow waters in this study, may be a result of these groups tracking nutritional resource availability (Russ 2003; Russ et al. 2015; Bellwood et al. 2018), as the productivity of algae (Klumpp and McKinnon 1989; Tebbett and Bellwood 2021) and nutritional quality of particulate detritus (Crossman et al. 2001; Purcell and Bellwood 2001) are known to peak in shallow water reef habitats, where light levels are high. It has even been suggested that the evolution of key locomotor and feeding traits allowed herbivorous and detritivorous fishes to move into such habitats to exploit these nutritional resources (Bellwood et al. 2018). In this study, the highest relative abundances of planktivorous fishes were observed in depths < 50 m. Light availability is also likely to limit many planktivorous fishes to these shallower depths as they rely heavily on visual acuity to procure their planktonic prey (Rickel and Genin 2005; Johansen and Jones 2013). Similarly, corallivorous chaetodontids made up a large component of the sessile invertivore feeding group at Linden Bank, and the abundance of many of these fishes is heavily dependent on the availability of their specific coral prey (Pratchett et al. 2006; Graham et al. 2009). Coral dominated habitats were not detected beyond 32 m at Linden Bank, and accordingly, coral feeding fishes were not recorded at depths > 32 m.
In contrast to the trophic groups that dominated shallow water areas, the higher trophic level carnivorous fishes (piscivores and predators of mobile invertebrates) are not as limited by light or nutritional resource acquisition and can readily exist in the deeper water habitats (Bejarano et al. 2014; Andradi-Brown et al. 2016; Sih et al. 2017). However, it is important to note that while the relative abundance (compared to other groups) of some taxa and carnivorous functional groups increased with depth, the abundance and species richness of the fish community declined markedly with depth. So rather than gaining different taxa or trophic groups across the depth range examined, our results suggest that in many cases taxa and trophic groups may be lost at different rates along this gradient. Evidence from stable isotopes suggests deeper water predators remain heavily reliant on the productivity of shallow water fish assemblages between 0 and 30 m (Smith and Parrish 2002; Asher et al. 2017). As such, these piscivores and predators of mobile invertebrates may act as important nutrient conduits channeling nutrients from photic to mesophotic depths (Meyer et al. 2001; Wetherbee et al. 2004). For example, predators such as Carangids and Lethrinids that remain relatively abundant at depths between 30 and 70 m, are large and highly mobile allowing them to easily transition between depths (Currey et al. 2015; Asher et al. 2017), and thus move energy across this depth gradient (Smith & Parrish 2002), particularly by moving into shallower waters during crepuscular or nocturnal periods for foraging (Bosiger & McCormick 2014). Although, any relationships between habitat connectivity and mobile fishes may be species-specific, with recent studies showing limited depth use for other mobile predators such as Plectropomus leopardus (Matley et al. 2015; Scott et al. unpublished). It is also worth noting that schools of herbivorous parrotfishes (Scarus ghobban) were still observed at depths beyond 60 m. This particular species appears to have broken the standard parrotfish mold, potentially being more flexible in its dietary requirements, as it frequently inhabits more marginal reef environments (Bariche & Bernardi 2009; Bennett et al. 2015). Further understanding of the capacity for reef fishes to move energy across depths and exist in deeper, light limited environments offers an interesting avenue for additional research (see Hilting et al. 2013; Fukunaga et al. 2016).
Temperature often varies greatly with depth (Bertolo et al., 2011) and is expected to influence the distribution, abundance and composition of fishes. In this study, absolute temperature differed markedly between sampling periods (February and August 2017) but varied relatively little with depth within sampling periods. Moreover, depth-related differences in the fish assemblage were seemingly independent of temperature. Depth-related refuges are hypothesised to buffer fishes and other coral reef organisms from localised stressors (i.e. elevated temperatures and wave energy) that are generally most pronounced in upper surface waters (Bonagerts et al. 2010; Smith et al. 2016). However, our data provides little support for the redistribution of fish species in accordance with changing temperatures. Rather, the depth distributions of most fish species appeared to be constrained by the availability of specific habitats and resources. It is possible that limited changes in the depth-distribution of fishes were attributable to the apparent lack of strong temperature gradients with depth (especially in February 2017), and it remains to be seen if our results (where there was consistent high temperature to a depth of > 50 m) were linked to anomalous conditions. If, however, elevated temperatures during marine heatwaves are linked to limited changes in temperature then this may limit the capacity of fishes to exploit depth-refugia as a means of moderating exposure to supra-optimal temperatures.
