Abstract
Atlantic salmon Salmo salar is a socio-economically important anadromous fish species that has suffered synchronous population declines around the North Atlantic over the last five decades. Reduced marine survival has been implicated as a key driver of the declines, yet the relative importance of different stressors causing mortality at sea is not well understood. This review presents a synopsis of the principal stressors impacting Atlantic salmon in estuarine and marine environments. It also applies a semi-quantitative 2-D classification system to assess the relative effects of these stressors on English salmon stocks and their likely development over the next decade. Climate change and predation were identified as the biggest threats at present and over the next decade. Poor water quality and bycatch were classified as relatively high impact stressors, but with a lower likelihood of becoming more prevalent in the future due to available mitigation measures. Other, less influential, stressors included tidal barrages, artificial light at night, impingement in power-station cooling waters and thermal discharges, pile-driving noise pollution, invasive non-native species, electromagnetic fields, salmon mariculture, and tidal lagoons. Salmon fisheries exploitation was not regarded as an important stressor currently because effective exploitation rate controls have been implemented to substantially reduce fishing pressure. Future research priorities include addressing knowledge gaps on expanding stressor impacts from climate change, predation, renewable energy developments, and artificial light at night. Local management actions directed towards improving freshwater and estuarine habitats to maximise ecosystem resilience to stressors and minimise their cumulative impacts are recommended.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atlantic salmon Salmo salar (hereafter salmon) is a socio-economically important anadromous fish species exploited in commercial, subsistence, and recreational fisheries (ICES 2019). After hatching in spring, juveniles remain in fresh water for 1–8 years, with an increase in age-at-smoltification with increasing latitude, before migrating to sea to feed and grow prior to returning as adults to reproduce in rivers (Hansen and Quinn 1998; Klemetsen et al. 2003). This species is genetically structured into reproductively isolated populations of European and North American origin that diverged over 600,000 years ago (King et al. 2007). Today, more than 2000 genetically distinct salmon populations are distributed in water courses flowing towards the North Atlantic Ocean (Verspoor et al. 2007).
Despite marked improvements in freshwater habitats and substantial reductions in fisheries exploitation (Friedland et al. 2003; Jonsson and Jonsson 2004; ICES 2020a), salmon have suffered synchronous population declines around the North Atlantic over the last five decades (Parrish et al. 1998; Limburg et al. 2009; Lehnert et al. 2019; Olmos et al. 2019). The temporal coherence observed in the population declines across large geographic areas implies common factors operating simultaneously on the freshwater and marine phases of the life cycle (Parrish et al. 1998; Potter and Crozier 2000; Friedland et al. 2009). Salmon suffer the highest numbers of mortalities in fresh water (Smialek et al. 2021), where the biggest threats to population persistence are climate change, modified river discharge, overexploitation, pollution, land-use change, and invasive non-native species (Dudgeon 2019). Freshwater conditions affect the physiological development of juvenile salmon, subsequently influencing the growth and survival of older life stages in the marine environment (Russell et al. 2012a). However, increased marine mortality has been a key driver of the observed population declines in recent decades (Chaput 2012; Olmos et al. 2019; Thorstad et al. 2021). Marine return rates, commonly defined as the proportion of emigrating juvenile salmon (smolts) that survive to return to rivers as adults, have fallen to record lows since the 1980s (Chaput 2012), with typically < 5% of smolts now surviving (ICES 2020b).
Juvenile salmon are generally growing faster and migrating to sea at younger ages and smaller sizes (Jonsson and Jonsson 2004; Russell et al. 2012a), and smaller fish can have a lower probability of marine survival (Gregory et al. 2019). Differences in juvenile growth can arise from varying environmental conditions and maternal effects in fresh water (Einum and Flemming 2000; Kennedy et al. 2008; Burton et al. 2013), subsequently influencing the age and size of smolts migrating to sea (Russell et al. 2012a). Most mortality between smolt and adult stages is generally considered to take place during the first year of life at sea when survival, maturation, and migration trajectories are being defined (Hansen and Quinn 1998; Potter and Crozier 2000; Friedland et al. 2009). Fluctuations in the sea age composition of adult salmon have been reported for more than 100 years, with periods where stocks were dominated by fish maturing after one-sea-winter or multi-sea-winters (Bielak and Power 1986; Summers 1995; Heddell-Cowie 2005). In the North East Atlantic, the annual proportion of one-sea-winter salmon increased from the 1980s to the late-1990s and then decreased after 2000 (ICES 2020b). In contrast, the annual proportion of one-sea-winter salmon in the North West Atlantic has increased steadily since the 1970s, but the underlying mechanisms remain unclear. Adult salmon are now tending to return to rivers (hereafter adult returns) in the North East Atlantic at older ages and in poorer condition due to reductions in marine feeding opportunities limiting the growth and maturation potential of the fish (Bacon et al. 2009; Jonsson et al. 2016; Bal et al. 2017).
Reduced marine survival is widely accepted to be an important contributary factor to the observed salmon population declines in recent decades (Chaput 2012; Olmos et al. 2019; Thorstad et al. 2021). Identifying which biotic and abiotic stressors have the biggest impacts on marine survival has therefore been subject to a substantial research effort over the last 30 years (Friedland et al. 1993; Cairns 2001; Jonsson and Jonsson 2004). Multiple stressors have been implicated, including, inter alia, climate change, predation, pollution, broad-scale oceanographic changes, overexploitation, and salmon mariculture (Parrish et al. 1998; Cairns 2001; Forseth et al. 2017). However, incomplete knowledge of the nature, severity, and extent of the different stressors impacting marine survival has hindered salmon conservation efforts (Jonsson and Jonsson 2004). To remedy this situation, several multidisciplinary efforts have been made to explore marine survival issues, identify knowledge gaps, and prioritise research directions (Mills 2000; Crozier et al. 2018; ICES 2020a). Understanding the relative importance of different stressors impacting marine survival has never been more important to prioritise research and management initiatives to protect and restore salmon stocks.
Many of the freshwater stressors impacting salmon are known, and management actions are available to mitigate them (Thorstad et al. 2021). In contrast, the marine stressors impacting salmon remain poorly understood, and management actions are being sought to lessen their impacts and improve the marine survival of salmon (Crozier et al. 2018). Accordingly, the present review had two objectives: 1) present a synopsis of the principal marine stressors impacting salmon survival between the time juveniles leave fresh water and return to rivers as adults prior to reproducing; and (2) apply a semi-quantitative classification system based on the approach devised by Forseth et al. (2017) to assess the major threats to English salmon stocks in the marine environment and prioritise management actions.
Methods
An extensive literature review on the marine stressors impacting salmon survival was undertaken by scrutinising peer-reviewed articles and grey literature. A marine stressor was defined as an activity that impacts salmon in estuaries, nearshore waters, and/or the open ocean; these stressors were primarily human generated.
Study areas
The study areas included all 42 ‘principal salmon rivers’ in England, which were selected to assess the effects of different marine stressors on salmon and their likely development over the next decade (Fig. 1). Each river was grouped into one of four ‘regional marine plan areas’ designated by the UK’s Marine Management Organisation (MMO). These regional marine plan areas were the North East, South, South West, and North West.
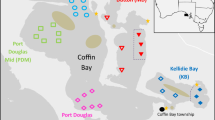
A map showing the location of 42 rivers (Environment Agency 2020) and neighbouring regional marine plan areas (MMO 2017) selected to assess the impacts of marine stressors on Atlantic salmon that originate from England (inset map; Ordnance Survey 2021a, b). Rivers include: 1 = Coquet; 2 = Tyne; 3 = Wear; 4 = Tees; 5 = Yorkshire Esk (NE: North East); 6 = Itchen; 7 = Test; 8 = Hampshire Avon; 9 = Stour; 10 = Piddle; 11 = Frome; 12 = Axe; 13 = Exe; 14 = Teign; 15 = Dart (S: South); 16 = Devon Avon; 17 = Erme; 18 = Yealm; 19 = Plym; 20 = Tavy; 21 = Tamar; 22 = Lynher; 23 = Fowey; 24 = Camel; 25 = Torridge; 26 = Taw; 27 = Lyn; 28 = Severn (SW: South West); 29 = Ribble; 30 = Wyre; 31 = Lune; 32 = Kent; 33 = Leven; 34 = Crake; 35 = Duddon; 36 = Cumbria Esk; 37 = Irt; 38 = Calder; 39 = Ehen; 40 = Derwent; 41 = Eden; and 42 = Border Esk (NW: North West). All the rivers were designated ‘principal salmon rivers’ by the Environment Agency on the basis of the prospect of annual rod catches of at least 50 fish around the time of the development of Salmon Action Plans (SAPs) for stock management (Cefas et al. 2019b)
The classification system
A semi-quantitative 2-D classification system based on the approach developed by Forseth et al. (2017) for Norwegian rivers was applied to determine the relative impact of marine stressors on salmon originating from English rivers at present and projected over the next decade. Thus, the first dimension, the effects axis, describes the assessed effect of each stressor on salmon, and the second dimension, the development axis, represents the likelihood of development over the next decade. Combined, the effects and development axes form a 2-D classification system that can be used to categorise stressors into four major impact groups (Fig. 2a):
-
(1)
Expanding high impact stressors included factors affecting salmon to a relatively high extent at present and with the potential to become more prevalent over the next decade. Mitigation measures implemented are unable to limit expansion of negative effects in the future.
-
(2)
Stabilised high impact stressors were factors that negatively affect salmon at present but are expected to be less detrimental over the next decade. Mitigation measures implemented are expected to limit expansion of negative impacts in the future.
-
(3)
Expanding low impact stressors represented factors that have low effects on salmon at present but are likely to expand over the next decade. Mitigation measures implemented are unable to limit expansion of negative impacts in the future.
-
(4)
Stabilised low impact stressors were factors with low impacts on salmon at present and over the next decade. Mitigation measures implemented are expected to limit expansion of negative impacts in the future.
a The classification system used to rank different marine stressors that impact English Atlantic salmon along the effects and development axes. The four major stressor categories are indicated, but the system is continuous. Background colouring indicates the severity of the impacts, with dark as the most severe. Modified from Forseth et al. (2017). b Location within the classification system of the 13 marine stressors based on mean scores across the four regional marine plan areas in 2018. For illustration, knowledge of each stressor and the uncertainty of future development are indicated by the marker’s colour. Green squares = extensive knowledge and small uncertainty (sum of scores 2–3), yellow circles = moderate knowledge and moderate uncertainty (sum of scores 4), and red triangles = poor knowledge and high uncertainty (sum of scores 5–6)
Five major modifications were made to the original classification system devised by Forseth et al. (2017) to ensure effective application to English salmon stocks given that different marine stressors with varying severity and extent of impact were expected. First, the classification system in the present assessment focused solely on marine stressors rather than those operating across aquatic systems. Second, evidence from the literature review and expert local knowledge were combined to assess the effects and future development of the stressors instead of relying only on expert judgement. Third, the habitat zone where each stressor occurs was evaluated. Fourth, separate scores were provided for salmon in the early stages of their marine phase (smolt/post-smolt) when the fish were relatively small and migrating through estuarine and nearshore waters and for adults in the oceanic phase of the life cycle and on their return migration, as opposed to scoring the percentage reduction in adult returns and the number of lost or critically endangered populations. Lastly, the nomenclature for the four major impact groups was revised.
Stressors considered
To identify stressors with the potential to impact salmon during their marine phase, the literature review was complemented by consultations with England’s Environment Agency fisheries officers responsible for the management of river stocks. The information was compiled into the semi-quantitative classification system (Table 1), which identified thirteen stressors: climate change, predation, water quality, bycatch, artificial light at night, tidal barrages, power station impingement and thermal discharge, noise pollution from pile-driving, invasive non-native species, electromagnetic fields, fisheries exploitation, salmon mariculture, and tidal lagoons. Although this review focuses on the impacts of these stressors on salmon in estuarine and marine environments, freshwater literature was drawn upon where necessary to illustrate important issues. National experts were consulted to check the accuracy and comprehensiveness of the literature review and the assessment of individual stressors.
Scoring approach
Different metrics were used to describe each stressor along the effects and development axes. The scores allocated to each metric were based on a range of semi-quantitative or qualitative criteria, each operating on a four-point scale, in accordance with Forseth et al. (2017). Unlike Forseth et al. (2017), however, impact and development metrics were scored from zero (no impact or development) to three (high impact or development potential) because not all the stressors considered in the present assessment were deemed to have impact or development potential. Habitat zone and mitigation metrics were scored on a scale of one to four because the origin of a stressor impact could not be zero and the application of extensive measures does not guarantee the complete alleviation of a stressor impact. Assuming a stressor was considered appropriate for inclusion in an assessment (i.e., it was considered to apply in a particular region), then all scores were summed and expressed as a proportion of the potential maximum score. If a stressor was absent from a region, then the effects and development scores were zero.
Several metrics adapted from Forseth et al. (2017) were considered for each stressor on the effects axis. First, the percentage of affected river stocks measured the severity of the stressor impact, which is analogous to the number of affected populations considered by Forseth et al. (2017). Assessments of the percentage of affected river stocks were based on local knowledge compiled following consultation with the respective Environment Agency fisheries officer responsible for the management of each river stock. The criteria ranged from zero to > 50% of river stocks affected to account for instances where a stressor effect was absent to prevalent amongst river stocks. Second, unlike Forseth et al. (2017), the habitat zone where the stressor effect occurs was assessed using information from the literature review combined with local knowledge. Effects were assumed to diminish with increasing distance offshore for most of the stressors because encounter probability was generally considered lower in offshore waters than in shallower estuarine or nearshore waters. Climate change was the exception to this assumption given that this stressor could not be assigned to a specific habitat zone due to its broad spatial extent. The four habitat zones were defined as offshore, nearshore, estuarine, and all marine habitats. Third, the spatial extent of each stressor was assessed by evaluating the geographic distribution of the stressor from published articles and online sources (Fig. S1–S9), matching the approach taken by Forseth et al. (2017). If geographic distribution maps were unavailable for a particular stressor, then alternative data sources (e.g., documented occurrence) were obtained to provide an indication of the spatial extent. Each stressor was classed as either point source or diffuse, with its spatial extent ranging from absent to small, moderate, or large. Finally, the existence, scope, and utility of mitigation measures were evaluated to determine the capacity to alleviate stressor effects on salmon, in line with Forseth et al. (2017). Assessments were based on documented effects in published articles, reports, and online sources. Mitigation scores ranged from extensive measures with large beneficial effects to very few or no measures with apparent effects.
Three metrics modified from Forseth et al. (2017) were considered for each stressor on the development axis. First, the main area for likely development was assessed based on evidence from the literature review combined with local knowledge, and ranged from offshore to nearshore, estuarine, or all marine habitats. In line with the habitat zone assessment, the likely development of all the stressors considered, except climate change, were assumed to diminish with increasing distance offshore. Second, the likelihood of each stressor becoming more prevalent over the next decade was based on a combination of evidence from the literature review, supporting information from UK government documents and planning websites, and local knowledge. The likelihood of increasing stressor prevalence over the next decade was ranked from no change to a high probability of increase. This differed from the approach taken by Forseth et al. (2017), where the likelihoods of future losses in adult returns and additional populations becoming critically endangered or lost were considered, because this assessment focused on stressor rather than impact development. Third, the potential for effective mitigation was based on a projection of the present situation and the scope for implementing measures to alleviate stressor impacts, which matched the Forseth et al. (2017) approach. This was primarily derived from expert judgement of public information, including UK government white papers, regulations, and guidelines from relevant management bodies. Classification of mitigation measures ranged from the availability and likely development of extensive and very effective measures to very few or no effective measures.
In line with Forseth et al. (2017), each stressor was also classified based on the extent of the knowledge informing the assessment of its effect and the uncertainty related to its projected development. In relation to the former, the extent of knowledge for the different stressors was classified as extensive, moderate, or poor. This classification was based on the evidence from published sources and local information from Environment Agency fisheries officers. The uncertainty of the projected development was classified as small, moderate, or large using published evidence and expert opinion. These knowledge and uncertainty scores were subsequently combined to categorise each stressor assessment as having extensive knowledge and small uncertainty, moderate knowledge and moderate uncertainty, or poor knowledge and high uncertainty.
Initial scoring was undertaken by the expert who completed the evidence review for each stressor, taking account of the separate regional information in each case. The scores were then extensively discussed and reviewed by all the experts before reaching consensus. Final scores were averaged across all four regional marine plan areas in England to provide an overall stressor ranking at the national level.
Results
The results are presented in order of the highest to lowest overall stressor ranking, giving an indication of their relative importance for English salmon stocks.