While we found limited evidence for the redistribution of species between sampling periods despite a > 4 °C difference in recorded temperatures, the overall relative abundance of fishes was ~ 26% higher in February 2017 compared to August 2017. The higher relative abundance of fishes in February may be due to a number of factors. For example, fishes may make seasonal reproductive movements to Linden Bank to spawn in summer (Samoilys and Squire 1994; Zeller 1998). Alternatively, temperature induced increases in metabolic demands during summer may increase foraging activity (Scott et al. 2017) making individuals more likely to approach the bait from the BRUV. However, temporal contrasts in this study cannot be clearly attributed to seasons or temperature differences due to simultaneous variation in the areal extent of sampling. Although, there were no significant differences in the range of habitat type or depths sampled in February versus August 2017.
Fisheries species are particularly vulnerable to the effects of fishing if there is limited habitat or constrained depth-ranges for target species (Jennings and Polunin 1996). Shallow waters have been selectively and heavily impacted by fishing due to gear constraints and accessibility to coastal fisheries (Fry et al. 2006). However, technological advances in gear type and fuel efficiency mean the exploitation of deeper reefs, further from shore, is becoming more commonplace (Schiller et al. 2015; Friedlander et al. 2019). In contrast to previous studies which highlight the potential for mesophotic coral reef ecosystems to act as refugia from disturbances and potential sinks for fisheries productivity (Bridge et al. 2011, 2013; Sih et al. 2017), our data tend to suggest a lack of any apparent or effective depth refuge down to 50 m, at least at this location. Again, the depth-distribution of fisheries target species in this study was strongly correlated with depth and habitat type rather than temperature and there appeared to be no overall differences in the depth-distribution between February and August 2017. This notion is supported by the direct dependence of many taxonomic and functional fisheries groups from the coral reef finfish fishery on the resources present in shallow-water habitats.
It is important to note that while we did find that the larger-bodied, mobile, carnivorous fisheries target species such as Lethrinids, Carangids and some Lutjanids (i.e. L. sebae) tended to characterise deeper locations, this was largely because their abundances remained relatively similar across the depth gradient, while the relative abundances of all other groups tended to decline. As such, this does not necessarily suggest they prefer deeper environments, but instead their distribution across this depth gradient could be decoupled from the factors constraining other groups (Smith and Parrish 2002; Asher et al. 2017). Given that Linden Bank is open to fishing, and subject to intensive fishing for pelagic fishes between September – December, the distribution of some groups could also be impacted by fisheries effects (i.e. limited variation in abundance across depths could be due to fishing pressure in the shallows reducing the relative abundance of some groups). However, the isolation and exposure of Linden Bank are likely to limit fisheries pressure on benthic fishes. Therefore, if environmental stressors lead to the degradation of highly-productive shallow water reef areas, there could be bottom-up ecosystem effects impacting the food chain and culminating in reduced fisheries productivity (e.g. Graham et al. 2007; Pratchett et al. 2014; Hempson et al. 2017). Even if deeper areas offered some refuge for some fish species, they are often habitat and resource limited compared with shallow reef areas and, therefore, may be unable to counteract a decline in fisheries productivity. Under future climate change scenarios, more frequent thermal anomalies coupled with limited water movement due to changing ocean currents are expected to impact the structure and function of coral reef ecosystems (IPCC 2022). Given the predicted increase in the frequency of such anomalies in the coming decades, there may be very limited opportunity for reef fishes to find thermal or structural refuge, thereby undermining the long-term productivity of wild fish stocks (Pratchett et al. 2016).
Overall, this study provides one of the few assessments of depth-related changes in the relative abundance as well as taxonomic and trophic structure of a coral reef fish assemblage (including important fisheries species) across a shallow to upper mesophotic depth gradient (but see Asher et al. 2017). Extensive sampling at Linden Bank in February and August 2017, revealed marked changes in the taxonomic and functional composition of fishes with depth that were largely attributable to changes in habitat structure. Contrary to expectations, there was minimal variation in temperature with depth (at least down to 50 m) and no obvious major temporal changes in the depth distributions of larger and more mobile fishes. Given that shallow-water coral reef habitats are highly vulnerable to increasing anthropogenic stressors (Hughes et al. 2017; IPCC 2022), and that our data highlight a limited propensity for depth to act as a refuge for fishes at this location, it is likely that any stressors that degrade the shallow-water habitats in this location could jeopardise the viability and sustainability of associated fisheries. The significantly higher relative abundance and species richness of fishes in shallow water (< 30 m) areas at Linden Bank, suggests that effectively managing such areas is essential to sustain fisheries productivity into the future.
Data availability
All data used in this study are available in.csv (supplementary file S1) and.pdf (supplementary file S2).
Code availability
Custom code for our analyses is available in an.Rmd format supplementary file (S3).