Climate change
Anthropogenic greenhouse gas emissions have increased the global average surface temperature by about 0.85 °C over the twentieth century (IPCC 2014), with many regions of the world already experiencing warming in excess of 1.5 °C in at least one season (IPCC 2018). Warmer temperatures in the North Atlantic have modified oceanic conditions, reducing the growth and survival of salmon by decreasing marine feeding opportunities (Peyronnet et al. 2007; Todd et al. 2008; Friedland et al. 2009). Salmon forage in marine feeding areas that experienced climate-driven regime shifts in biophysical conditions during the late-1980s (Friedland et al. 2012; Mills et al. 2013; Almodóvar et al. 2019). Several studies have postulated that a climate-driven shift in zooplankton community composition towards more temperature tolerant species might be associated with reductions in the marine growth and survival of salmon due to decreases in food abundance and nutritional content (Beaugrand and Reid 2003; Todd et al. 2008; Jonsson et al. 2016). The marine survival of post-smolts is strongly influenced by fluctuations in sea surface temperature and primary production in the Labrador Sea/Grand Banks regions for North American populations and the Norwegian Sea for southern European populations (Olmos et al. 2020). Increased ocean acidification due to higher levels of CO2 caused by climate change may have diminished the capacity of salmonids to detect olfactory cues used to find prey, avoid predators, and locate natal rivers during homing migrations (Ou et al. 2015; Williams et al. 2019). Little doubt therefore remains that broad-scale, climate-induced changes in oceanic conditions have contributed significantly to recent salmon population declines (Beaugrand and Reid 2012; Friedland et al. 2014; Nicola et al. 2018).
The relative proportion of one-sea-winter to multi-sea-winter adult returns has fluctuated over time (Bielak and Power 1986; Summers 1995; Heddell-Cowie 2005). Since the 2000s, the trend in the North East Atlantic has generally been towards an increasing proportion of older, later maturing individuals (ICES 2019). Salmon are believed to be delaying sexual maturity in response to reduced marine feeding opportunities, thereby limiting the growth and maturation potential of the fish (Bacon et al. 2009; Otero et al. 2012; Jonsson et al. 2016; Bal et al. 2017). Age-at-maturity in salmon is a classic evolutionary trade-off to optimise lifetime fitness (Flemming 1996). Larger, later-maturing individuals, which spend more time at sea, have higher fecundity but also run a greater risk of mortality before first reproduction (Fleming and Einum 2011). In contrast, smaller, earlier-maturing individuals, which spend less time at sea, have lower fecundity but a higher probability of surviving to reproduce. Declines in growth during the first summer at sea have been linked to changes in the sex composition of adult returns (Tréhin et al. 2021), because females may need to spend more years at sea than males to achieve the minimum body size required to attain sexual maturity (Barson et al. 2015; Mobley et al. 2020) and therefore might be subject to higher mortality rates.
Increased temperatures resulting from climate change have modified salmon migration timing. Smolts are understood to synchronise the timing of their seaward migration to coincide with the arrival of optimal thermal conditions at sea to maximise survival (McCormick et al. 1998; Hvidsten et al. 1998, 2009). Otero et al. (2014) found that, on average, smolts are initiating their seaward migration 2.5 days earlier per decade in response to increased temperatures in fresh water. Earlier seaward migration can result in a mismatch with optimal conditions for post-smolt growth and survival at sea (Kennedy and Crozier 2010; Russell et al. 2012a; Simmons et al. 2021). Climate change is also believed to affect the timing of adult returns, although the mechanisms remain unclear. Adult salmon are generally returning to rivers earlier in the North West Atlantic (Huntington et al. 2003; Juanes et al. 2004; Dempson et al. 2017) and later in the North East Atlantic (Solomon and Sambrook 2004; Valiente et al. 2011; Bal et al. 2017), with potentially detrimental effects on reproductive success (Jonsson and Jonsson 2009).
Knowledge about climate change impacts on the marine migration routes of salmon is limited. Salmon migrate northwards towards marine feeding areas that are geographically segregated among populations (Mackenzie et al. 2012; Soto et al. 2018; Gilbey et al. 2021). Local movements in estuaries and nearshore waters are influenced by tidal currents (Holm et al. 2003; Lacroix and Knox 2005; Lacroix et al. 2005), while movements in offshore waters are governed by surface currents (Dadswell et al. 2010; Mork et al. 2012; Strøm et al. 2018). Interannual variation in climatic conditions shapes marine migration routes by changing surface currents, surface temperatures, and salinities that guide salmon towards feeding areas with different environmental conditions and prey availability (Friedland et al. 2012; Mork et al. 2012; Byron et al. 2014). Over the next century, climate models predict a global weakening of surface currents, reducing nutrient availability, and primary production, with negative effects on fish stocks in the North Atlantic (Gröger et al. 2013). Climate change could modify the spatial positioning of migration corridors leading to drastic changes in salmon growth and survival at sea (ICES 2017a).
Geographic overlap in the spatial distribution of pelagic fish interacting with salmon as competitors, predators, or prey has changed due to climate-driven changes in oceanic conditions (ICES 2017b). For example, mackerel Scomber scombrus and herring Clupea harengus have expanded their spatial distribution northwards in response to warmer temperatures over the past three decades (Rose 2005; Hughes et al. 2014), potentially increasing their interactions with salmon (Haugland et al. 2006; Utne et al. 2020). Indeed, it has been hypothesised that the change in mackerel distribution might be a contributory factor to the declines in salmon growth and survival at sea due to greater competition for food resources and predation on post-smolts (Holst 2018). However, there is currently a lack of clear evidence to support such effects (Utne et al. 2020). The risk of incidental capture by pelagic fisheries that have now moved into salmon migration paths to target these species has also increased (ICES 2014), but the population-level effects are assessed to be small at present (ICES 2017b).
Climate change is not expected to result in the extinction of salmon across the North Atlantic because projected future temperatures are unlikely to exceed their thermal optimum in all areas (Jonsson and Jonsson 2009; ICES 2017a). However, the distribution of salmon is projected to contract by the end of the twenty-first century due to climate change reducing the availability of suitable thermal habitat (Lassalle and Rochard 2009). Suitable thermal habitat for salmon is expected to extend northwards with the invasion of new spawning, nursery, and feeding areas north of the species’ present distributional range but with the extirpation of southern populations (Todd et al. 2011; Hedger et al. 2013; Hastings et al. 2020). Salmon are already responding to warmer temperatures by expanding their range northwards into the Arctic Ocean (Jensen et al. 2014; Bilous and Dunmall 2020), and disappearing from the southern edge of their distribution area (Parrish et al. 1998; Jonsson and Jonsson 2009).
Exactly how salmon will respond to future climate change is highly uncertain because oceanic conditions in the North Atlantic are now beyond a historical context (ICES 2017a). Furthermore, it is likely that climate change will interact with other stressors (e.g., predation, water quality, and invasive non-native species) to have synergistic negative impacts on salmon (Graham and Harrod 2009).
Predation
Salmon are vulnerable to predatory mammals, birds, and fish (Ward and Hvidsten 2011). Direct predation effects are usually less obvious during the early freshwater life stages, when most losses of salmon occur, and density-dependent processes are more likely to regulate recruitment and compensate for impacts at the population-level (Jonsson et al. 1998; Milner et al. 2003). However, the potential for such compensatory processes typically weakens through the early life stages, and older life stages (smolts, post-smolts, and adults) are not thought to be regulated by density-dependent processes nor subject to compensation (Ward and Hvidsten 2011). Losses of smolts and post-smolts are thus expected to have a direct proportional impact on adult returns (Thorstad et al. 2012a).
Predation on smolts during the first months at sea is a major source of natural mortality impacting salmon abundance (Hansen et al. 2003). Smolts leaving fresh water and entering the sea are particularly vulnerable to predation due to their relatively small body size and naivety with regards to predator avoidance (Friedland et al. 2012; Flávio et al. 2020). For example, on average 49% (23–79%) of smolts are estimated to be lost to predation each year in the lower reaches of rivers and estuaries in Denmark (Jepsen et al. 2019). Furthermore, 59% of smolts have been estimated to suffer predation-related mortality in the Miramichi River estuary and Bay in Canada (Daniels et al. 2019). The extent and location of losses of post-smolts and pre-spawning adults to predators is largely unknown beyond estuaries. Recent developments in archival tag technology have shed some light on the mortality of post-spawning adult salmon returning to the sea (known as kelts). Strøm et al. (2019) reported 22 predation events (14%) and 38 undetermined mortalities (24%) from 156 tagged kelts released from 12 rivers around the North Atlantic, with 59% of the predation events attributed to endothermic fishes.
Marine mammals including seals and cetaceans have been reported aggregating in river mouths, estuaries, and nearshore waters to prey on emigrating smolts and returning adult salmon (Thorstad et al. 2012a; Civil et al. 2019). Adult salmon are most at risk of predation by marine mammals when returning from sea to reproduce (Butler et al. 2008). Smaller salmon stocks and population units, such as early-running spring salmon, are particularly vulnerable to seal predation (Butler et al. 2006). Seals are perceived to have contributed to some local salmon population declines, leading to calls from fisheries stakeholders for seal populations to be controlled in the vicinity of salmon fisheries (Butler et al. 2006, 2008, 2011; Graham et al. 2009). However, seal predation on salmon may be limited to a relatively small proportion of individuals that specialise in targeting salmonids (Hammill and Stenson 2000; Bowen et al. 2002; Graham et al. 2011), or fishing in particular areas around nets (Graham et al. 2011; Harris et al. 2014), and predation levels can be exacerbated when the fish aggregate below migration barriers (Bendall and Moore 2008).
Piscivorous birds are known to consume salmon in estuaries and nearshore waters (Dieperink et al. 2002; Harris et al. 2008). Cormorants Phalacrocorax spp. have been observed aggregating in estuaries during smolt runs, when they may represent a major mortality source (Jepsen et al. 2019; Flávio et al. 2020). For example, salmon smolts have been shown to comprise a large percentage of the diet of cormorants in Northern Ireland (66%) and Scotland (18%) (Kennedy and Greer 1988; Marquiss et al. 1998). Harris et al. (2008) reported that goosander Mergus merganser predation removes a moderate percentage of total annual smolt production on the rivers North Esk (3–16%) and Spey (3–5%) in Scotland. Other investigations in Scotland indicate latitudinal trends in the diet of goosanders and cormorants, with salmon comprising a larger percentage of the prey items in northern (41% and 18%, respectively) than southern (9% and < 1%, respectively) rivers (Marquiss et al. 1998), consistent with the relative abundance of different fish species. Avian predation is potentially exacerbated at migration barriers, where other bird species (e.g., herons and gulls) could also aggregate to prey on smolts. Gulls have been identified as a potentially important predator on Pacific salmonids around North American dams (Ruggerone et al. 1986; Collis et al. 2002), but this has been little studied in the context of Atlantic salmon (Jepsen et al. 2006).
Piscivorous fish predate on salmon in the marine environment (Friedland et al. 2012). However, this remains poorly understood because it is not readily observable. Salmon are known to be consumed by sharks and large pelagic fish (Lacroix 2014; Daniels et al. 2018; Strøm et al. 2019). Predation could occur in nearshore waters from a range of fish species, such as Atlantic cod Gadus morhua and saithe Pollachius virens, which have been identified as important predators of salmon smolts and post-smolts in some areas (Jepsen et al. 2006; Thorstad et al. 2012b; Friedland et al. 2017). Sea bass Dicentrarchus labrax is another notable predator of salmon smolts that has expanded its geographical distribution range in the North Sea since the 1980s (Colman et al. 2008), and thus it is conceivable that this might have increased predation pressure on salmon smolts. A study carried out in the tidal reaches of the River Frome in South England postulated that 5% of total annual smolt production could be lost to sea bass predation (Riley et al. 2011). The creation of sea bass nursery areas in estuaries (Anon, 1990) could therefore negatively affect smolt production from rivers, whilst affording some protection against bycatches of returning adult salmon by prohibiting the operation of bass fisheries. Similarly, it has been inferred that 1.9–17.5% of smolts are lost to striped bass Morone saxatilis predation in the Miramichi River estuary in Canada, thus it is plausible that the observed increase in the abundance of this predator might have contributed to salmon population declines over the last two decades (Daniels et al. 2018).
Predation is a natural process regulating salmon stock size in balance with predator levels. However, the evidence indicates that predation is a relatively major source of mortality for salmon (Hansen et al. 2003), which can have disproportionate negative impacts on depleted stocks (Ward and Hvidsten 2011).
Water quality
The water quality characteristics with the greatest potential to have sublethal and lethal effects on salmon in marine waters were broadly categorised into dissolved oxygen, suspended sediment, and contaminants. All three characteristics have been shown to be important regulatory factors, with the potential to interact synergistically to impact salmon, often exacerbated by other stressors such as increased temperature (Alabaster and Lloyd 1982; Cabral et al. 2019).
Dissolved oxygen governs the development, growth, and survival of salmon (Brett 1972). The behavioural responses of salmon to dissolved oxygen fluctuations include changes in movement and activity, increased use of surface respiration and air breathing, and altered habitat use (Kramer et al. 1987). Salmon consume more oxygen at higher temperatures due to an increased metabolic rate (Beamish 1964). Interactions between temperature and dissolved oxygen concentrations influence migration success by modifying the aerobic scope of salmon during muscular exercise (Alabaster and Gough 1986; Farrell 2009). High temperatures lower dissolved oxygen concentrations in estuaries delaying the upstream migration of adult salmon into rivers, thereby increasing estuarine residency time (Solomon and Sambrook 2004; Bendall et al. 2012). Adult salmon avoid entering estuaries when dissolved oxygen levels are below 5.5 mg L−1 (Priede et al. 1988). If dissolved oxygen falls below the lethal limit of 1.5 mg L−1, then salmon mortality due to asphyxiation can occur (Kazakov and Khalyapina 1981). For example, exposure to dissolved oxygen below the lethal limit was identified as a major contributory factor to salmon mortality in the River Tamar estuary in South West England between 1975 and 1995 (Solomon and Sambrook 2004). For smolts, the lethal dissolved oxygen concentration that produces 50% mortality over a 3-day period is 2.5 mg L−1 (Alabaster et al. 1979).
Fine sediment entering the marine environment from land drainage, erosion, and construction activities can become mobilised and suspended in the water column by turbulence (Winterwerp and Kranenburg 2002). Salmon exhibit behavioural and physiological responses to changes in suspended sediment (Newcombe and Jensen 1996; Robertson et al. 2007; Kemp et al. 2011). Most investigations into the effects of suspended sediment have focussed on freshwater life stages, during which sediment size and loading rates influence spawning habitat availability, egg and embryo survival, fry emergence timing, juvenile growth, and smoltification age (MacCrimmon and Gots 1986; Lisle and Lewis 1992; Armstrong et al. 2003; Suttle et al. 2004). In contrast, limited information exists on the impacts of suspended sediment in estuarine or nearshore waters, where salmon would most likely encounter high suspended sediment levels during the smolt migration or as returning adults. Possible impacts could range from a temporary behavioural avoidance reaction to more serious physiological effects, potentially resulting in impaired migration and, in extreme conditions, mortality. However, salmon successfully migrate through estuaries that have naturally high suspended sediment levels to enter rivers, up to several thousand mg L−1 in the case of the Severn Estuary in South West England (Gibson 1933), and increased turbidity can improve salmon survival by lowering predation rates (Gregory and Levings 1998). Extrapolating to population-level impacts is difficult because limited attention has been given to the long-term effects of increased suspended sediment (Kjelland et al. 2015).
Water-borne contaminants, including pesticides, heavy metals, and synthetic hydrocarbons, can adversely affect salmon (Giattina and Garton 1983). Salmonids can bioaccumulate contaminants from their prey (Burreau et al. 2006). For example, juvenile chinook salmon Oncorhynchus tshawytscha accumulate synthetic hydrocarbons from their prey along estuarine migration routes (Johnson et al. 2007). In the open ocean, Atlantic salmon are potentially at similar risk of accumulating contaminants. For instance, the historically polluted River Tees in North East England is a source for polybrominated diphenylethers in the North Sea food web, with biomagnification occurring from invertebrates through fish to seals (Boon et al. 2002). Adult returns to the River Tees accumulate polybrominated diphenylethers, polychlorinated biphenyls, and hexachlorobenzene over an unknown portion of their lifetime (Assunção et al. 2020). Multiple contamination events can cumulatively affect salmon, particularly in smolts prior to seawater entry (Russell et al. 2012a). Smolts exposed to atrazine pesticides in fresh water, and then exposed to oestrogenic chemicals in estuaries, exhibit reduced growth and survival during marine residency (Moore et al. 2003; Waring and Moore 2004). Physiological effects of sublethal contaminant exposure on smolts include reduced salinity tolerance when entering marine waters (Kroglund and Finstad 2003; Moore et al. 2008), with the effects of some contaminants such as aluminium increasing with higher water acidity (Kroglund et al. 2007; McCormick et al. 2012; Thorstad et al. 2013). Adult returns may also be impacted by sublethal contaminant levels modifying olfactory cues required for successful homing to spawning grounds (Saunders and Sprague 1967).