References
Adey WH (1998) Coral reefs: algal structured and mediated ecosystems in shallow, turbulent, alkaline waters. J Phycol 34:393–406
Alanara A, Burns MD, Metcalfe NB (2001) Intraspecific resource partitioning in brown trout: the temporal distribution of foraging is determined by social rank. J Anim Ecol 70:980–986. https://doi.org/10.1046/j.0021-8790.2001.00550.x
Andradi-Brown DA, Gress E, Wright G, Exton DA, Rogers AD (2016) Reef fish community biomass and trophic structure changes across shallow to upper-mesophotic reefs in the Mesoamerican barrier reef. Caribbean Plos One 11:e0156641. https://doi.org/10.1371/journal.pone.0156641
Asher J, Williams ID, Harvey ES (2017) Mesophotic depth gradients impact reef fish assemblage composition and functional group partitioning in the Main Hawaiian Islands. Front Mar Sci 4:98. https://doi.org/10.3389/fmars.2017.00098
Azumaya T, Ishida Y (2005) Mechanism of body cavity temperature regulation of chum salmon (Oncorhynchus keta) during homing migration in the North Pacific Ocean. Fish Oceanogr 14:81–96. https://doi.org/10.1111/j.1365-2419.2004.00323.x
Bariche M, Bernardi G (2009) Lack of a genetic bottleneck in a recent Lessepsian bioinvader, the blue-barred parrotfish, Scarus ghobban. Mol Phylogenet Evol 53:592–595. https://doi.org/10.1016/j.ympev.2009.06.017
Barton K (2020) MuMIn: Multi-Model Inference. R package version 1.43.17. https://CRAN.R-project.org/package=MuMIn
Bejarano I, Appeldoorn RS, Nemeth M (2014) Fishes associated with mesophotic coral ecosystems in La Parguera, Puerto Rico. Coral Reefs 33:313–328. https://doi.org/10.1007/s00338-014-1125-6
Bell JD, Kronen M, Vunisea A, Nash WJ, Keeble G, Demmke A, Pontifex S, Andréfouët S (2009) Planning the use of fish for food security in the Pacific. Mar Policy 33:64–76. https://doi.org/10.1016/j.marpol.2008.04.002
Bellwood DR, Tebbett SB, Bellwood O, Mihalitsis M, Morais RA, Streit RP, Fulton CJ (2018) The role of the reef flat in coral reef trophodynamics: past, present, and future. Ecol Evol 8:4108–4119. https://doi.org/10.1002/ece3.3967
Bennett S, Wernberg T, Harvey ES, Santana-Garcon J, Saunders BJ (2015) Tropical herbivores provide resilience to a climate-mediated phase shift on temperate reefs. Ecol Lett 18:714–723. https://doi.org/10.1111/ele.12450
Bertolo A, Pepino M, Adams J, Magnan P (2011) Behavioural thermoregulatory tactics in lacustrine brook Charr. Salvelinus Fontinalis Plos One. https://doi.org/10.1371/journal.pone.0018603
Bond T, Partridge JC, Taylor MD, Cooper TF, McLean DL (2018) The influence of depth and a subsea pipeline on fish assemblages and commercially fished species Ed M. (Gee) G. Chapman. Plos One 13:e0207703. https://doi.org/10.1371/journal.pone.0207703
Bongaerts P, Ridgway T, Sampayo EM, Hoegh-Guldberg O (2010) Assessing the ‘deep reef refugia’ hypothesis: focus on Caribbean reefs. Coral Reefs 29:309–327. https://doi.org/10.1007/s00338-009-0581-x
Bongaerts P, Muir P, Englebert N, Bridge TL, Hoegh-Guldberg O (2013) Cyclone damage at mesophotic depths on Myrmidon Reef (GBR). Coral Reefs 32:935–935
Bosiger YJ, McCormick MI (2014) Temporal links in daily activity patterns between coral reef predators and their prey. PLoS ONE. https://doi.org/10.1371/journal.pone.0111723
Bouchet P, Meeuwig J, Huveneers C, Langlois T, Letessier T, Lowry M, Rees M, Santana-Garcon J, Scott M, Taylor M, Thompson C, Vigliola L, Whitmarsh S (2018) Marine sampling field manual for pelagic stereo BRUVS (baited remote underwater videos) [Version 1]. In: Przeslawski R, Foster S (eds) Field manuals for marine sampling to monitor Australian waters. NESP Marine Biodiversity Hub, Canberra, Australia, pp 105–132. https://doi.org/10.11636/9781925297669