Over the last 50 years, improvements in water quality have been an important contributory factor to the recovery of many salmon stocks (Kroglund et al. 2001; Doughty and Gardiner 2003; Mawle and Milner 2003; Saltveit et al. 2014). A striking example of improved water quality aiding stock recovery has been provided by the River Tyne in North East England (Mawle and Milner 2003). After being almost lost in the twentieth century, the Tyne salmon stock has undergone a remarkable recovery following de-industrialisation and better sewage treatment, which ameliorated low dissolved oxygen and high ammonia concentrations resulting in improved estuarine water quality (Milner et al. 2008). Improvements in estuarine water quality have also led to the recovery of other salmon stocks in England, such as those on the rivers Wear, Mersey, and Tamar (Nuttall and Purves 1974; Mawle and Milner 2003; Jones 2006). Nonetheless, just 50 out of 166 (≈30%) estuaries and nearshore waters in England had acceptable water quality in 2018 according to the standards set out in the EU’s Water Framework Directive (Defra 2020), with sewage effluent, agricultural run-off, and industrial waste identified as key pollution sources. Indeed, good chemical status in 2018 was only achieved in 2441 out of 6537 (≈37%) European estuaries and nearshore waters, where the main pressures included atmospheric deposition of contaminants and discharges from urban wastewater treatment plants (EEA 2018). Restoring and maintaining water quality has and will continue to be challenging because many pollutants remain dormant in benthic substrate and aquifers for extended periods of time before being mobilised into aquatic systems. Further remediation work is therefore necessary to ensure that the quality of estuarine and nearshore waters is suitable to sustain salmon populations.
Poor water quality is a concern for salmon in riverine, estuarine, and nearshore habitats (Solomon and Sambrook 2004; Thorstad et al. 2008). Low dissolved oxygen concentrations can have severe impacts on salmon over relatively large spatial and temporal scales in rivers, estuaries, and nearshore waters. In contrast, high suspended sediment levels typically have localised, short duration, and low severity impacts on salmon. Contaminant exposure is an important perturbation due to the widespread occurrence and persistence of chemical compounds along migration routes.
Bycatch
Salmon are at risk of incidental capture by marine fisheries targeting other species. Bycatches of salmon are taken in marine fisheries targeting pelagic species in estuarine and nearshore waters, which can result in the mortality of injured fish (ICES 2005). For example, marine fisheries exploiting mullet (Mugilidae) and sea bass are known to take bycatches of salmon in England’s nearshore waters (Sumner 2015). Emigrating smolts are less at risk of bycatch than returning adult salmon because their small size, surface-swimming behaviour, and peak spring emigration render them less likely to be intercepted by most fishing gears operating in nearshore waters. In contrast, adult salmon reach larger sizes and return to rivers over a large part of the year (Thorstad et al. 2008), which makes them more vulnerable to being taken as bycatch in a wider range of marine fisheries than smolts. The greatest risk comes from marine fisheries targeting pelagic species of similar size.
High seas pelagic fisheries in the North Atlantic, particularly those targeting mackerel and herring, are known to take bycatches of salmon (ICES 2014). Annual bycatch estimates for salmon from European countries range from 300 to 800 fish in Icelandic mackerel fisheries, representing a very small percentage (0.01–0.03%) of the estimated total salmon abundance in the North East Atlantic. Other fishery-independent bycatch estimates from summer surveys of the Nordic Seas between 2010 and 2013 indicated that less than 2% of the total salmon abundance in the North East Atlantic was taken as bycatch. ICES (2014) recognised that catches in North Atlantic pelagic fisheries, potentially overlapping geographically with the movements of salmon, had increased in recent years. For example, concerns were raised over the northward expansion of the blue whiting Micromesistius poutassou fishery in the North East Atlantic. However, much of the catch in this fishery is taken prior to smolts emigrating into the ocean and at a depth greater than generally occupied by salmon. Therefore, the blue whiting fishery is likely to have little impact on salmon stocks, but uncertainties remain (ICES 2017b).
The severity and extent of salmon bycatch by marine fisheries is of concern but remains difficult to quantify accurately. Losses of adult returns to bycatch will have a greater impact on stock sustainability than losses of emigrating smolts, given that returning adults are emigrated smolts that have survived to maturity.
Artificial light at night
Emissions of artificial light at night (ALAN) are estimated to be increasing globally by 6% per annum (Hölker et al. 2010), raising concerns about potential environmental impacts. More than 20% of the world’s nearshore regions (excluding Antarctica) experience some form of artificial light pollution (Davies et al. 2014), and some of the largest urban conurbations are located adjacent to the tidal reaches, estuaries, and ports of rivers that support salmon stocks.
Light perception by salmon depends not only on the intensity and wavelength of the light source, but also the light-transmitting qualities of the water and the depth at which the fish is located. Visual sensitivity in salmon alters during development (Cheng et al. 2006), with major changes occurring during smoltification (Beatty 1966; Folmar and Dickhoff 1981; Alexander et al. 1994), which render smolts and post-smolts sensitive to the blue-green end of the visible spectrum (Migaud et al. 2007; Vera et al. 2010). Experimental studies focusing on the effects of ALAN on salmon have demonstrated significant disruption to the diel migratory pattern of smolts leaving fresh water, as well as to fry dispersing from spawning redds (Riley et al. 2012, 2013, 2015). As these patterns are believed to have evolved primarily as anti-predation measures, any disruption caused by ALAN is speculated to decrease recruitment success to the adult population.
Little research has been undertaken into the effects of ALAN on salmon in estuaries and nearshore waters. In recent years, interest on the effects of ALAN on marine life has increased (Davies et al. 2014; Alter et al. 2021). Behavioural effects have been observed in marine fish, including increased activity levels and a cessation of normal circadian and circatidal activity patterns in intertidal rockfish Girella laevifrons (Pulgar et al. 2019), as well as changes in swimming behaviour and susceptibility to nocturnal predation resulting in increased overall mortality in coral reef fish larvae (O’Connor et al. 2019). Lighting from ships has been shown to modify fish behaviour to depths of at least 200 m (Berge et al. 2020). No studies of the effects of ALAN on wild salmon in the marine environment have been published, but artificial lighting at fish farms increases the swimming depth of salmon in sea cages (Juell et al. 2003; Hansen et al. 2017).
Given the difficulty of extrapolating experimental evidence from aquaria or streams, the potential effects of ALAN on wild smolts and adults migrating through estuaries or marine waters are unclear. This potential stressor is widespread across many estuaries, and emissions of ALAN are likely to increase in the future. However, impacts may be minor in estuaries, where turbid water limits light penetration, and in areas where salmon can descend into deep water beyond the light penetration depth. Light emissions from oil and gas platforms may have a pronounced local impact on salmon behaviour at sea, particularly in the North Sea, which contains over 500 of such installations (OSPAR 2015), but the probability or likely severity of the impact is poorly understood. Salmon that have adapted to detect and respond to extremely low levels of natural light during the polar night might be susceptible to artificial light in Arctic seas (Berge et al. 2020).
Tidal barrages
Barrages have been constructed across rivers, bays, and estuaries for renewable energy generation, the provision of recreational facilities, freshwater storage, flood protection, and urban regeneration (Sitharam et al. 2020). Tidal barrages are quite rare, with relatively few examples in the world. Detrimental impacts have been attributed to their construction and operation, including but not limited to loss of intertidal habitats and associated biota due to modified flow patterns and sediment dynamics (Drinkwater and Frank 1994; Wolf et al. 2009; Frid et al. 2012; Hooper and Austen 2013; Kidd et al. 2015). For example, tidal barrages at La Rance in North West France and on the River Wansbeck in North East England decreased tidal ranges by 40% and 80%, respectively, causing changes to flow and sediment regimes, thus affecting the quality and quantity of suitable habitat available for wading birds and fish (Worrall and McIntyre 1998; Hooper and Austen 2013). Changes in fish species composition can occur following the construction of a tidal barrage, such as in the case of the Geum estuary barrage in South Korea, where freshwater species increased and diadromous species decreased upstream of the barrage (Yoon et al. 2017).
Salmon can be directly impacted by tidal barrages creating a physical barrier that disrupts migrations, and therefore these structures have the potential to cause local extirpation of stocks if fish passage is severely compromised (Drinkwater and Frank 1994; Gough 1996; Russell et al. 1998; Moore and Potter 2014). Barrier operations can indirectly impact salmon through modifications of the river’s natural discharge regime, thereby affecting migration cues and fish passage (Gillson 2011). Modified discharge regimes can impose additional energetic costs for migrating salmon in their attempt to negotiate the physical barrier, with potentially deleterious impacts at the population-level (Gough 1996; Russell et al. 1998). In addition, tidal barrages will alter hydrological conditions by reducing the tidal range upstream of the structure, resulting in a loss of suitable estuarine habitats for salmon (Frid et al. 2012; Hooper and Austen 2013; Kidd et al. 2015). Reduced tidal ranges can alter migratory cues crucial for initiating and orientating salmon during their upstream and downstream migrations. Aside from these issues, salmon can be susceptible to mechanical injuries due to turbine passage in power generating barrages, increased predation pressure and higher stress levels, with lesser effects posed by factors such as noise, habitat fragmentation, and water quality (Dadswell and Rulifson 1994; Dadswell et al. 2018).
The evidence indicates that tidal barrages can negatively impact salmon stocks. However, given that there are only few operating tidal barrages in the world, the severity and extent of their impact on salmon stocks are regarded as low at present but there is high potential for development over the next decade.
Power station impingement and thermal discharge
Power stations dispose of waste heat created in the electricity generation process typically using a water supply for cooling (Sarkar 2015). During this process, water is abstracted from a nearby water body to dissipate the produced heat, and the thermal effluent (usually 8–12.5 °C above ambient water temperature) is discharged into the same water body (Langford 2001) or into a heated-water effluent reservoir. Detrimental ecological impacts resulting from water intake and disposal include the trapping of organisms against screens that prevent debris entering water intakes in a process known as impingement (Turnpenny 1988), and the mechanical and thermal stresses imposed on organisms drawn through cooling systems in a phenomenon known as entrainment (Briand 1975; Bamber et al. 1994; Bamber and Seaby 2004). Thermal and chemically altered effluents can also have ecological impacts on receiving waters and adjacent biota (Bamber 1990; Langford 1990; Pawson and Eaton 1999). Increased temperatures from thermal discharge will rarely cause the mortality of fish species in isolation but can affect their performance as temperatures often surpass optimal conditions for growth and development (Coutant and Brook 1970; Wither et al. 2012). High chlorine levels in thermal discharges can also negatively affect fish, with increased avoidance behaviour or mortality detected in salmon at levels as low as 0.01 mg L−1 (Brungs 1973). The severity of the ecological effect depends on the location of the power plant (Turnpenny et al. 2010), with confined water bodies such as estuaries more prone to long-term effects than offshore waters (Wither et al. 2012). In addition, the position of the intake influences fish entrapment rates and the design of the outlet determines the velocity, quantity, and the temperature of the effluent released into the recipient water body (Turnpenny et al. 2010).
Salmon is a cold-water species that has among the lowest temperature threshold of any teleost fish in temperate regions (Jobling 1981), and therefore is expected to be highly sensitive to thermal effluents. Exposure to water temperatures above 22 °C can impede salmon migration (Alabaster and Gough 1986; Alabaster 1991; Alabaster et al. 1991). Delays in the upstream migration of adult salmon have been correlated with low river discharge, high temperatures, and reduced oxygen content (Solomon and Sambrook 2004), with oxygen content postulated as the main factor governing migration success (Rosten et al. 2010). Increased temperatures from thermal discharges can affect the reproductive success of salmon, particularly if exposure occurs in late autumn or early winter when ovulation and spermiation takes place in adults (Taranger and Hansen 1993; Vikingstad et al. 2008; Pankhurst and King 2010). Interactions between water temperature, river discharge, and oxygen content are complex, resulting in high levels of uncertainty about the potential impacts of thermal discharges on salmon migration. Limited information is available on the impacts of increased temperatures on the downstream migration of smolts, but it is expected to be severe if the transition from fresh to ‘heated’ salt water was abrupt.
Power station impingement and thermal discharge impacts on salmon will be location and time specific. Detrimental impacts are expected during seaward migration in areas where the power station outlet and/or thermal plume intersects with salmon migration routes.
Noise pollution from pile-driving
Underwater noise pollution is a growing concern, with detrimental effects on marine life noted from both continuous noise sources such as shipping and impulsive noise sources such as seismic surveys and pile-driving (Merchant et al. 2016; Faulkner et al. 2018). Despite this, there is a paucity of information on the effects of underwater noise on fish in the marine environment (Popper et al. 2020). Concern has been expressed about the ecological impacts of underwater pile-driving sounds on salmon (reviewed by Hawkins et al. 2015). Salmon have been classified as ‘hearing generalists’ with low sensitivity to sound pressure under a narrow range of frequencies and limited auditory response thresholds compared to other fish species (Hawkins and Johnstone 1978; Popper and Fay 1999; Hawkins and Popper 2014). Hearing in salmon is restricted to frequencies ranging from 32 to 380 Hz and the species lacks specialist auditory apparatus, indicating sensitivity for particle motion rather than sound pressure (Hawkins and Johnstone 1978).
Fishes generally avoid harmful pile-driving sounds to minimise adverse physiological responses and physical damage (Putland et al. 2019). Behavioural responses to pile-driving sounds include short-term directional movement away from the noise source and long-term avoidance reactions resulting in potential changes to migration patterns and access to feeding areas (Hawkins 2006; Gill et al. 2012; Hawkins et al. 2015). However, the behavioural responses of salmon to pile-driving sounds remain poorly understood. During the construction of a liquefied natural gas terminal in the Baltic Sea, pile-driving sounds above the lowest hearing threshold of salmon by 122 dB were postulated to have the potential to impede spawner passage and damage the hearing of fish at a distance less or equal to 40 m from the source (Bagočius 2015). Another study found that pile-driving sounds within the hearing range of juvenile pink Oncorhynchus gorbuscha and chum salmon O. keta, which were emitted over a radius of at least 600 m in the Snohomish River (Washington State, USA), modified the abundance and distribution of both species by producing avoidance reactions that reduced schooling behaviour (Feist et al. 1996). Nonetheless, salmon have also been found to lack behavioural responses to pile-driving sounds ranging from 160 to 194 dB (Nedwell et al. 2003; Harding et al. 2016). For example, a tagging study indicated that the behaviour of salmon migrating through the River Tyne estuary was similar irrespective of whether they were exposed to pile-driving sounds (Moore and Bendall 2011). However, individuals entering the river to spawn might have been delayed by pile-driving operations, potentially negatively affecting the salmon stock.
Despite recent advancements, information gaps are too substantial to draw firm conclusions about the impacts of underwater pile-driving sounds on salmon. Although salmon have been shown to exhibit behavioural and physiological responses to pile-driving sounds, impacts are likely to be restricted to periods when smolts and adults move through estuaries and nearshore waters where construction schemes are mostly situated.
Invasive non-native species
Introductions of invasive non-native species in the marine environment are increasing globally, with detrimental ecological consequences being reported (Molnar et al. 2008). Non-native species are classed as invasive if they establish, spread, and cause a significant change in species communities or ecosystem processes, or cause severe economic losses to human activities (Copp et al. 2005). Invasive non-native species can affect native taxa and ecosystems through alteration of trophic pathways (Mooney and Cleland 2001), habitat modification (Bax et al. 2003), competition and predation, and the spread of pathogens and parasites (Bax et al. 2003; Eno et al. 1997; Tidbury et al. 2014). However, little is known about the possible impacts of invasive non-native species on salmon in estuarine and marine environments.
Concerns have been expressed about the potential ecological impacts of invading pink salmon on indigenous Atlantic salmon stocks due to competition and predation (ICES 2018). Pink salmon is a Pacific salmonid species that had been deliberately introduced to Russian rivers discharging into the Barents Sea before spreading to other rivers in the North Atlantic, in particular those of Norway (Sandlund et al. 2019). Occasional pink salmon captures have been reported in rivers outside Russia since the early-1960s, but unprecedented numbers were caught in 2017 across a wide geographic area (ICES 2018). In Norway, record numbers of pink salmon were captured in 2017 (Mo et al. 2018; Sandlund et al. 2019), with reports of 6594 individuals caught in 262 rivers (Berntsen et al. 2020). Similarly, the reported numbers of pink salmon caught in the UK (341), Sweden (80), Iceland (66), and Ireland (36) in 2017 were unparalleled (Armstrong et al. 2018; ICES 2018; Millane et al. 2019). The pink salmon invasion of rivers in the North Atlantic is in its early stages and characterised by considerable inter-annual variation in the numbers of captured individuals mainly due to spawning runs occurring on a two-year cycle. Recent unpublished data on pink salmon captures in 2019 and 2021 indicate an emerging increasing trend in the species’ prevalence across the North East Atlantic (NINA 2021). Although competition with Atlantic salmon in fresh water might be limited by pink salmon’s use of different spawning habitats, an earlier spawning season, and short juvenile residency before seaward migration (Copp 2017), there is evidence of competition between adults and overwintered autumn-run salmon in Russian rivers (ICES 2013). With colonisation in its early stages, there is no evidence for marine competition, but this could occur if the abundance of pink salmon increases, and a European-funded study to address this knowledge gap will commence in 2021 (University of Gdańsk 2021). A recent study has found that pink salmon eggs and carcasses can increase the amount of marine-derived nutrients and energy available to juvenile Atlantic salmon in rivers, but little is known about the potential population-level effects at present (Dunlop et al. 2021).
Other invasive non-native species could potentially affect the marine life stages of salmon via changes to food supply, with these likely to operate in conjunction with other stressors such as climate change. One species of note is the invasive ctenophore Mnemiopsis leidyi, which has caused trophic cascades in plankton communities in the Mediterranean and Black Seas (Tiselius and Møller 2017). Hamer et al. (2011) warn of the possibility of this ctenophore affecting fish recruitment in the North Sea through zooplankton predation and competition with larval fish, and such effects could, if on a large enough scale, affect salmon.
Invasive non-native species in the marine environment are not presently considered a prominent stressor for salmon stocks. However, climate change could increase the likelihood of a wider range of non-native species impacting salmon stocks over the next decade.
Electromagnetic fields
Rapid development of ‘green’ energy from renewable energy installations and increases in subsea cable networks have increased the range of sources of artificial electromagnetic fields (EMF) in the marine environment (Gill et al. 2014). Interest in the potential effects of artificial EMF on salmonids has consequently grown in recent years (Formicki et al. 2019; Gill and Desender 2020). Salmonids are electromagnetically sensitive due to the presence of magnetic receptors, containing microscopic crystals of magnetite, which may facilitate behavioural and physiological responses to fluctuations in EMF (Walker et al. 1985; Moore et al. 1990). They use EMF to navigate during long-distance migrations to marine feeding areas and direction-find when returning to their natal rivers (Putman et al. 2013; Scanlan et al. 2018; Minkoff et al. 2020). Artificial EMF emitted from subsea cables could therefore have marked effects on salmonids that rely on the Earth’s natural geo-magnetic fields for spatial navigation and orientation at sea (Gill et al. 2012).
Exposure to EMF can generate behavioural and physiological responses in salmonids (Walker et al. 1997), but most of the available information is limited to Pacific salmonids (Yano et al. 1997; Klimley et al. 2017; Fey et al. 2019; Jakubowska et al. 2021). For example, Wyman et al. (2018) demonstrated that artificial EMF emanating from a high-voltage (200 kV) subsea cable in the field did not significantly alter the migration success of juvenile Chinook salmon through the San Francisco Bay in California, USA. After activation, however, higher proportions of juveniles crossed the cable location, and fish were more likely to be detected south of their normal migration route, suggesting that artificial EMF could influence migratory cues. The limited information available for Atlantic salmon shows a lack of detectable behavioural or physiological responses to artificial EMF under laboratory conditions (Armstrong et al. 2015), particularly at low frequencies (Richardson et al. 1976).
Salmon may encounter artificial EMF from marine renewable energy installations along their nearshore migration routes, where these overlap with subsea cable networks, particularly in shallow waters < 20 m deep (Gill et al. 2012). As both smolts and adults primarily use the surface layers of the water column, this preferred habitat, combined with diminishing EMF with distance from the cable, is expected to reduce the likelihood of interactions with artificial EMF from buried cables. However, the advent of floating offshore renewable energy installations will require consideration of interactions with migratory fish in surface waters (Hutchison et al. 2020). In the context of existing subsea cables, the risk of exposure to artificial EMF will be greatest when salmon undertake periodic deep dives to the seabed in estuaries and nearshore waters. Depending on dive frequency and the magnitude and persistence of any artificial EMF encountered, the effects on salmon could range from short-term behavioural responses (e.g. a change in swimming speed or direction) to a more serious, long-term response including disruption or delay to migration (Öhman et al. 2007).
Drawing firm conclusions on the impacts of artificial EMF from subsea cables on salmon is challenging due to the scarcity of available information on population-level effects (Boehlert and Gill 2010; Hutchison et al. 2020). However, the potential impacts are considered to be relatively low at present, with possible greater effects confined to the shallow waters of estuaries and nearshore areas, noting that this evaluation has large uncertainty and will require revisiting as more extensive cable networks and new renewable energy technologies increase in prevalence.
Fisheries exploitation
Concerns have been expressed over the population impacts of fisheries exploiting salmon in the North Atlantic (Parrish et al. 1998; Jonsson and Jonsson 2004). Mean annual exploitation rates have decreased for one-sea-winter and multi-sea-winter salmon retained in commercial and recreational fisheries from Europe and North America since the early-1970s (ICES 2019). However, these reductions in exploitation rates have not produced the expected gains in adult returns, indicating that factors other than fishing are responsible for increased levels of marine mortality (Klemetsen et al. 2003; ICES 2019). It has been speculated that illegal fishing at sea might account for the shortfall in adult returns (Dadswell et al. 2021), but evidence to support this hypothesis is lacking. For many years, surveillance flights provided no evidence of illegal fishing for salmon on the high seas (e.g. ICES 2013), and there have been no reports of such activity more recently.
Salmon have been exploited in home-water and distant-water fisheries (ICES 2019). Mean annual exploitation rates for one-sea-winter and multi-sea-winter salmon in home-water fisheries operating in most countries throughout Europe and North America have declined to the lowest levels over the last 50 years. This reflects the closure of many fisheries and increasingly restrictive measures to reduce levels of exploitation in most countries. For example, mean annual exploitation rates in home-water net and rod fisheries in England and Wales have decreased substantially for one-sea-winter (57–10%) and multi-sea-winter (42–5%) salmon since the early-1970s (Cefas et al. 2019a). Various fisheries regulations have been introduced to ensure that the exploitation of English salmon stocks in home-water fisheries is at low levels (Cefas et al. 2019b), with no exploitation by commercial fisheries permitted since 2019 (Cefas et al. 2020). Mixed-stock fisheries at sea in the Republic of Ireland closed in 2007. Furthermore, the exploitation of salmon stocks in distant-water, high-seas fisheries off West Greenland and the Faroes has decreased markedly since the late-1980s (ICES 2019). The West Greenland salmon fishery has been restricted to an internal-use subsistence fishery, with the commercial export of salmon prohibited since 1998 (NASCO 2014). No commercial salmon fishery has operated at the Faroes since 1991 and the research fishery was closed in 2001 (NASCO 2020).
Fisheries exploitation has the potential to impact the marine feeding, growth, and survival of salmon indirectly through bottom-up processes modifying the abundance of other fish species they interact with as competitors, predators, or prey, such as sandeels Ammodytes spp. and herring (Jacobsen and Hansen 2000; Haugland et al. 2006; Rikardsen and Dempson 2011). The North Sea herring stock has recovered from overfishing to reach the highest abundance levels since the 1970s (ICES 2020c). High abundances of herring within the feeding areas of salmon might act as a ‘double-edged sword’, being both an important prey as juveniles and a competitor with post-smolts for food resources as adults (Rikardsen and Dempson 2011). In contrast, the relationship between salmon and sandeels is considered a more straightforward predator–prey relationship, although the impact of fishing pressure on sandeel stock dynamics is less clear than with herring. Therefore, the degree to which fisheries exploitation may impact the growth and survival of salmon at sea by altering marine feeding conditions remains unclear.
Exploitation in home-water and distant-water fisheries is regarded as less of a threat to salmon stocks than in the past because effective controls have been implemented to substantially reduce fishing pressure in the North Atlantic.
Salmon mariculture
Farmed salmon production has increased rapidly since the early-1980s, with over 1.5 million tonnes of salmon farmed in the North Atlantic in 2018 (ICES 2019). Most farmed salmon are produced in Norway (81%) and Scotland (10%). In contrast, farmed salmon production is considerably smaller in the Faroes (4%), Canada (2%), Republic of Ireland (1%), Iceland (1%), Russia (1%), the USA and Northern Ireland (data unavailable, but production understood to be very small in both countries). No farmed salmon are produced in mariculture facilities elsewhere in the North Atlantic.
Salmon farming can impact the productivity of wild salmon populations by transferring diseases, but the most pronounced effects on wild salmon stocks are believed to result from sea lice Lepeophtheirus salmonis infestations and genetic introgression between escaped farmed fish and wild conspecifics (ICES 2016). Sea lice are an ectoparasitic crustacean that occurs naturally in low levels on wild salmon (Torrissen et al. 2013; Serra-Llinares et al. 2014). However, higher levels of sea lice on wild salmon have been observed near mariculture facilities (Serra-Llinares et al. 2014; Helland et al. 2015). Infestations of sea lice on salmon can increase their marine mortality (Ford and Myers 2008; Vollset et al. 2018), sensitivity to ocean warming (Shephard and Gargan 2020), and susceptibility to viruses (Barker et al. 2019). Mortality of post-smolts due to sea lice infestations can cause sizeable (0.6–39%) reductions in adult returns (Gargan et al. 2012; Krkošek et al. 2013; Skilbrei et al. 2013). Lice-induced mortality is higher in years of low marine survival, suggesting an additive effect of lice infestation with other stressors (Connors et al. 2012; Jackson et al. 2013; Godwin et al. 2015). Salmon mariculture has been identified as a major stressor for wild salmon stocks in Norway (Forseth et al. 2017).
Escapees from salmon farms pose a genetic threat to the fitness and viability of wild salmon stocks (McGinnity et al. 2003). The numbers of farmed salmon escaping mariculture facilities can be high, with 2.86 million escapes reported in Scotland between 1999 and 2014 (Ellis et al. 2016), and more than 26,000 escaped farmed salmon confirmed by scale reading in Norway between 1989 and 2013 (Diserud et al. 2019). Genetic introgression between wild and farmed salmon is known to occur extensively in Norwegian rivers (Karlsson et al. 2016) and has also been demonstrated elsewhere, such as in Canada (Wringe et al. 2018). In rivers where the density of wild spawning fish is low, the spawning success of escapees is likely to increase and therefore stressed populations are likely to suffer a greater level of introgression (Glover et al. 2012, 2013; Heino et al. 2015). Such introgression can lead to phenotypic changes in the wild population, reducing fitness due to loss of local adaptations (Fraser et al. 2010; Bolstad et al. 2017), and over the longer-term reducing population resilience to environmental perturbations (Lande and Shannon 1996; McGinnity et al. 2009; ICES 2016). Genetic introgression is unlikely to occur at a significant magnitude in areas away from mariculture facilities, such as English rivers where numbers of escaped fish are in general comparatively low (Milner and Evans 2003; Walker et al. 2006). It is possible that escaped farmed salmon from neighbouring facilities on the west coast of Scotland impact wild salmon stocks in North West England, but these impacts are likely to be small given that post-smolts are believed to move offshore rapidly to pick up the shelf edge current and migrate northwards towards marine feeding areas (Shelton et al. 1997; Holm et al. 2000, 2003).
The severity and extent of the impacts of salmon mariculture on wild salmon stocks are location specific depending on the size of the production facilities. Impacts on salmon stocks in Norway and Scotland are considered to be high because most of the world’s salmon mariculture facilities exist in these areas (Forseth et al. 2017; Tett et al. 2018). In contrast, salmon mariculture is likely to have low, if any, impacts on English salmon stocks at present given the lack of production facilities in the country. However, salmon mariculture is expected to expand in Scotland and Norway over the next decade (Norwegian Ministry of Trade, Industry and Fisheries 2015; Scotland Food and Drink 2016), and the potential risks for English salmon stocks remain under review.
Tidal lagoons
Growing interest in marine renewable energy installations has led to the proposed development of tidal lagoons that convert kinetic energy from tidal currents into electricity (Chowdhury et al. 2021). A high degree of uncertainty exists over the ecological impacts of tidal lagoons because few such developments exist globally (Roche et al. 2016), but concerns have been expressed over their potential effects on salmon (Dadswell and Rulifson 1994). The principal risks of tidal lagoons for salmon would be physical injury and/or mortality due to turbine passage rather than as an impediment to migration (Davies 1988; Wilson et al. 2006). Salmon from rivers in the vicinity of tidal lagoons may encounter these structures during the smolt migration and when adults return to rivers to spawn. Losses of salmon due to turbine entrainment depend upon the probability of individuals: (a) moving or being drawn into the vicinity of the turbine intake; (b) failing to take avoidance action; and (c) being killed or mortally wounded as they pass through. Aside from turbine-related mortality, tidal lagoons could impact salmon by modifying water quality in the surrounding region (Xia et al. 2010; Cornett et al. 2013) and attracting predators. Potential impacts may be location-specific depending on factors such as device design, volume of water impounded, tidal range, current speeds, turbine approach velocities, the proximity of rivers and key migration routes, and the status of local salmon stocks.
Little information exists on the ecological impacts of tidal lagoons on salmon. Many of the risks have been too poorly quantified to draw firm conclusions on the behavioural and physiological responses of salmon to tidal lagoons and any subsequent population-level effects. Given that few of these developments exist in the world, the present impacts on salmon stocks are considered to be low or non-existent. However, there is a growing interest in generating renewable energy from tidal lagoons, which might pose a threat to salmon over the next decade.
Overview of the ranking of marine stressor impacts on English salmon stocks
At the national level, climate change and predation were classified as expanding high impact stressors (Fig. 2; Table 1), with implemented or planned mitigation measures having small effects. Climate change ranked highest on the effects and development axes, representing the stressor with the greatest overall impact. However, the knowledge supporting the effects and future development of both these stressors was poor with high levels of uncertainty. Other stressors with relatively high effects scores included poor water quality and bycatch, although these all had lower development scores due to the availability of effective mitigation measures. In contrast, ALAN and tidal barrages were classified as expanding low impact stressors, attracting relatively low effects scores but representing a high likelihood of further development over the next decade given that planned mitigation measures are expected to have limited effects. Fisheries exploitation of salmon stocks and salmon mariculture represented stabilised low impact stressors with relatively low scores on both axes. The fisheries exploitation score reflected the major reductions in fishing mortality resulting from the implementation of extensive management measures, while salmon mariculture had a low score given its absence around the nearshore waters of England. Other factors with relatively low effects scores, but slightly higher development scores, included power station impingement and thermal discharge, noise pollution from pile-driving, invasive non-native species, and EMF. Tidal lagoons scored zero on the effects axis because no such structures exist in England, although there is a potential for future development.
Spatial variation in the proportion of votes by local fisheries officers for each stressor impacting salmon stocks was evident among the four marine plan areas in England (Fig. 3). Differences therefore existed in the regional stressor rankings (Fig. 4; Tables S1–S4). Climate change scored highest on the effects and development axes with the largest impact in all regions except the North West, where predation ranked highest on the effects axis. In the South and South West regions, climate change had markedly higher scores along both axes than any other stressor due to the absence of effective mitigation measures. Other stressors with relatively high effects scores included poor water quality and bycatch. Invasive non-native species and EMF had among the lowest effects scores across regions. Stressors with relatively low effects scores among regions included power station impingement and thermal discharge, ALAN, noise pollution from pile-driving, and fisheries exploitation. However, ALAN was the stressor considered to have the greatest potential to expand over the next decade, along with predation and climate change because planned mitigation measures are anticipated to have small effects. Salmon mariculture scored zero on the effects axis in all regions, except the North West region where there are concerns about the potential impact on English salmon stocks of fish emanating from Scottish salmon farms. Tidal barrages also scored zero on the effects axis in all regions, except the North East region due to the presence of a tidal barrage on the River Tees estuary, but there is potential for future development elsewhere.
Location within the classification system of the 13 marine stressors assessed for Atlantic salmon in the North East (a), South (b), South West (c), and North West (d) marine plan areas of England in 2018. For illustration, knowledge of each stressor and the uncertainty of future development are indicated by the marker’s colour. Green squares = extensive knowledge and small uncertainty (sum of scores 2–3), yellow circles = moderate knowledge and moderate uncertainty (sum of scores 4), and red triangles = poor knowledge and high uncertainty (sum of scores 5–6)
Discussion
Salmon are vulnerable to stressors operating across life stages and environments, which makes their conservation challenging (McDowall 1999). Management prioritisation of key stressors is therefore crucial. While it is more feasible to address stressor impacts in fresh water, marine environmental issues must not be overlooked, where density-dependent effects are nominal, resulting in proportional losses of adult returns. The present assessment identified climate change and predation to exert the biggest impacts on English salmon stocks in the marine environment both currently and over the next decade. Regional differences in both climate change and predation prevalence were evident across England. Specifically, greater climate change impacts were expected in the South due to higher temperatures projected under future climatic scenarios (Lowe et al. 2019), while predation ranked highest in the North West given local concerns about rising levels of bird and seal predation on salmon stocks in estuaries. As knowledge on the effects and projected development of both stressors was poor, careful monitoring, assessment, and mitigation is required to inform management decisions to protect and restore salmon stocks. Climate change and predation are challenging issues to address given that both stressors have a high uncertainty of projected development, low mitigation potential due to their large spatial extent and many predator species have protected status, and they interact with other factors to generate synergistic and additive effects. However, targeted management actions to address specific stressors are possible in some circumstances.