Bridge TCL, Done TJ, Beaman RJ, Friedman A, Williams SB, Pizarro O, Webster JM (2011) Variability in mesophotic coral reef communities along the Great Barrier Reef, Australia. Mar Ecol Prog Ser 428:63–75. https://doi.org/10.3354/meps09046
Bridge TCL, Hughes TP, Guinotte JM, Bongaerts P (2013) Call to protect all coral reefs. Nat Clim Chang 3:528. https://doi.org/10.1038/nclimate1879
Brokovich E, Einbinder S, Shashar N, Kiflawi M, Kark S (2008) Descending to the twilight-zone: changes in coral reef fish assemblages along a depth gradient down to 65 m. Mar Ecol Prog Ser 371:253–262. https://doi.org/10.3354/meps07591
Brown BE (1997) Adaptations of reef corals to physical environmental stress. In: Blaxter JHS, Southward AJ (eds) Advances in marine biology, vol 31. Academic Press, Singapore, pp 221–299
Brown CJ, Taylor W, Wabnitz CC, Connolly RM (2020) Dependency of Queensland and the great barrier Reef’s tropical fisheries on reef-associated fish. Sci Rep 10(1):1–11. https://doi.org/10.1038/s41598-020-74652-2
Cappo M, De’ath G, Speare P (2007) Inter-reef vertebrate communities of the Great Barrier Reef Marine Park determined by baited remote underwater video stations. Marine Ecol Prog Ser 350:209–221. https://doi.org/10.3354/meps07189
Cartamil DP, Lowe CG (2004) Diel movement patterns of ocean sunfish Mola mola off southern California. Mar Ecol Prog Ser 266:245–253. https://doi.org/10.3354/meps266245
Crossman DJ, Choat JH, Clements KD, Hardy T, McConochie J (2001) Detritus as food for grazing fishes on coral reefs. Limnol Oceanogr 46:1596–1605. https://doi.org/10.4319/lo.2001.46.7.1596
Cuetos-Bueno J, Houk P (2015) Re-estimation and synthesis of coral-reef fishery landings in the Commonwealth of the Northern Mariana Islands since the 1950s suggests the decline of a common resource. Rev Fish Biol Fisheries 25:179–194. https://doi.org/10.1007/s11160-014-9358-6
Cundy ME, Santana-Garcon J, Ferguson AM, Fairclough DV, Jennings P, Harvey ES (2017) Baited remote underwater stereo-video outperforms baited downward-facing single-video for assessments of fish diversity, abundance and size composition. J Exp Mar Biol Ecol 497:19–32. https://doi.org/10.1016/j.jembe.2017.09.004
Currey L, Heupel M, Simpfendorfer C, Williams A (2015) Assessing environmental correlates of fish movement on a coral reef. Coral Reefs 34:1267–1277. https://doi.org/10.1007/s00338-015-1318-7
Currey-Randall LM, Cappo M, Simpfendorfer CA, Farabaugh NF, Heupel MR (2020) Optimal soak times for Baited Remote Underwater Video Station surveys of reef-associated elasmobranchs. PLoS ONE 15:e0231688. https://doi.org/10.1371/journal.pone.0231688
Dustan P (1979) Distribution of zooxanthellae and photosynthetic chloroplast pigments of the reef-building coral Montastraea annularis Ellis and Solander in relation to depth on a West Indian coral reef. Bull Mar Sci 29:79–95
Fitzpatrick BM, Harvey ES, Heyward AJ, Twiggs EJ, Colquhoun J (2012) Habitat specialization in tropical continental shelf demersal fish assemblages Ed R. K. F. Unsworth. PLoS ONE. 7:e39634. https://doi.org/10.1371/journal.pone.0039634
Friedlander AM, Giddens J, Ballesteros E, Blum S, Brown EK, Caselle JE, Henning B, Jost C, Salinas-de-León P, Sala E (2019) Marine biodiversity from zero to a thousand meters at Clipperton Atoll (Île de La Passion). Tropical Eastern Pacific Peerj 7:e7279. https://doi.org/10.7717/peerj.7279