Efforts to limit climate change impacts on salmon stocks depend on international actions to reduce greenhouse gas emissions, with legally binding targets set at national (HMSO 2008) and international (UNFCCC 2015) levels. While such actions are necessary to tackle climate change globally, alleviating locally operating stressors will be more practical to implement than addressing broad-scale environmental changes. This is crucial to maximise ecosystem resilience to stressors and minimise their cumulative impacts (Falkenberg et al. 2013; Scheffer et al. 2015; Ramírez et al. 2018). Priority should be given to protecting and improving estuaries due to the high likelihood of future anthropogenic development (Kennish 2002), which might threaten salmon stocks, especially in heavily modified environments where multiple stressors operate concurrently (Toft et al. 2018; Hodgson et al. 2020). However, it must be recognised that fully improving and protecting the resilience of estuaries will also require the rehabilitation and restoration of whole catchments from headwaters to the river mouth (Riley et al. 2018). Given that stressor exposure during one life stage can affect subsequent survival (Fenkes et al. 2016), effective management of freshwater habitats is equally important to the maintenance and enhancement of salmon stocks (Mainstone et al. 2012), especially as fisheries managers have a limited ability to control factors that influence salmon survival at sea (Russell et al. 2012a), particularly beyond territorial waters. Improvements in salmon stock status, predominantly in North East England (e.g., the Rivers Tyne, Tees, and Wear), have occurred against wide-scale population declines in many areas of the North Atlantic (Mawle and Milner 2003), indicating that freshwater effects on spawners and recruits can be dominant controls of stock size. Management actions in freshwater habitats should endeavour to maximise the number of smolts entering the ocean in the best condition and minimise stressor impacts that compromise marine survival (Russell et al. 2012a; Gregory et al. 2019). Local actions to combat the impacts of climate change on salmon in freshwater habitats should focus on ensuring access to cold-water refugia, improving habitat connectivity, and maintaining natural discharge regimes in rivers (Smialek et al 2021; Thorstad et al. 2021).
Losses to predators can be ameliorated through compensatory processes, especially for the earliest freshwater life stages, but these losses are also dependent on the timing and extent of predation, stock size, and other human-generated stressors (Ward and Hvidsten 2011). Most salmon stocks in England are in a depleted state (Cefas et al. 2019a), therefore the population-level impacts of predation will be proportionally greater than for stocks at full reproductive capacity. Addressing predation will be more pragmatic than tackling climate change in the short-term and at local management scales. The key will be to identify potential predation ‘bottlenecks’ in rivers and estuaries, and where seasonal concentrations of migrating salmon are particularly vulnerable to predators (Halfyard et al. 2012; Thorstad et al. 2012b; Lothian et al. 2018). This vulnerability is exacerbated when salmon are exposed to other stressors such as migration barriers (Moore et al. 1996) and poor water quality (Moore et al. 2007, 2008). Alleviating other stressors could mitigate predation impacts on salmon, but more coordinated actions will typically be required to deter predators at key life stages and identified bottlenecks. Given that predation is probably the most important source of mortality when salmon leave fresh water and enter the marine phase of their life cycle (Hansen et al. 2003), regulation of avian and mammal predation is likely to be beneficial at those times using harassment techniques (e.g., non-lethal acoustic/visual deterrents), local population reductions where necessary, justified, and legally approved (Russell et al. 1996; Butler et al. 2008), and habitat improvement (e.g., barrier removal) as an integrated control strategy (Russell et al. 2012b; Harris et al. 2014; Götz and Janik 2015).
Stressors with relatively high effects scores and moderate likelihoods of increasing in prevalence over the next decade included poor water quality and bycatch. Although improved water quality has enhanced salmon stock status in many English rivers over the last 50 years (Mawle and Milner 2003), concerns about the impacts of poor water quality on the passage of smolts and adult salmon through estuaries exist (Hugman et al. 1984), especially given the potential for synergistic effects in combination with other stressors including climate change (Teichert et al. 2016). Measures to alleviate poor water quality impacts have been implemented under the EU’s Water Framework and Marine Strategy Framework Directives, which aim to safeguard the good ecological status of aquatic ecosystems (EC 2000, 2008, 2017). Maintaining established water quality standards and ensuring that adequate controls are placed on construction schemes (e.g., timing restrictions and regular water quality monitoring; DECC 2011) is essential to minimise impacts on salmon stocks in estuaries and nearshore waters in the future. Salmon are taken as bycatch in high-seas pelagic fisheries targeting other species (ICES 2006, 2014), but these losses are considered nominal at present (ICES 2017b). Nevertheless, bycatch rates could increase over the next decade because of climate-related geographic shifts in pelagic fish distribution. Less is known about the bycatch of returning adults in estuarine and nearshore fisheries, and local concerns were expressed over possible impacts on salmon. Some mitigation measures have been established to restrict salmon bycatch (e.g., seasonal fishing restrictions and mesh size limits), but enhanced monitoring and regulation are required to minimise incidental captures in non-target fisheries.
All the other stressors considered were assessed to have relatively low effects on English salmon stocks at present, but differences existed in their potential prevalence over the next decade. These included marine renewable energy installations (tidal barrages and lagoons) that have become the focus for ‘green’ energy generation and EMF closely related to their development (EC 2009; DECC 2009). Tidal barrages were assessed to pose a larger risk to salmon than tidal lagoons due to their higher future development and lower mitigation potentials. Electromagnetic fields appeared to be a relative minor issue for salmon due to effective measures and transient nature of possible impacts. Given the growing interest in marine renewable energy installations, the effects of impingement and thermal discharge from power stations on salmon were expected to increase less rapidly in the future than in the past. Invasive non-native species and ALAN both had low impacts on salmon given the lack of documented effects in the marine environment, with a moderate-to-high likelihood of becoming more prevalent over the next decade. Noise pollution from pile-driving had a high likelihood of increasing in prevalence over the next decade. Impacts are, however, likely to be low because of the poor hearing sensitivity of salmon (Hawkins and Popper 2014), transient nature of their migration (Klemetsen et al. 2003), and the wide range of mitigation measures available (e.g., noise free periods, soft-start procedures, and acoustic barriers; JNCC 2010). Fisheries exploitation and salmon mariculture were considered to pose a relatively small threat to English salmon stocks at present, with salmon mariculture having a greater likelihood of development over the next decade. However, it is expected that mitigation measures to control fisheries exploitation and salmon mariculture will continue to emerge (Cefas et al. 2019b; Lekang et al. 2016). Identifying the impacts of salmon mariculture on wild salmon stocks is an area of ongoing investigation (NASCO 2021), which requires further progress towards achieving the international goals of effective sea lice management and the containment of farmed fish in production facilities (ICES 2016; NASCO 2016).
Stressor impacts vary depending on salmon life stage (Smialek et al. 2021; Thorstad et al. 2021). As autumn migrating parr can reside in the lower reaches of rivers and estuaries for extended periods of time (October–April) before migrating to sea as smolts in the spring (Pinder et al. 2007; Riley et al. 2009), they will likely be exposed to some potential stressor effects for longer durations than life stages undertaking more transient migrations. Autumn migrating parr have been found to be important stock components in some rivers on both sides of the North Atlantic (Cunjak et al. 1989; Pinder et al. 2007; Riley et al. 2009), but their significance is unknown in others where it has not been assessed. Smolts transiting through the tidal reaches of rivers and estuaries for one day to several weeks during spring and early summer could be subject to potential stressor effects for relatively short durations (Thorstad et al. 2012a). In contrast, post-smolts and adult salmon moving over large geographic areas through estuaries, nearshore waters, and the open ocean for one or more years are likely to be exposed to potential stressor effects for comparatively long durations (Hansen et al. 2003; Klemetsen et al. 2003).
Differences existed in the stressors considered and the scoring approach used to assess their impacts on salmon stocks in England and Norway. The present assessment focused solely on marine stressors impacting English salmon stocks, whereas Forseth et al. (2017) considered stressors operating across freshwater and marine environments in Norway. In terms of the scoring approach, the present assessment combined evidence from the literature review and expert local knowledge instead of only relying on expert judgement. Nevertheless, a broad comparison of stressor impacts is possible because both assessments were informed by a 2-D classification system with similar principles. Escaped farmed salmon and sea lice were assessed to have the biggest impacts on Norwegian salmon stocks at present and in the future given the scale of the mariculture industry there. In contrast, no salmon mariculture facilities exist in England, and therefore the risks from escaped farmed salmon and sea lice were considered low. In Norway, climate change and predation were assessed as having relatively low impacts on salmon stocks compared to the results of the present assessment. This could be due to latitudinal effects and the presence of strong evidence on the key stressors in Norway, thereby lowering climate change and predation scores in the overall ranking, as well as high uncertainty on their projected development. However, both assessments agreed that fisheries exploitation at present rates posed a relatively small threat to salmon stocks given the effective controls imposed.
Six caveats should be noted in relation to the present review. First, not all the marine stressors potentially impacting salmon were considered. For example, trophic cascades, disease outbreaks, parasites, marine plastics, and pharmaceuticals were not considered because they were not deemed to be important anthropogenic factors impacting salmon survival at sea. Second, stressor impacts were broadly scored for smolts and adult salmon. Partitioning these scores into finer time intervals during the migratory phase might have provided better insight into stressor impacts. Third, determining stressor impacts at the population-level was challenging because studies tend to focus on the biological responses of individuals or groups rather than entire populations. Fourth, knowledge on the effects of seven out of thirteen stressors was classified as poor and the uncertainty of the future development high, therefore it is inevitably difficult to draw firm conclusions about the impacts of these stressors. Fifth, stressor scores were semi-quantitative based on evidence from the literature review, local fisheries officers, and expert judgement. Although a more quantitative classification would be preferable, the limited amount of data available for all salmon stocks makes this an unrealistic aspiration. Sixth, evaluation of synergistic and additive stressor effects was beyond the scope of the present review, but this remains an important consideration in the long-term sustainability of salmon stocks (Russell et al. 2012a; Wright et al. 2017; Hodgson et al. 2019). Despite these caveats, the present review identified climate change and predation as the most important threats to English salmon stocks in the marine environment, thereby contributing to a growing body of evidence that suggests broad-scale environmental factors are key drivers of the reductions in the marine survival of salmon.
An important next step for research is to understand better how the synergistic and additive effects of multiple stressors impact salmon across aquatic ecosystems (Sobocinksi et al. 2018; Hodgson et al. 2019). Salmon are subjected to multiple stressors at differing spatial and temporal scales, and stressor exposure at one life stage can have subsequent implications later in life and can have inter-generational effects, thereby altering life-history traits and population-level processes (Cline et al. 2019). It is well established that stressors acting in fresh water, including altered temperature regimes, migration obstacles, and contaminants, can have ‘carry-over’ effects on smolt physiological development, post-smolt behaviour, sea survival, growth, and homing ability (McCormick et al. 2009). A reduction in stressor impacts throughout the salmon life cycle is therefore crucial. A priority for the future is to develop an integrated management approach that addresses both anthropogenic and natural drivers of change, including complex interactions and cumulative effects, at the ecosystem level (Wells et al. 2020). Knowledge gaps and uncertainties are evident for all the stressors considered in this review. The prioritisation of evidence needs to address these stressors is challenging, not least because the scale and extent of the stressors varies over time. New evidence is constantly emerging, so it will be essential to maintain a watching brief of future developments. Research should focus both on how better to address and mitigate prominent stressors at present and on those stressors for which knowledge is limited and are likely to expand in severity and/or extent in the future.
Conclusion
The findings from this review can aid in the prioritisation of management actions and to direct future research to protect and restore salmon stocks. It is clear that the nature, severity, and extent of the stressors impacting the marine survival of salmon are location and time specific. Management actions should therefore focus on alleviating locally operating stressors that reduce the number and condition of smolts entering the ocean. In England, the stressors assessed to have the largest impacts on salmon stocks in the marine environment are climate change and predation. Although tackling both these stressors is and will remain challenging, targeted management actions to alleviate the highest-impact stressors in estuaries and to ensure freshwater habitats remain suitable to sustain salmon stocks are possible. Salmon have persisted for millennia by adapting to environmental change, but the present rate of environmental change is unprecedented (ICES 2017b). Safeguarding as many returning adults as possible to provide more time for salmon populations to adapt to environmental change has therefore never been more important. Future research should address knowledge gaps on expanding stressor impacts from climate change, predation, renewable energy developments, and artificial light at night, as well as possible synergistic effects. The findings from this review are relevant to other diadromous species of fish and lamprey, given that these have similar life traits involving migrations through aquatic ecosystems and exposure to multiple anthropogenic pressures. The semi-quantitative 2-D classification system used in the present review to rank stressor impacts on salmon represents a useful tool with which to prioritise research and management initiatives, and it is an approach that can be readily transferred to other species and systems (Forseth et al. 2017). To provide a more detailed overview of different threats and help prioritise conservation efforts at appropriate spatial and temporal scales, it is recommended that a dynamic ecosystem-based assessment be developed with which to evaluate regularly, as new evidence emerges, the cumulative risks across ecosystem components and salmon life stages.
Availability of data and material
Raw data presented at regional scale are available in the Supplementary Information.