Fry GC, Brewer DT, Venables WN (2006) Vulnerability of deepwater demersal fishes to commercial fishing: Evidence from a study around a tropical volcanic seamount in Papua New Guinea. Fish Res 81:126–141. https://doi.org/10.1016/j.fishres.2006.08.002
Fukunaga A, Kosaki RK, Wagner D, Kane C (2016) Structure of mesophotic reef fish assemblages in the Northwestern Hawaiian Islands. PLoS ONE 11:e0157861
Furey NB, Dance MA, Rooker JR (2013) Fine-scale movements and habitat use of juvenile southern flounder Paralichthys lethostigma in an estuarine seascape. J Fish Biol 82:1469–1483. https://doi.org/10.1111/jfb.12074
Glynn PW (1996) Coral reef bleaching: facts, hypotheses and implications. Glob Change Biol 2:495–509. https://doi.org/10.1111/j.1365-2486.1996.tb00063.x
González-Sansón G, Aguilar C, Hernández I, Cabrera Y (2009) Effects of depth and bottom communities on the distribution of highly territorial reef fish in the northwestern region of Cuba. J Appl Ichthyol 25:652–660. https://doi.org/10.1111/j.1439-0426.2009.01332.x
Goyer K, Bertolo A, Pepino M, Magnan P (2014) Effects of Lake Warming on Behavioural Thermoregulatory Tactics in a Cold-Water Stenothermic Fish. PLoS ONE. https://doi.org/10.1371/journal.pone.0092514
Graham NAJ, Wilson SK, Jennings S, Polunin NVC, Robinson J, Bijoux JP, Daw TM (2007) Lag effects in the impacts of mass coral bleaching on coral reef fish, fisheries, and ecosystems. Conserv Biol 21:1291–1300. https://doi.org/10.1111/j.1523-1739.2007.00754
Graham NAJ, Wilson SK, Pratchett MS, Polunin NVC, Spalding MD (2009) Coral mortality versus structural collapse as drivers of corallivorous butterflyfish decline. Biodivers Conserv 18:3325–3336. https://doi.org/10.1007/s10531-009-9633-3
Harasti D, Malcolm H, Gallen C, Coleman MA, Jordan A, Knott NA (2015) Appropriate set times to represent patterns of rocky reef fishes using baited video. J Exp Mar Biol Ecol 463:73–180. https://doi.org/10.1016/j.jembe.2014.12.003
Hemingson CR, Bellwood DR (2018) Biogeographic patterns in major marine realms: function not taxonomy unites fish assemblages in reef, seagrass and mangrove systems. Ecography 41:174–182. https://doi.org/10.1111/ecog.03010
Hempson TN, Graham NAJ, MacNeil MA, Williamson DH, Jones GP, Almany GR (2017) Coral reef mesopredators switch prey, shortening food chains, in response to habitat degradation. Ecol Evol 7:2626–2635. https://doi.org/10.1002/ece3.2805
Hicks CC, Cohen PJ, Graham NA, Nash KL, Allison EH, D’Lima C, Mills DJ, Roscher M, Thilsted SH, Thorne-Lyman AL, MacNeil MA (2019) Harnessing global fisheries to tackle micronutrient deficiencies. Nature 574:95–98. https://doi.org/10.1038/s41586-019-1592-6
Hilting AK, Currin CA, Kosaki RK (2013) Evidence for benthic primary production support of an apex predator-dominated coral reef food web. Mar Biol 160:1681–1695. https://doi.org/10.1007/s00227-013-2220-x
Hixon MA, Menge BA (1991) Species diversity: Prey refuges modify the interactive effects of predation and competition. Theor Popul Biol 39:178–200. https://doi.org/10.1016/0040-5809(91)90035-E
Holt RD (1987) Prey communities in patchy environments. Oikos. https://doi.org/10.2307/3565488
Hussey NE, Kessel ST, Aarestrup K, Cooke SJ, Cowley PD, Fisk AT, Harcourt RG, Holland KN, Iverson SJ, Kocik JF, Flemming JEM (2015) Aquatic animal telemetry: a panoramic window into the underwater world. Science. https://doi.org/10.1126/science.1255642
IPCC, 2022: Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [H.-O. Pörtner, D.C. Roberts, M. Tignor, E.S. Poloczanska, K. Mintenbeck, A. Alegría, M. Craig, S. Langsdorf, S. Löschke, V. Möller, A. Okem, B. Rama (eds.)]. Cambridge University Press.
Jennings S, Polunin NVC (1996) Effects of fishing effort and catch rate upon the structure and biomass of Fijian reef fish communities. J Appl Ecol 33:400–412. https://doi.org/10.2307/2404761
Johansen JL, Jones GP (2013) Sediment-induced turbidity impairs foraging performance and prey choice of planktivorous coral reef fishes. Ecol Appl 23:1504–1517. https://doi.org/10.1890/12-0704.1
Kahler TH, Roni P, Quinn TP (2001) Summer movement and growth of juvenile anadromous salmonids in small western Washington streams. Can J Fish Aquat Sci 58:1947–1956. https://doi.org/10.1139/f01-134
Kahng SE, García-Sais JR, Spalding HL, Brokovich E, Wagner D, Weil E, Hinderstein L, Toonen RJ (2010) Community ecology of mesophotic coral reef ecosystems. Coral Reefs 29:255–275. https://doi.org/10.1007/s00338-010-0593-6
Kerry JT, Bellwood DR (2015) The functional role of tabular structures for large reef fishes: avoiding predators or solar irradiance? Coral Reefs 34:693–702. https://doi.org/10.1007/s00338-015-1275-1
Khan JA, Goatley CHR, Brandl SJ, Tebbett SB, Bellwood DR (2017) Shelter use by large reef fishes: long-term occupancy and the impacts of disturbance. Coral Reefs 36:1123–1132. https://doi.org/10.1007/s00338-017-1604-7
Klumpp DW, McKinnon AD (1989) Temporal and spatial patterns in primary production of a coral-reef epilithic algal community. J Exp Mar Biol Ecol 131:1–22. https://doi.org/10.1016/0022-0981(89)90008-7
Langlois T, Williams J, Monk J, Bouchet P, Currey L, Goetze J, Harasti D, Huveneers C, Malcolm H. Whitmarsh S (2018) Marine sampling field manual for benthic stereo BRUVS (Baited Remote Underwater Videos). In book: Field Manuals for Marine Sampling to Monitor Australian Waters Chapter: 5 Publisher: National Environmental Science Programme, Marine Biodiversity Hub Editors: Rachel Przeslawski, Scott Foster
Lenth R (2020) emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.4.8. https://CRAN.R-project.org/package=emmeans
Lesser MP, Slattery M, Leichter JJ (2009) Ecology of mesophotic coral reefs. J Exp Mar Biol Ecol 375:1–8. https://doi.org/10.1016/j.jembe.2009.05.009
Lindberg WJ, Frazer TK, Portier KM, Vose F, Loftin J, Murie DJ, Mason DM, Nagy B, Hart MK (2006) Density-dependent habitat selection and performance by a large mobile reef fish. Ecol Appl 16:731–746
Lindfield SJ, Harvey ES, Halford AR, McIlwain JL (2016) Mesophotic depths as refuge areas for fishery-targeted species on coral reefs. Coral Reefs 35:125–137. https://doi.org/10.1007/s00338-015-1386-8
Loya Y, Eyal G, Treibitz T, Lesser MP, Appeldoorn R (2016) Theme section on mesophotic coral ecosystems: advances in knowledge and future perspectives. Coral Reefs 35:1–9. https://doi.org/10.1007/s00338-016-1410-7
MacDonald C, Bridge TCL, Jones GP (2016) Depth, bay position and habitat structure as determinants of coral reef fish distributions: Are deep reefs a potential refuge? Mar Ecol Prog Ser 561:17–231. https://doi.org/10.3354/meps11953
Matley JK, Heupel MR, Simpfendorfer CA (2015) Depth and space use of leopard coralgrouper Plectropomus leopardus using passive acoustic tracking. Mar Ecol Prog Ser 521:201–216. https://doi.org/10.3354/meps11122
McClanahan TR, Graham NAJ, MacNeil MA, Cinner JE (2015) Biomass-based targets and the management of multispecies coral reef fisheries. Conserv Biol 29:409–417. https://doi.org/10.1111/cobi.12430
McGehee MA (1994) Correspondence between assemblages of coral-reef fishes and gradients of water motion, depth, and habitat type size off Puerto-Rico. Mar Ecol Prog Ser 105:243–255
McLean DL, Harvey ES, Meeuwig JJ (2011) Declines in the abundance of coral trout (Plectropomus leopardus) in areas closed to fishing at the Houtman Abrolhos Islands, Western Australia. J Exp Mar Biol Ecol 406:71–78. https://doi.org/10.1016/j.jembe.2011.06.009
Meyer CG, Holland KN, Wetherbee BM, Lowe CG (2001) Diet, resource partitioning and gear vulnerability of Hawaiian jacks captured in fishing tournaments. Fish Res 53:05–113. https://doi.org/10.1016/S0165-7836(00)00285-X
Neal BP, Condit C, Liu G, dos Santos S, Kahru M, Mitchell BG, Kline DI (2014) When depth is no refuge: cumulative thermal stress increases with depth in Bocas del Toro, Panama. Coral Reefs 33:193–205. https://doi.org/10.1007/s00338-013-1081-6
Neverman D, Wurtsbaugh WA (1994) The thermoregulatory function of diel vertical migration for a juvenile fish, Cottus extensus. Oecologia 98:247–256. https://doi.org/10.1007/bf00324211
Newton K, Côté IM, Pilling GM, Jennings S, Dulvy NK (2007) Current and Future Sustainability of Island Coral Reef Fisheries. Curr Biol 17:655–658. https://doi.org/10.1016/j.cub.2007.02.054
Oksanen J, Guillaume F, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin P, O'Hara G, Simpson G, Solymos P, Stevens H, Szoecs E, Wagner H (2019) vegan:Community Ecology Package. R package version 2.5–6. https://CRAN.R-project.org/package=vegan