References
Alabaster JS (1991) Water quality criteria for freshwater fish – review of progress. In: Proceedings of the institute of fisheries management conference, Fisheries in the year 2000. Royal Holloway and Bedford New College, University of London, Egham, UK
Alabaster JS, Gough PJ (1986) The dissolved oxygen and temperature requirements of Atlantic salmon, Salmo salar L., in the Thames estuary. J Fish Biol 29(5):613–621
Alabaster JS, Gough PJ, Brooker WJ (1991) The environmental requirements of Atlantic salmon, Salmo salar L., during their passage through the Thames estuary, 1982–1989. J Fish Biol 38(5):741–762
Alabaster JS, Lloyd RS (1982) Water quality criteria for freshwater fish, 2nd edn. Butterworths, London
Alabaster JS, Shurben DG, Knowles G (1979) The effect of dissolved oxygen and salinity on the toxicity of ammonia to smolts of salmon, Salmo salar L. J Fish Biol 15:705–712
Alexander G, Sweeting R, McKeown B (1994) The shift in visual pigment dominance in the retinae of juvenile coho salmon (Oncorhynchus kisutch): an indicator of smolt status. J Exp Biol 195:185–197
Almodóvar A, Ayllón D, Nicola GG, Jonsson B, Elvira B (2019) Climate-driven biophysical changes in feeding and breeding environments explain the decline of southernmost European Atlantic salmon populations. Can J Fish Aquat Sci 76(9):1581–1595
Alter SE, Tariq L, Creed JK, Megafu E (2021) Evolutionary responses of marine organisms to urbanized seascapes. Evol Appl 14:210–232
Anon (1990) Bass nursery areas and other conservation measures. Ministry of Agriculture, Fisheries and Food (MAFF) Publications, London, p 15
Armstrong JD, Bean CW, Wells A (2018) The Scottish invasion of pink salmon in 2017. J Fish Biol 93(1):8–11
Armstrong JD, Hunter D-C, Fryer RJ, Rycroft P, Orpwood JE (2015) Behavioural responses of atlantic salmon to mains frequency magnetic fields. Marine Scotland. https://data.marine.gov.scot/dataset/behavioural-responses-atlantic-salmon-mains-frequency-magnetic-fields. Accessed 20 June 2019
Armstrong JD, Kemp PS, Kennedy GJA, Ladle M, Milner NJ (2003) Habitat requirements of Atlantic salmon and brown trout in rivers and streams. Fish Res 62:143–170
Assunção MG, Ives M, Davison P, Barber JL, Moore A, Law RJ (2020) Persistent contaminants in adipose fins of returning adult salmonids to the river Tees (UK). Mar Pollut Bull 153:110945. https://doi.org/10.1016/j.marpolbul.2020.110945
Bacon PJ, Palmer SCF, MacLean JC, Smith GW, Whyte BDM, Gurney WSC, Youngson AF (2009) Empirical analyses of the length, weight, and condition of adult Atlantic salmon on return to the scottish coast between 1963 and 2006. ICES J Mar Sci 66(5):844–859
Bagočius D (2015) Piling underwater noise impact on migrating salmon fish during Lithuanian LNG terminal construction (Curonian Lagoon, Eastern Baltic Sea Coast). Mar Pollut Bull 92:45–51
Bal G, Montorio L, Rivot E, Prévost E, Baglinière JL, Nevoux M (2017) Evidence for long-term change in length, mass and migration phenology of anadromous spawners in French Atlantic salmon Salmo salar. J Fish Biol 90(6):2375–2393
Bamber RN (1990) Power station thermal effluents and marine crustaceans. J Therm Biol 15(1):91–96
Bamber RN, Seaby RMH (2004) The effects of entrainment passage on three species of marine planktonic crustacean, Acartia tonsa (Copepoda), Crangon (Decapoda) and Homarus gammarus (Decapoda). Mar Environ Res 57:281–294
Bamber RN, Seaby RMH, Fleming JM, Taylor CJL (1994) The effects of entrainment passage on embryonic development of the Pacific oyster Crassostrea gigas. Nucl Energy 33:353–357
Barker SE, Bricknell IR, Covello J, Purcell S, Fast MD, Wolters W, Bouchard DA (2019) Sea lice, Lepeophtheirus salmonis (Krøyer 1837), infected Atlantic salmon (Salmo salar L.) are more susceptible to infectious salmon anemia virus. PLoS ONE 14(1):e0209178. https://doi.org/10.1371/journal.pone.0209178
Barson NJ, Aykanat T, Hindar K, Baranski M, Bolstad GH, Fiske P, Jacq C, Jensen AJ, Johnston SE, Karlsson S, Kent M (2015) Sex-dependent dominance at a single locus maintains variation in age at maturity in salmon. Nature 528(7582):405–408
Bax N, Williamson A, Aguero M, Gonzalez E, Geeves W (2003) Marine invasive alien species: a threat to global biodiversity. Mar Policy 27(4):313–323
Beamish FWH (1964) Respiration of fishes with special emphasis on standard oxygen consumption: II. Influence of weight and temperature on respiration of several species. Can J Zool 42:177–188
Beatty DD (1966) A study of the succession of visual pigments in Pacific salmon (Oncorhynchus). Can J Zool 44(3):429–455
Beaugrand G, Reid PC (2012) Relationships between North Atlantic salmon, plankton, and hydroclimatic change in the Northeast Atlantic. ICES J Mar Sci 69:1549–1562
Beaugrand G, Reid PC (2003) Long-term changes in phytoplankton, zooplankton and salmon related to climate. Glob Chang Biol 9:801–817
Bendall B, Moore A (2008) Temperature-sensing telemetry – possibilities for assessing the feeding ecology of marine mammals and their potential impacts on returning salmonid populations. Fish Manag Ecol 15:339–345
Bendall B, Moore A, Maxwell D, Davison P, Edmonds N, Archer D, Solomon D, Greest V, Wyatt R, Broad K (2012) Modelling the migratory behaviour of salmonids in relation to environmental and physiological parameters using telemetry data. Fish Manag Ecol 19:475–483
Berge J, Geoffroy M, Daase M, Cottier F, Priou P, Cohen JH, Johnsen G, McKee D, Kostakis I, Renaud PE, Vogedes D (2020) Artificial light during the polar night disrupts Arctic fish and zooplankton behaviour down to 200 m depth. Commun Biol 3:102–108
Berntsen HH, Sandlund OT, Thorstad EB, Fiske P (2020) Pukkellaks i Norge, 2019. Norwegian Institute for Nature Research Report 1821. https://hdl.handle.net/11250/2651741. Accessed 28 Oct 2021
Bielak AT, Power G (1986) Changes in mean weight, sea-age composition, and catch-per-unit-effort of Atlantic salmon (Salmo salar) angled in the Godbout River, Quebec, 1859–1983. Can J Fish Aquat Sci 43(2):281–287
Bilous M, Dunmall K (2020) Atlantic salmon in the Canadian Arctic: potential dispersal, establishment, and interaction with Arctic char. Rev Fish Biol Fish 30:463–483
Boehlert GW, Gill AB (2010) Environmental and ecological effects of ocean renewable energy development: a current synthesis. Oceanography 23(2):68–81
Bolstad GH, Hindar K, Robertsen G, Jonsson B, Sægrov H, Diserud OH, Fiske P, Jensen AJ, Urdal K, Næsje TF, Barlaup BT (2017) Gene flow from domesticated escapes alters the life history of wild Atlantic salmon. Nat Ecol Evol 1(5):0124
Boon JP, Wilma EL, Tjoen-A-Choy MR, Allchin CR, Law RJ, De Boer J, Ten Hallers-Tjabbes CC, Zegers BN (2002) Levels of polybrominated diphenyl ether (PBDE) flame retardants in animals representing different trophic levels of the North Sea food web. Environ Sci Technol 36:4025–4032
Bowen WD, Tully D, Boness DJ, Bulheier BM, Marshall GJ (2002) Prey-dependent foraging tactics and prey profitability in a marine mammal. Mar Ecol Prog Ser 244:235–245
Brett JR (1972) The metabolic demand for oxygen in fish, particularly salmonids, and a comparison with other vertebrates. Respir Physiol 14(1–2):151–170
Briand FJ-P (1975) Effects of power-plant cooling systems on marine phytoplankton. Mar Biol 33(2):135–146
Brungs WA (1973) Effects of residual chlorine on aquatic life. J Water Pollut Control Fed 45(10):2180–2193
Burreau S, Zebühr Y, Broman D, Ishaq R (2006) Biomagnification of PBDEs and PCBs in food webs from the Baltic Sea and the northern Atlantic Ocean. Sci Total Environ 366(2–3):659–672
Burton T, McKelvey S, Stewart DC, Armstrong JD, Metcalfe NB (2013) Early maternal experience shapes offspring performance in the wild. Ecology 94(3):618–626
Butler JRA, Middlemas SJ, Graham IM, Harris RN (2011) Perceptions and costs of seal impacts on Atlantic salmon fisheries in the Moray Firth, Scotland: implications for the adaptive co-management of seal-fishery conflict. Mar Policy 35:317–323
Butler JRA, Middlemas SJ, Graham IM, Thompson PM, Armstrong JD (2006) Modelling the impacts of removing seal predation from Atlantic salmon, Salmo salar, rivers in Scotland: a tool for targeting conflict resolution. Fish Manag Ecol 13:285–291
Butler JRA, Middlemas SJ, McKelvey SA, McMyn I, Leyshon B, Walker I et al (2008) The moray firth seal management plan: an adaptive framework for balancing the conservation of seals, salmon, fisheries and wildlife tourism in the UK. Aquat Conserv Mar Freshw Ecosyst 18(6):1025–1038
Byron CJ, Pershing AJ, Stockwell JD, Xue H, Kocik J (2014) Migration model of post-smolt Atlantic salmon (Salmo salar) in the Gulf of Maine. Fish Oceanogr 23:172–189
Cabral H, Fonseca V, Sousa T, Costa Leal M (2019) Synergistic effects of climate change and marine pollution: an overlooked interaction in coastal and estuarine areas. Int J Environ Res Public Health 16:1–17
Cairns DK (2001) An evaluation of possible causes of the decline in pre-fishery abundance of North American Atlantic salmon. Canadian Technical Report of Fisheries and Aquatic Sciences No. 2358
Cefas, Environment Agency, Natural Resources Wales (2019a) Salmon stocks and fisheries in England and Wales in 2018. Preliminary assessment prepared for ICES, March 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/907284/SalmonReport-2019-summary.pdf. Accessed January 2020
Cefas, Environment Agency, Natural Resources Wales (2019b) Assessment of salmon stocks and fisheries in England and Wales. Standing report on methods, approaches and wider stock conservation and management considerations. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/907289/SalmonReport-2019-background.pdf. Accessed January 2020
Cefas, Environment Agency, Natural Resources Wales (2020) Salmon stocks and fisheries in England and Wales in 2019. Preliminary assessment prepared for ICES, March 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/907284/SalmonReport-2019-summary.pdf. Accessed August 2020
Chaput G (2012) Overview of the status of Atlantic salmon (Salmo salar) in the North Atlantic and trends in marine mortality. ICES J Mar Sci 69:1538–1548
Cheng CL, Novales Flamarique I, Hárosi FI, Rickers-Haunerland J, Haunerland NH (2006) Photoreceptor layer of salmonid fishes: transformation and loss of single cones in juvenile fish. J Comp Neurol 495:213–235
Chowdhury MS, Rahman KS, Selvanathan V, Nuthammachot N, Suklueng M, Mostafaeipour A, Habib A, Akhtaruzzaman M, Amin N, Techato K (2021) Current trends and prospects of tidal energy technology. Environ Dev Sustain 23:8179–8194. https://doi.org/10.1007/s10668-020-01013-4
Civil MA, Quick NJ, Cheney B, Pirotta E, Thompson PM, Hammond PS (2019) Changing distribution of the east coast of Scotland bottlenose dolphin population and the challenges of area-based management. Aquat Conserv Mar Freshw Ecosyst 29:178–196
Cline TJ, Ohlberger J, Schindler DE (2019) Effects of warming climate and competition in the ocean for life-histories of Pacific salmon. Nat Ecol Evol 3(6):935–942
Collis K, Roby DD, Craig DP, Adamany S, Adkins JY, Lyons DE (2002) Colony size and diet composition of piscivorous waterbirds on the lower Columbia River: implications for losses of juvenile salmonids to avian predation. Trans Am Fish Soc 131:537–550
Colman JE, Pawson MG, Holmen J, Haugen TO (2008) European sea bass in the North Sea: past, present and future status, use and management challenges. In: Aas Ø, Arlinghaus R, Ditton RB, Policansky D, Schramm HL (eds) Global challenges in recreational fisheries. Blackwell Publishing Ltd, Oxford, pp 111–129
Connors BM, Braun DC, Peterman RM, Cooper AB, Reynolds JD, Dill LM, Ruggerone GT, Krkošek M (2012) Migration links ocean-scale competition and local ocean conditions with exposure to farmed salmon to shape wild salmon dynamics. Conserv Lett 5:304–312
Copp GH (2017) GB Non-native Species Rapid Risk Assessment (NRRA): rapid risk assessment of: Oncorhynchus gorbuscha (Walbaum) (pink or humpback salmon). https://www.cefas.co.uk/media/w0notcdi/rrav4_oncorhynchus_gorbuscha_pinksalmon_release_v2_07-03-18-passed-dj.pdf. Accessed 13 December 2017
Copp GH, Bianco PG, Bogutskaya NG, Erős T, Falka I, Ferreira MT, Fox MG, Freyhof J, Gozlan RE, Grabowska JO, Kováč V (2005) To be, or not to be, a non-native freshwater fish? J Appl Ichthyol 21:242–262
Cornett A, Cousineau J, Nistor I (2013) Assessment of hydrodynamic impacts from tidal power lagoons in the Bay of Fundy. Int J Mar Energy 1:33–54
Coutant CC, Brook AJ (1970) Biological aspects of thermal pollution I. Entrainment and discharge canal effects. Crit Rev Environ Sci Technol 1(1–4):341–381
Crozier W, Whelan K, Buoro M, Chaput G, Daniels J, Grant S, Hyatt K, Irvine J, Ó'maoiléidigh N, Prévost E, Rivot E (2018) Atlantic salmon mortality at sea: developing an evidence-based “likely suspects” framework. https://atlanticsalmontrust.org/wp-content/uploads/2018/08/Blue-Journal-June-2018.pdf. Accessed 10 August 2019
Cunjak RA, Chadwick EMP, Shears M (1989) Downstream movements and estuarine residence by Atlantic salmon parr (Salmo salar). Can J Fish Aqua Sci 46(9):1466–1471
Dadswell M, Spares A, Reader J, McLean M, McDermott T, Samways K, Lilly J (2021) The decline and impending collapse of the Atlantic salmon (Salmo salar) population in the North Atlantic Ocean: a review of possible causes. Rev Fish Sci Aquac. https://doi.org/10.1080/23308249.2021.1937044
Dadswell MJ, Rulifson RA (1994) Macrotidal estuaries: A region of collision between migratory marine animals and tidal power development. Biol J Linn Soc Lond 51:93–113
Dadswell MJ, Spares AD, Mclean MF, Harris PJ, Rulifson RA (2018) Long-term effect of a tidal, hydroelectric propeller turbine on the populations of three anadromous fish species. J Fish Biol 93(2):192–206
Dadswell MJ, Spares AD, Reader JM, Stokesbury MJW (2010) The North Atlantic subpolar gyre and the marine migration of Atlantic salmon Salmo salar: the ‘Merry-Go-Round’ hypothesis. J Fish Biol 77:435–467
Daniels J, Chaput G, Carr J (2018) Estimating consumption rate of Atlantic salmon smolts (Salmo salar) by striped bass (Morone saxatilis) in the Miramichi River estuary using acoustic telemetry. Can J Fish Aqua Sci 75(11):1811–1822
Daniels J, Sutton S, Webber D, Carr J (2019) Extent of predation bias present in migration survival and timing of Atlantic salmon smolt (Salmo salar) as suggested by a novel acoustic tag. Anim Biotelemetry 7(16):1–11
Davies JK (1988) A review of information relating to fish passage through turbines: implications to tidal power schemes. J Fish Biol 33:111–126
Davies TW, Duffy JP, Bennie J, Gaston KJ (2014) The nature, extent, and ecological implications of marine light pollution. Front Ecol Environ 12:347–355
Department for Environment, Food & Rural Affairs (Defra) (2020) Biodiversity 2020: a strategy for England’s wildlife and ecosystem services - Indicators. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/926056/England_biodiversity_indicators_2020_REVISED.pdf. Accessed 10 June 2020
Dempson B, Schwarz CJ, Bradbury IR, Robertson MJ, Veinott G, Poole R, Colbourne E (2017) Influence of climate and abundance on migration timing of adult Atlantic salmon (Salmo salar) among rivers in Newfoundland and Labrador. Ecol Freshw Fish 26:247–259
Department of Energy and Climate Change (DECC) (2009) The UK renewable energy strategy. Presented to Parliament by the Secretary of State for Energy and Climate Change by command of Her Majesty. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/228866/7686.pdf. Accessed 10 June 2020
Department of Energy and Climate Change (DECC) (2011) National policy statement for renewable energy infrastructure (EN-3). Presented to Parliament pursuant to section 5(9) of the Planning Act 2008. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/47856/1940-nps-renewable-energy-en3.pdf. Accessed 10 June 2020
Dieperink C, Bak BD, Pedersen LF, Pedersen MI, Pedersen S (2002) Predation on Atlantic salmon and sea trout during their first days as postsmolts. J Fish Biol 61(3):848–852
Diserud OH, Fiske P, Sægrov H, Urdal K, Aronsen T, Lo H, Barlaup BT, Niemelä E, Orell P, Erkinaro J, Lund RA (2019) Escaped farmed Atlantic salmon in Norwegian rivers during 1989–2013. ICES J Mar Sci 76(4):1140–1150
Doughty R, Gardiner R (2003) The return of salmon to cleaner rivers: a scottish perspective. In: Mills D (ed) Salmon at the Edge. Blackwell Science, Oxford, pp 175–185
Drinkwater KF, Frank KT (1994) Effects of river regulation and diversion on marine fish and invertebrates. Aquat Conserv Mar Freshw Ecosyst 4(2):135–151
Dudgeon D (2019) Multiple threats imperil freshwater biodiversity in the Anthropocene. Curr Biol 29(19):R960–R967
Dunlop K, Eloranta AP, Schoen E, Wipfli M, Jensen JL, Muladal R, Christensen GN (2021) Evidence of energy and nutrient transfer from invasive pink salmon (Oncorhynchus gorbuscha) spawners to juvenile Atlantic salmon (Salmo salar) and brown trout (Salmo trutta) in northern Norway. Ecol Freshw Fish 30(2):270–283
European Commission (EC) (2000) Directive 2000/60/EC of the European Parliament and of the Council establishing a framework for the Community action in the field of water policy, 23rd October 2000. http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32000L0060. Accessed 20 August 2019
European Commission (EC) (2008) Directive 2008/56/EC of the European Parliament and the Council of 17 June 2008 establishing a framework for community action in the field of marine environmental policy (Marine Strategy Framework Directive). https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32008L0056. Accessed 20 August 2020
European Commission (EC) (2009) Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009 on the promotion of the use of energy from renewable sources and amending and subsequently repealing Directives 2001/77/EC and 2003/30/EC. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32009L0028. Accessed 20 August 2020
European Commission (EC) (2017) Decision (EU) 2017/848 of 17 May 2017 laying down criteria and methodological standards on good environmental status of marine waters and specifications and standardised methods for monitoring and assessment, and repealing Decision 2010/477/EU. https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A32017D0848. Accessed 20 August 2020
European Environment Agency (EEA) (2018) European waters: assessment of status and pressures 2018. European Environment Agency Report No. 7/2018. www.eea.europa.eu/publications/state-of-water. Accessed 29 October 2021
Einum S, Fleming IA (2000) Selection against late emergence and small offspring in Atlantic salmon (Salmo salar). Evol 54(2):628–639
Ellis T, Turnbull JF, Knowles TG, Lines JA, Auchterlonie NA (2016) Trends during development of Scottish salmon farming: an example of sustainable intensification? Aquac 458:82–99
Eno NC, Clark RA, Sanderson WG (1997) Non-native marine species in British waters: a review and directory. Joint Nature Conservation Committee, Peterborough, UK
Environment Agency (2020) WFD Surface Water Management Catchments Cycle 2 [shapefile geospatial data]. Scale not given. https://data.gov.uk/dataset/1a494e3e-e414-456c-9c2e-ca367a2945b6/wfd-surface-water-management-catchments-cycle-2. Accessed 1 October 2020. Using: ArcMap GIS. Version 10.5. Redlands, CA: Environmental Systems Research Institute, Inc., 1999–2016. All content is available under the Open Government licence v3.0. © Crown Copyright 2021
Falkenberg LJ, Connell SD, Russell BD (2013) Disrupting the effects of synergies between stressors: Improved water quality dampens the effects of future CO2 on a marine habitat. J Appl Ecol 50(1):51–58
Farrell AP (2009) Environment, antecedents and climate change: lessons from the study of temperature physiology and river migration of salmonids. J Exp Biol 212:3771–3780
Faulkner RC, Farcas A, Merchant ND (2018) Guiding principles for assessing the impact of underwater noise. J Appl Ecol 55:2531–2536
Feist BE, Anderson JJ, Miyamoto R (1996) Potential impacts of pile driving on juvenile pink (Oncorhynchus gorbuscha) and chum (O. keta) salmon behaviour and distribution. Dissertation, University of Washington
Fenkes M, Shiels HA, Fitzpatrick JL, Nudds RL (2016) The potential impacts of migratory difficulty, including warmer waters and altered flow conditions, on the reproductive success of salmonid fishes. Comp Biochem Physiol A Mol Integr Physiol 193:11–21
Fey DP, Jakubowska M, Greszkiewicz M, Andrulewicz E, Otremba Z, Urban-Malinga B (2019) Are magnetic and electromagnetic fields of anthropogenic origin potential threats to early life stages of fish? Aquat Toxicol 209:150–158
Flávio H, Kennedy R, Ensing D, Jepsen N, Aarestrup K (2020) Marine mortality in the river? Atlantic salmon smolts under high predation pressure in the last kilometres of a river monitored for stock assessment. Fish Manag Ecol 27:92–101
Fleming IA (1996) Reproductive strategies of Atlantic salmon: ecology and evolution. Rev Fish Biol Fish 6:349–416
Fleming IA, Einum S (2011) Reproductive ecology: a tale of two sexes. In: Aas Ø, Einum S, Klemetsen A, Skurdal J (eds) Atlantic salmon ecology. Blackwell Publishing Ltd, Oxford, pp 33–65
Folmar LC, Dickhoff WW (1981) Evaluation of some physiological parameters as predictor indices of smoltification. Aquaculture 23:309–324
Ford JS, Myers RA (2008) A global assessment of salmon aquaculture impacts on wild salmonids. PLoS Biol 6(2):e33. https://doi.org/10.1371/journal.pbio.0060033
Formicki K, Korzelecka-Orkisz A, Tański A (2019) Magnetoreception in fish. J Fish Biol 95(1):73–91
Forseth T, Barlaup BT, Finstad B, Fiske P, Gjoaester H, Falkegard M, Hindar A, Mo TA, Rikardsen AH, Thorstad EB, Vøllestad LA (2017) The major threats to Atlantic salmon in Norway. ICES J Mar Sci 74(6):1496–1513
Fraser DJ, Minto C, Calvert AM, Eddington JD, Hutchings JA (2010) Potential for domesticated-wild interbreeding to induce maladaptive phenology across multiple populations of wild Atlantic salmon (Salmo salar). Can J Fish Aquat Sci 67(11):1768–1775
Frid C, Andonegi E, Depestele J, Judd A, Rihan D, Rogers SI, Kenchington E (2012) The environmental interactions of tidal and wave energy generation devices. Environ Impact Assess Rev 32(1):133–139
Friedland KD, MacLean JC, Hansen LP, Peyronnet AJ, Karlsson L, Reddin DG, Maoiléidigh ÓN, JL, McCarthy (2009) The recruitment of Atlantic salmon in Europe. ICES J Mar Sci 66:289–304
Friedland KD, Shank BV, Todd CD, McGinnity P, Nye JA (2014) Differential response of continental stock complexes of Atlantic salmon (Salmo salar) to the Atlantic Multidecadal Oscillation. J Mar Syst 133:77–87
Friedland KD, Dannewitz J, Romakkaniemi A, Palm S, Pulkkinen H, Pakarinen T, Oeberst R (2017) Post-smolt survival of Baltic salmon in context to changing environmental conditions and predators. ICES J Mar Sci 74:1344–1355
Friedland KD, Manning JP, Link JS, Gilbert JR, Gilbert AT, O’Connell AF (2012) Variation in wind and piscivorous predator fields affecting the survival of Atlantic salmon, Salmo salar, in the Gulf of Maine. Fish Manag Ecol 19:22–35
Friedland KD, Reddin DG, Kocik JF (1993) Marine survival of North American and European Atlantic salmon: effects of growth and environment. ICES J Mar Sci 50(4):481–492
Friedland KD, Reddin DG, McMenemy JR, Drinkwater KF (2003) Multidecadal trends in North American Atlantic salmon (Salmo salar) stocks and climate trends relevant to juvenile survival. Can J Fish Aquat Sci 60:563–583
Gargan PG, Forde G, Hazon N, Russell DJF, Todd CD (2012) Evidence for sea lice-induced marine mortality of Atlantic salmon (Salmo salar) in western Ireland from experimental releases of ranched smolts treated with emamectin benzoate. Can J Fish Aquat Sci 69:343–353
Giattina JD, Garton RR (1983) A review of the preference-avoidance responses of fishes to aquatic contaminants. Residue Rev 87:43–90
Gibson AM (1933) Construction and operation of a tidal model of the Severn Estuary. His Majesty’s Stationery Office, London
Gilbey J, Utne KR, Wennevik V, Beck AC, Kausrud K, Hindar K, de Garcia Leaniz C, Cherbonnel C, Coughlan J, Cross TF, Dillane E, Ensing D, García-Vázquez E, Hole LR, Holm M, Holst JC, Jacobsen JA, Jensen AJ, Karlsson S, Maoiléidigh ÓN, Mork KA, Eg Nielsen E, Nøttestad L, Primmer CR, Prodöhl P, Prusov S, Stevens JR, Thomas K, Whelan K, McGinnity P, Verspoor E (2021) The early marine distribution of Atlantic salmon in the North-east Atlantic: a genetically informed stock-specific synthesis. Fish Fish 22:1274–1306
Gill AB, Bartlett M, Thomsen F (2012) Potential interactions between diadromous fishes of UK conservation importance and the electromagnetic fields and subsea noise from marine renewable energy developments. J Fish Biol 81:664–695
Gill A, Desender M (2020) Risk to animals from electro-magnetic fields emitted by electric cables and marine renewable energy devices. In: Copping AE, Hemery LG (eds) OES-Environmental 2020 state of the science report: environmental effects of marine renewable energy development around the world. Ocean Energy Systems (OES), Lisbon, pp 86–103
Gill AB, Gloyne-Philips I, Kimber J, Sigray P (2014) Marine renewable energy, electromagnetic (EM) fields and EM-sensitive animals. Marine Renewable Energy Technology and Environmental Interactions. Springer, Netherlands, pp 61–79
Gillson J (2011) Freshwater flow and fisheries production in estuarine and coastal systems: where a drop of rain is not lost. Rev Fish Sci 19(3):168–186
Glover KA, Pertoldi C, Besnier F, Wennevik V, Kent M, Skaala Ø (2013) Atlantic salmon populations invaded by farmed escapees: quantifying genetic introgression with a Bayesian approach and SNPs. BMC Genet 14:74
Glover KA, Quintela M, Wennevik V, Besnier F, Sørvik AGE, Skaala Ø (2012) Three decades of farmed escapees in the wild: a spatio-temporal analysis of population genetic structure throughout Norway. PLoS ONE 7(8):e43129. https://doi.org/10.1371/journal.pone.0043129
Godwin SC, Dill LM, Reynolds JD, Krkošek M (2015) Sea lice, sockeye salmon, and foraging competition: lousy fish are lousy competitors. Can J Fish Aquat Sci 72(7):1113–1120
Gözt T, Janik VM (2015) Target-specific acoustic predator deterrence in the marine environment. Anim Conserv 18:102–111
Gough PJ (1996) Potential impacts of estuarine barrages on migratory fish in England and Wales. In: Burt N and Watts J (eds) Barrages: engineering design and environmental impacts. International Conference, 10–13/09/1996, John Wiley and Sons Ltd, Cardiff, UK
Graham CT, Harrod C (2009) Implications of climate change for the fishes of the British Isles. J Fish Biol 74:1143–1205
Graham IM, Harris RN, Denny B, Fowden D, Pullan D (2009) Testing the effectiveness of an acoustic deterrent device for excluding seals from Atlantic salmon rivers in Scotland. ICES J Mar Sci 66(5):860–864
Graham IM, Harris RN, Matejusová I, Middlemas SJ (2011) Do ‘rogue’ seals exist? Implications for seal conservation in the UK. Anim Conserv 14:587–598
Gregory RS, Levings CD (1998) Turbidity reduces predation on migrating juvenile Pacific salmon. Trans Am Fish Soc 127:275–285
Gregory SD, Ibbotson AT, Riley WD, Nevoux M, Lauridsen RB, Russell IC, Britton JR, Gillingham PK, Simmons OM, Rivot E (2019) Atlantic salmon return rate increases with smolt length. ICES J Mar Sci 76:1702–1712
Gröger M, Maier-Reimer E, Mikolajewicz U, Moll A, Sein D (2013) NW European shelf under climate warming: implications for open ocean–shelf exchange, primary production, and carbon absorption. Biogeosciences 10:3767–3792
Halfyard EA, Gibson AJF, Ruzzante DE, Stokesbury MJW, Whoriskey FG (2012) Estuarine survival and migratory behaviour of Atlantic salmon Salmo salar smolts. J Fish Biol 81:1626–1645
Hamer HH, Malzahn AM, Boersma M (2011) The invasive ctenophore Mnemiopsis leidyi: a threat to fish recruitment in the North Sea? J Plankton Res 33(1):137–144
Hammill MO, Stenson GB (2000) Estimated prey consumption by harp seals (Phoca groenlandica), hooded seals (Cystophora cristata), grey seals (Halichoerus grypus) and harbour seals (Phoca vitulina) in Atlantic Canada. J Northwest Atl Fish Sci 26:1–24
Hansen LP, Holm M, Holst JC, Jacobsen JA (2003) The ecology of post-smolts of Atlantic salmon. In: Mills D (ed) Salmon at the Edge. Blackwell Science, Oxford, pp 25–39
Hansen LP, Quinn TP (1998) The marine phase of the Atlantic salmon (Salmo salar) life cycle, with comparisons to Pacific salmon. Can J Fish Aquat Sci 55(S1):104–118
Hansen TJ, Fjelldal PG, Folkedal O, Vågseth T, Oppedal F (2017) Effects of light source and intensity on sexual maturation, growth and swimming behaviour of Atlantic salmon in sea cages. Aquac Environ Interact 9:193–204
Harding H, Bruintjes R, Radford AN, Simpson SD (2016) Measurement of hearing in the Atlantic salmon (Salmo salar) using auditory evoked potentials, and effects of pile driving playback on salmon behaviour and physiology. Scottish Marine and Freshwater Science Report Volume 7, No 11, pp 47. https://digital.nls.uk/pubs/scotgov/2016/9781786521460.pdf. Accessed 12 May 2021.
Harris CM, Calladine JR, Wernham CV, Park KJ (2008) Impacts of piscivorous birds on salmonid populations and game fisheries in Scotland: a review. Wildlife Biol 14(4):395–411
Harris RN, Sievers C, Northridge S (2014). Seal diet at salmon net fisheries. Seal and salmon research project. Sea Mammal Research Unit, University of St. Andrews. http://www.smru.st-andrews.ac.uk/files/2016/08/Seal-diet-at-salmon-net-fisheries.pdf. Accessed 26 Aug 2020
Hastings RA, Rutterford LA, Freer JJ, Collin RA, Simpson SD, Genner MJ (2020) Climate change drives poleward increases and equatorward declines in marine species. Curr Biol 30:1572–1577
Haugland M, Holst JC, Holm M, Hansen LP (2006) Feeding of Atlantic salmon (Salmo salar L.) post-smolts in the Northeast Atlantic. ICES J Mar Sci 63:1488–1500
Hawkins A (2006) Assessing the impact of pile driving upon fish. In: Proceedings of the 2005 international conference on ecology and transportation. Irwin CL, Garrett P, McDermott KP (eds). Center for Transportation and the Environment, North Carolina State University, Raleigh, NC, p 22
Hawkins AD, Johnstone ADF (1978) The hearing of the Atlantic salmon, Salmo salar. J Fish Biol 13:655–673
Hawkins AD, Pembroke AE, Popper AN (2015) Information gaps in understanding the effects of noise on fishes and invertebrates. Rev Fish Biol Fish 25:39–64
Hawkins AD, Popper AN (2014) Assessing the impacts of underwater sounds on fishes and other forms of marine life. Acoust Today 10:30–41
Heddell-Cowie M (2005) Importance of the River Teviot to Atlantic salmon, Salmo salar, rod catches in the River Tweed, Scotland. Fish Manag Ecol 12:137–142
Hedger RD, Sundt-Hansen LE, Forseth T, Ugedal O, Diserud OH, Kvambekk ÅS, Finstad AG (2013) Predicting climate change effects on subarctic–Arctic populations of Atlantic salmon (Salmo salar). Can J Fish Aquat Sci 70:159–168
Heino M, Svåsand T, Wennevik V, Glover KA (2015) Genetic introgression of farmed salmon in native populations: quantifying the relative influence of population size and frequency of escapees. Aquac Environ Interact 6:185–190
Helland IP, Uglem I, Jansen PA, Diserud OH, Bjørn PA, Finstad B (2015) Statistical and ecological challenges of monitoring parasitic sea lice infestations in wild salmonid fish stocks. Aquac Environ Interact 7:267–280
Her Majesty’s Stationary Office (HMSO) (2008) Climate Change Act (2050 Target Amendment) Order 2019. Public General Acts, chapter 27. Her Majesty’s Stationary Office, London, UK. https://www.legislation.gov.uk/ukpga/2008/27/contents. Accessed 10 July 2019
Hodgson EE, Halpern BS, Essington TE (2019) Moving beyond silos in cumulative effects assessment. Front Ecol Evol 7:1–8
Hodgson EE, Wilson SM, Moore JW (2020) Changing estuaries and impacts on juvenile salmon: a systematic review. Glob Chang Biol 26(4):1986–2001
Hölker F, Wolter C, Perkin EK, Tockner K (2010) Light pollution as a biodiversity threat. Trends Ecol Evol 25:681–682
Holm M, Holst JC, Hansen LP (2000) Spatial and temporal distribution of post-smolts of Atlantic salmon (Salmo salar L.) in the Norwegian Sea and adjacent areas. ICES J Mar Sci 57:955–964
Holm M, Holst JC, Hansen LP, Jacobsen JA, O’Maoileidigh N, Moore A (2003) Migration and distribution of Atlantic salmon post-smolts in the North Sea and North-East Atlantic. In: Mills D (ed) Salmon at the Edge. Blackwell Science, Oxford, pp 7–23
Holst JC (2018) Let's bring back the salmon, sea birds and G6 mackerel! The hypothesis on overgrazing and predation. Towards an ecosystem-based management of the Norwegian Sea ecosystem, p 34. https://doi.org/10.13140/RG.2.2.12317.13288
Hooper T, Austen M (2013) Tidal barrages in the UK: ecological and social impacts, potential mitigation, and tools to support barrage planning. Renew Sustain Energy Rev 23:289–298
Hughes KM, Dransfeld L, Johnson MP (2014) Changes in the spatial distribution of spawning activity by north-east Atlantic mackerel in warming seas: 1977–2010. Mar Biol 161:2563–2576
Hugman SJ, O’Donnell AR, Mance G (1984) A survey of estuarine oxygen concentrations in relation to the passage of migratory salmonids. Water Resources Centre Report 745-M, p 38
Huntington TG, Hodgkins GA, Dudley RW (2003) Historical trend in river ice thickness and coherence in hydroclimatological trends in Maine. Clim Change 61:217–236