Pauly D, Watson R, Alder J (2005) Global trends in world fisheries: impacts on marine ecosystems and food security. Philosophical Transactions of the Royal Society of London. Series b, Biological Sciences 360:5–12. https://doi.org/10.1098/rstb.2004.1574
Pearson R, Stevens T (2015) Distinct cross-shelf gradient in mesophotic reef fish assemblages in subtropical eastern Australia. Mar Ecol Prog Ser 532:185–196. https://doi.org/10.3354/meps11351
Pratchett MS, Wilson SK, Baird AH (2006) Declines in the abundance of Chaetodon butterflyfishes following extensive coral depletion. J Fish Biol 69:1269–1280. https://doi.org/10.1111/j.1095-8649.2006.01161.x
Pratchett MS, Hoey AS, Wilson SK (2014) Reef degradation and the loss of critical ecosystem goods and services provided by coral reef fishes. Current Opinion in Environmental Sustainability 7:37–43. https://doi.org/10.1016/j.cosust.2013.11.022
Purcell SW, Bellwood DR (2001) Spatial patterns of epilithic algal and detrital resources on a windward coral reef. Coral Reefs 20:117–125. https://doi.org/10.1007/s003380100150
Pyle RL (2000) Assessing undiscovered fish biodiversity on deep coral reefs using advanced self-contained diving technology. Mar Technol Soc J 34:82–91. https://doi.org/10.4031/MTSJ.34.4.11
Queensland Fisheries (Coral Reef Fin Fish) Management Plan (2003) Queensland Subordinate Legislation 2003 No. 212. Fisheries Act 1994. https://www.legislation.qld.gov.au/view/pdf/asmade/sl-2003-0212
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Richards BL, Williams ID, Vetter OJ, Williams GJ (2012) Environmental factors affecting large-bodied coral reef fish assemblages in the Mariana Archipelago. PLoS ONE. https://doi.org/10.1371/journal.pone.0031374
Rickel S, Genin A (2005) Twilight transitions in coral reef fish: The input of light-induced changes in foraging behaviour. Anim Behav 70:133–144. https://doi.org/10.1016/j.anbehav.2004.10.014
Robinson JP, Wilson SK, Robinson J, Gerry C, Lucas J, Assan C, Govinden R, Jennings S, Graham NA (2019) Productive instability of coral reef fisheries after climate-driven regime shifts. Nature Ecol Evol 3(2):183–190. https://doi.org/10.1038/s41559-018-0715-z
Rocha LA, Pinheiro HT, Shepherd B, Papastamatiou YP, Luiz OJ, Pyle RL, Bongaerts P (2018) Mesophotic coral ecosystems are threatened and ecologically distinct from shallow water reefs. Science 361:281–284. https://doi.org/10.1126/science.aaq1614
Russ GR (2003) Grazer biomass correlates more strongly with production than with biomass of algal turfs on a coral reef. Coral Reefs 22:63–67. https://doi.org/10.1007/s00338-003-0286-5
Russ GR, Questel S-LA, Rizzari JR, Alcala AC (2015) The parrotfish–coral relationship: refuting the ubiquity of a prevailing paradigm. Mar Biol 162:2029–2045. https://doi.org/10.1007/s00227-015-2728-3
Samoilys MA, Squire LC (1994) Preliminary observations on the spawning behavior of coral trout, Plectropomus leopardus (Pisces, Serranidae), on the Great Barrier Reef. Bull Mar Sci 54:332–342
Schiller L, Alava JJ, Grove J, Reck G, Pauly D (2015) The demise of Darwin’s fishes: evidence of fishing down and illegal shark finning in the Galápagos Islands. Aquat Conserv Mar Freshwat Ecosyst 25:431–446. https://doi.org/10.1002/aqc.2458
Scott M, Heupel M, Tobin A, Pratchett M (2017) A large predatory reef fish species moderates feeding and activity patterns in response to seasonal and latitudinal temperature variation. Sci Rep 7:12966. https://doi.org/10.1038/s41598-017-13277-4
Scott ME, Heupel MR, Simpfendorfer CA, Matley JK, Pratchett MS (2019) Latitudinal and seasonal variation in space use by a large, predatory reef fish, Plectropomus leopardus. Funct Ecol 33:670–680. https://doi.org/10.1111/1365-2435.13271
Sih TL, Cappo M, Kingsford M (2017) Deep-reef fish assemblages of the Great Barrier Reef shelf-break (Australia). Sci Rep 7:10886. https://doi.org/10.1038/s41598-017-11452-1
Sih TL, Daniell JJ, Bridge TCL, Beaman RJ, Cappo M, Kingsford MJ (2019) Deep-reef fish communities of the great barrier reef shelf-break: trophic structure and habitat associations. Diversity 11:26. https://doi.org/10.3390/d11020026
Smith GC, Parrish JD (2002) Estuaries as Nurseries for the Jacks Caranx ignobilis and Caranx melampygus (Carangidae) in Hawaii. Estuar Coast Shelf Sci 55:347–359. https://doi.org/10.1006/ecss.2001.0909
Srinivasan M (2003) Depth distributions of coral reef fishes: the influence of microhabitat structure, settlement, and post-settlement processes. Oecologia 137:76–84. https://doi.org/10.1007/s00442-003-1320-6
State of Queensland Department of Agriculture Fisheries and Forestry. QFish data cube. https://qfish.fisheries.qld.gov.au/ (2020).
Stowar M, De’ath G, Doherty P, Johansson C, Speare P, Venables B (2008) Influence of zoning on midshelf shoals from the southern Great Barrier Reef. Report to the Marine and Tropical Sciences Research Facility.
Tebbett SB, Bellwood DR (2021) Algal turf productivity on coral reefs: a meta-analysis. Mar Environ Res 168:105311
Thresher RE, Colin PL (1986) Trophic structure, diversity and abundance of fishes of the deep reef (30–300m) at Enewetak, Marshall Islands. Bull Mar Sci 38:253–272
Thums M, Meekan M, Stevens J, Wilson S, Polovina J (2013) Evidence for behavioural thermoregulation by the world’s largest fish. J R Soc Interface. https://doi.org/10.1098/rsif.2012.0477
Tobin A, Currey L, Simpfendorfer C (2013) Informing the vulnerability of species to spawning aggregation fishing using commercial catch data. Fish Res 143:47–56. https://doi.org/10.1016/j.fishres.2013.01.011
Walsh AT, Barrett N, Hill N (2017) Efficacy of baited remote underwater video systems and bait type in the cool-temperature zone for monitoring ‘no-take’marine reserves. Mar Freshw Res 68:568–580. https://doi.org/10.1071/MF15165
Wetherbee BM, Holland KN, Meyer CG, Lowe CG (2004) Use of a marine reserve in Kaneohe Bay, Hawaii by the giant trevally, Caranx ignobilis. Fish Res 67:253–263. https://doi.org/10.1016/j.fishres.2003.11.004
White KN, Weinstein DK, Ohara T, Denis V, Montenegro J, Reimer JD (2017) Shifting communities after typhoon damage on an upper mesophotic reef in Okinawa. Japan Peerj 5:e3573. https://doi.org/10.7717/peerj.3573
Wickham HJ et al (2019) Welcome to the tidyverse. J Open Sour Softw 4:43–1686. https://doi.org/10.21105/joss.01686
Williams J, Jordan A, Harasti D, Davies P, Ingleton T (2019) Taking a deeper look: Quantifying the differences in fish assemblages between shallow and mesophotic temperate rocky reefs. PLoS ONE 14:e0206778. https://doi.org/10.1371/journal.pone.0206778
Willis TJ, Babcock RC (2000) A baited underwater video system for the determination of relative density of carnivorous reef fish. Mar Freshw Res 51:755–763. https://doi.org/10.1071/MF00010
Zeller DC (1998) Spawning aggregations: patterns of movement of the coral trout Plectropomus leopardus (Serranidae) as determined by ultrasonic telemetry. Mar Ecol Prog Ser 162:253–263. https://doi.org/10.3354/meps162253
Zintzen V, Anderson MJ, Roberts CD, Harvey ES, Stewart AL, Struthers CD (2012) Diversity and composition of demersal fishes along a depth gradient assessed by baited remote underwater stereo-video. PLoS ONE 7:e48522. https://doi.org/10.1371/journal.pone.0048522
Acknowledgements
We would like to sincerely thank Marcus Stowar, Rebecca Dennis and Matthew Knott as well as the captains and crew of the Kirby and Blue Planet Marine research vessels for their assistance and in-kind support for the field aspects of this study.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This study was funded by the Fisheries Research and Development Corporation (FRDC; Project 2018–034), Sea World Research and Rescue Foundation (Grant No. SWR/9/2017), and the Australian Research Council (ARC) Centre of Excellence for Coral Reef Studies (Grant No. CE140100020).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Molly Scott, Morgan Pratchett, Cassandra Thompson and Michelle Heupel, data analysis and footage watching was performed by Molly Scott, Sterling Tebbett. Frank Mancini and Kirsty Whitman. The first draft of the manuscript was written by Molly Scott and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare they have no conflicts of interest.
Consent to participate
All authors consent to participate.
Consent for publication
All authors consent to publication of this paper.
Ethics approval
This project was carried out under ethics permit number: CSE76 from James Cook University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Scott, M.E., Tebbett, S.B., Whitman, K.L. et al. Variation in abundance, diversity and composition of coral reef fishes with increasing depth at a submerged shoal in the northern Great Barrier Reef. Rev Fish Biol Fisheries 32, 941–962 (2022). https://doi.org/10.1007/s11160-022-09716-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11160-022-09716-9