Hutchison ZL, Secor DH, Gill AB (2020) The interaction between resource species and electromagnetic fields associated with electricity production by offshore wind farms. Oceanogr 33(4):96–107
Hvidsten NA, Heggberget TG, Jensen AJ (1998) Sea water temperatures at Atlantic salmon smolt entrance. Nord J Freshw Res 74:79–86
Hvidsten NA, Jensen AJ, Rikardsen AH, Finstad B, Aure J, Stefansson S, Fiske P, Johnsen BO (2009) Influence of sea temperature and initial marine feeding on survival of Atlantic salmon Salmo salar post-smolts from the Rivers Orkla and Hals. Norway J Fish Biol 74(7):1532–1548
International Council for the Exploration of the Sea (ICES) (2005) Report of the Study Group on the Bycatch of Salmon in Pelagic Trawl Fisheries (SGBYSAL), 8–11 February 2004, Bergen, Norway. https://www.ices.dk/sites/pub/Publication%20Reports/Expert%20Group%20Report/acfm/2005/sgbysal/SGBYSAL05.pdf. Accessed 1 Nov 2021
International Council for the Exploration of the Sea (ICES) (2006) Report of the Working Group on North Atlantic Salmon (WGNAS), 4–13 April 2006, ICES Headquarters. https://www.ices.dk/sites/pub/CM%20Doccuments/2006/ACFM/ACFM2306.pdf. Accessed 1 Oct 2019
International Council for the Exploration of the Sea (ICES) (2013) Report of the Working Group on North Atlantic Salmon (WGNAS), 3–12 April 2013 Copenhagen, Denmark. https://www.ices.dk/sites/pub/Publication%20Reports/Expert%20Group%20Report/acom/2013/WGNAS/wgnas_2013.pdf. Accessed 1 Oct 2019
International Council for the Exploration of the Sea (ICES) (2014) Report of the Working Group on North Atlantic Salmon (WGNAS), 19–28 March 2014, Copenhagen, Denmark. https://www.ices.dk/sites/pub/Publication%20Reports/Expert%20Group%20Report/acom/2014/WGNAS/wgnas_2014.pdf. Accessed 1 Oct 2019
International Council for the Exploration of the Sea (ICES) (2016) Report of the Workshop to address the NASCO request for advice on possible effects of salmonid aquaculture on wild Atlantic salmon populations in the North Atlantic (WKCULEF), 1–3 March 2016, Charlottenlund, Denmark. https://www.ices.dk/sites/pub/Publication%20Reports/Expert%20Group%20Report/acom/2016/WKCULEF/WKCULEF_2016.pdf. Accessed 1 Oct 2019
International Council for the Exploration of the Sea (ICES) (2017a) Report of the Workshop on Potential Impacts of Climate Change on Atlantic Salmon Stock Dynamics (WKCCISAL), 27–28 March 2017, Copenhagen, Denmark. https://www.ices.dk/sites/pub/Publication%20Reports/Expert%20Group%20Report/acom/2017/WKCCISAL/wkccisal_2017.pdf. Accessed 1 Oct 2019
International Council for the Exploration of the Sea (ICES) (2017b) Report of the Working Group on North Atlantic Salmon (WGNAS), 29 March–7 April 2017, Copenhagen, Denmark. https://www.ices.dk/sites/pub/publication%20reports/expert%20group%20report/acom/2017/wgnas/wgnas_2017.pdf. Accessed 1 Oct 2019
International Council for the Exploration of the Sea (ICES) (2018) Report of the Working Group on North Atlantic Salmon (WGNAS), 4–13 April 2018, Woods Hole, MA, USA. https://www.ices.dk/sites/pub/Publication%20Reports/Expert%20Group%20Report/acom/2018/WGNAS/wgnas_2018.pdf. Accessed 1 Oct 2019
International Council for the Exploration of the Sea (ICES) (2019) Working Group on North Atlantic Salmon (WGNAS). ICES Scientific Reports. https://www.ices.dk/sites/pub/Publication%20Reports/Expert%20Group%20Report/Fisheries%20Resources%20Steering%20Group/2019/WGNAS/WGNAS_2019.pdf. Accessed 10 Dec 2020
International Council for the Exploration of the Sea (ICES) (2020a) NASCO Workshop for North Atlantic Salmon At-Sea Mortality (WKSalmon, outputs from 2019 meeting). ICES Scientific Reports. https://nasco.int/wp-content/uploads/2020/08/ICES-wksalmon_2019.pdf. Accessed 5 Mar 2021
International Council for the Exploration of the Sea (ICES) (2020b) Working Group on North Atlantic Salmon (WGNAS). ICES Scientific Reports. https://www.ices.dk/sites/pub/Publication%20Reports/Forms/DispForm.aspx?ID=36572. Accessed 5 Mar 2021
International Council for the Exploration of the Sea (ICES) (2020c) Herring (Clupea harengus) in Subarea 4 and divisions 3.a and 7.d, autumn spawners (North Sea, Skagerrak and Kattegat, eastern English Channel). In: Report of the ICES Advisory Committee, 2020. ICES Advice 2020, her.27.3a47d. https://doi.org/10.17895/ices.advice.4716. Accessed 5 Mar 2021
Intergovernmental Panel on Climate Change (IPCC) (2014) Climate change 2014: synthesis report. Contribution of working groups I, II and III to the fifth assessment report of the Intergovernmental Panel on Climate Change. In: Pachauri RK, Meyer LA (eds) IPCC. Switzerland, Geneva, p 151
Intergovernmental Panel on Climate Change (IPCC) 2018 Global warming of 1.5 °C. An IPCC special report on the impacts of global warming of 1.5 °C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. Masson-Delmotte V, Zhai P, Pörtner H-O , Roberts D, Skea J, Shukla PR et al (eds) World Meteorological Organization, Geneva p 32
Jackson D, Cotter D, Newell J, McEvoy S, O’Donohoe P, Kane F, McDermott T, Kelly S, Drumm A (2013) Impact of Lepeophtheirus salmonis infestations on migrating Atlantic salmon, Salmo salar L., smolts at eight locations in Ireland with an analysis of lice-induced marine mortality. J Fish Dis 36(3):273–281
Jacobsen JA, Hansen LP (2000) Feeding habits of Atlantic salmon at different life stages at sea. In: Mills D (ed) The ocean life of Atlantic salmon: environmental and biological factors influencing survival. Fishing news books, Oxford, pp 170–192
Jakubowska M, Greszkiewicz M, Fey DP, Otremba Z, Urban-Malinga B, Andrulewicz E (2021) Effects of magnetic fields related to submarine power cables on the behaviour of larval rainbow trout (Oncorhynchus mykiss). Mar Freshw Res 72:1196–1207
Jensen AJ, Karlsson S, Fiske P, Hansen LP, Østborg GM, Hindar K (2014) Origin and life history of Atlantic salmon (Salmo salar) near their northernmost oceanic limit. Can J Fish Aquat Sci 71:1740–1746
Jepsen N, Flávio H, Koed A (2019) The impact of cormorant predation on Atlantic salmon and sea trout smolt survival. Fish Manag Ecol 26:183–186
Jepsen N, Holthe E, Økland F (2006) Observations of predation on salmon and trout smolts in a river mouth. Fish Manag Ecol 3(5):341–343
Jobling M (1981) Temperature preference and thermal preferendum—Rapid methods for assessing optimum growth temperatures. J Fish Biol 19:439–455
Johnson LL, Ylitalo GM, Arkoosh MR, Kagley AN, Stafford C, Bolton JL, Buzitis J, Anulacion BF, Collier TK (2007) Contaminant exposure in outmigrant juvenile salmon from Pacific Northwest estuaries of the United States. Environ Monit Assess 124:167–194
Joint Nature Conservation Committee (JNCC) (2010) Statutory nature conservation agency protocol for minimising the risk of injury to marine mammals from piling noise. https://hub.jncc.gov.uk/assets/31662b6a-19ed-4918-9fab-8fbcff752046. Accessed 10 June 2020
Jones PD (2006) Water quality and fisheries in the Mersey estuary, England: a historical perspective. Mar Pollut Bull 53:144–154
Jonsson B, Jonsson N (2004) Factors affecting marine production of Atlantic salmon (Salmo salar). Can J Fish Aquat Sci 61:2369–2383
Jonsson B, Jonsson N (2009) A review of the likely effects of climate change on anadromous Atlantic salmon Salmo salar and brown trout Salmo trutta, with particular reference to water temperature and flow. J Fish Biol 75(10):2381–2447
Jonsson B, Jonsson N, Albretsen J (2016) Environmental change influences the life history of salmon Salmo salar in the North Atlantic Ocean. J Fish Biol 88(2):618–637
Jonsson N, Jonsson B, Hansen LP (1998) The relative role of density-dependent and density-independent survival in the life-cycle of Atlantic salmon Salmo salar. J Anim Ecol 67:751–762
Juanes F, Gephard S, Beland KF (2004) Long-term changes in migration timing of adult Atlantic salmon (Salmo salar) at the southern edge of the species distribution. Can J Fish Aquat Sci 61:2392–2400
Juell JE, Oppedal F, Boxaspen K, Taranger GL (2003) Submerged light increases swimming depth and reduces fish density of Atlantic salmon Salmo salar L. in production cages. Aquacult Res 34:469–477
Karlsson S, Diserud OH, Fiske P, Hindar K (2016) Widespread genetic introgression of escaped farmed Atlantic salmon in wild salmon populations. ICES J Mar Sci 73(10):2488–2498
Kazako RV, Khalyapina LM (1981) Oxygen consumption of adult Atlantic salmon (Salmo salar L.) males and females in fish culture. Aquaculture 25(2–3):289–292
Kemp P, Sear D, Collins A, Naden P, Jones I (2011) The impacts of fine sediment on riverine fish. Hydrol Process 25:1800–1821
Kennedy BP, Nislow KH, Folt CL (2008) Habitat-mediated foraging limitations drive survival bottlenecks for juvenile salmon. Ecology 89(9):2529–2541
Kennedy GJA, Greer JE (1988) Predation by cormorants, Phalacrocorax carbo (L.), on the salmonid populations of an Irish river. Aquac Fish Manage 19(2):159–170
Kennedy RJ, Crozier WW (2010) Evidence of changing migratory patterns of wild Atlantic salmon Salmo salar smolts in the River Bush, Northern Ireland, and possible associations with climate change. J Fish Biol 76:1786–1805
Kennish MJ (2002) Environmental threats and environmental future of estuaries. Environ Conserv 29:78–107
Kidd IM, Fischer A, Chai S, Davis JA (2015) A scenario-based approach to evaluating potential environmental impacts following a tidal barrage installation. Ocean Coast Manag 116:9–19
King TL, Verspoor E, Spidle AP, Gross R, Phillips RB, Koljonen M-L, Sanchez JA, Morrison CL (2007) Biodiversity and population structure. In: Verspoor E, Stradmeyer L, Nielsen JL (eds) The Atlantic salmon: genetics, conservation and management. Blackwell Publishing Ltd, Oxford, pp 117–166
Kjelland ME, Woodley CM, Swannack TM, Smith DL (2015) A review of the potential effects of suspended sediment on fishes: potential dredging-related physiological, behavioral, and transgenerational implications. Environ Syst Decis 35:334–350
Klemetsen A, Amundsen PA, Dempson JB, Jonsson B, Jonsson N, O’Connell MF, Mortensen E (2003) Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.): a review of aspects of their life histories. Ecol Freshw Fish 12:1–59
Klimley AP, Wyman MT, Kavet R (2017) Chinook salmon and green sturgeon migrate through San Francisco Estuary despite large distortions in the local magnetic field produced by bridges. PLoS ONE 12(6):e0169031. https://doi.org/10.1371/journal.pone.0169031
Kramer DL (1987) Dissolved oxygen and fish behaviour. Environ Biol Fishes 18:81–92
Krkošek M, Revie CW, Gargan PG, Skilbrei OT, Finstad B, Todd CD (2013) Impact of parasites on salmon recruitment in the Northeast Atlantic Ocean. Proc R Soc B Biol Sci 280:20122359. https://doi.org/10.1098/rspb.2012.2359
Kroglund F, Finstad B (2003) Low concentrations of inorganic monomeric aluminium impair physiological status and marine survival of Atlantic salmon. Aquaculture 222:119–133
Kroglund F, Finstad B, Stefansson SO, Nilsen TO, Kristensen T, Rosseland BO, Teien HC, Salbu B (2007) Exposure to moderate acid water and aluminium reduces Atlantic salmon post-smolt survival. Aquaculture 273:360–373
Kroglund F, Kaste Ø, Rosseland BO, Poppe T (2001) The return of the salmon. Water Air Soil Pollut 130:1349–1354
Lacroix GL (2014) Large pelagic predators could jeopardize the recovery of endangered Atlantic salmon. Can J Fish Aquat Sci 71(3):343–350
Lacroix GL, Knox D (2005) Distribution of Atlantic salmon (Salmo salar) postsmolts of different origins in the Bay of Fundy and Gulf of Maine and evaluation of factors affecting migration, growth, and survival. Can J Fish Aquat Sci 62:1363–1376
Lacroix GL, Knox D, Stokesbury MJW (2005) Survival and behaviour of post-smolt Atlantic salmon in coastal habitat with extreme tides. J Fish Biol 66:485–498
Lekang OI, Salas-Bringas C, Bostock JC (2016) Challenges and emerging technical solutions in on-growing salmon farming. Aquacult Int 24(3):757–766
Lande R, Shannon S (1996) The role of genetic variation in adaptation and population persistence in a changing environment. Evol 50:434–437
Langford TE (1990) Ecological effects of thermal discharges. Elsevier Applied Science, London
Langford TE (2001) Thermal discharges and pollution. Encyclopedia of Oceanic Sciences, New York Academic, pp 2933–2940
Lassalle G, Rochard E (2009) Impact of twenty-first century climate change on diadromous fish spread over Europe, North Africa and the Middle East. Glob Chang Biol 15:1072–1089
Lehnert SJ, Kess T, Bentzen P, Kent MP, Lien S, Gilbey J, Clément M, Jeffery NW, Waples RS, Bradbury IR (2019) Genomic signatures and correlates of widespread population declines in salmon. Nat Commun 10:1–10
Limburg KE, Waldman JR (2009) Dramatic declines in North Atlantic diadromous fishes. Bioscience 59(11):955–965
Lisle TE, Lewis J (1992) Effects of sediment transport on survival of salmonid embryos in a natural stream: a simulation approach. Can J Fish Aquat Sci 49:2337–2344
Lothian AJ, Newton M, Barry J, Walters M, Miller RC, Adams CE (2018) Migration pathways, speed and mortality of Atlantic salmon (Salmo salar) smolts in a Scottish river and the near-shore coastal marine environment. Ecol Freshw Fish 27:549–558
Lowe JA, Bernie D, Bett P, Bricheno L, Brown S, Calvert D, Clark R, Eagle K et al. (2019) UKCP18 Science overview report. November 2018 (Updated March 2019). Met Office UK. https://www.metoffice.gov.uk/pub/data/weather/uk/ukcp18/science-reports/UKCP18-Overview-report.pdf. Accessed 15 August 2019
MacCrimmon HR, Gots BL (1986) Laboratory observations on emergent patterns of juvenile Atlantic salmon, Salmo salar, relative to sediment loadings of test substrate. Can J Zool 64:1331–1336
MacKenzie KM, Trueman CN, Palmer MR, Moore A, Ibbotson AT, Beaumont WR, Davidson IC (2012) Stable isotopes reveal age-dependent trophic level and spatial segregation during adult marine feeding in populations of salmon. ICES J Mar Sci 69:1637–1645
Mainstone CP, Thomas R, Bean CW, Waterman T (2012) The role of the UK conservation agencies in protecting river flows. Fish Manag Ecol 19:557–569
Marine Management Organisation (MMO) (2017) Marine plan areas [shapefile geospatial data]. Scale not given. https://data.gov.uk/dataset/ceecc6a3-297b-4a72-b2ca-d430324b546f/marine-management-organisation-marine-plan-areas. Accessed 1 November 2020. Using: ArcMap GIS. Version 10.5. Redlands, CA: Environmental Systems Research Institute, Inc., 1999–2016. All content is available under the Open Government licence v3.0. © Crown Copyright 2021
Marquiss M, Carss DN, Armstrong JD, Gardiner R (1998) Fish-eating birds and salmonids in Scotland. Report to The Scottish Office of Agriculture, Environment and Fisheries Department. The Scottish Office. https://www.researchgate.net/publication/338037364_Fish_Eating_Birds_and_Salmonids_in_Scotland. Accessed 1 Feb 2019
Mawle GW, Milner NJ (2003) The return of salmon to cleaner rivers - England and Wales. In: Mills D (ed) Salmon at the Edge. Blackwell Science, Oxford, pp 186–199
McCormick SD, Hansen LP, Quinn TP, Saunders RL (1998) Movement, migration, and smolting of Atlantic salmon (Salmo salar). Can J Fish Aquat Sci 55(S1):77–92
McCormick SD, Lerner DT, Monette MY, Nieves-Puigdoller K, Kelly JT, Björnsson BT (2009) Taking it with you when you go: how perturbations to the freshwater environment, including temperature, dams and contaminants, affect marine survival of salmon. Am Fish Soc Symp 69:195–214
McCormick SD, Lerner DT, Regish AM, O’Dea MF, Monette MY (2012) Thresholds for short-term acid and aluminium impacts on Atlantic salmon smolts. Aquaculture 362:224–231
McDowall RM (1999) Different kinds of diadromy: different kinds of conservation problems. ICES J Mar Sci 56:410–413
McGinnity P, Jennings E, DeEyto E, Allott N, Samuelsson P, Rogan G, Whelan K, Cross T (2009) Impact of naturally spawning captive-bred Atlantic salmon on wild populations: depressed recruitment and increased risk of climate-mediated extinction. Proc R Soc B Biol Sci 276:3601–3610
McGinnity P, Prodöhl P, Ferguson A, Hynes R, Maoiléidigh NO, Baker N, Cotter D, O’Hea B, Cooke D, Rogan G, Taggart J (2003) Fitness reduction and potential extinction of wild populations of Atlantic salmon, Salmo salar, as a result of interactions with escaped farm salmon. Proc R Soc B Biol Sci 270(1532):2443–2450
Merchant ND, Brookes KL, Faulkner RC, Bicknell AW, Godley BJ, Witt MJ (2016) Underwater noise levels in UK waters. Sci Rep 6:36942. https://doi.org/10.1038/srep36942
Migaud H, Cowan M, Taylor J, Ferguson HW (2007) The effect of spectral composition and light intensity on melatonin, stress and retinal damage in post-smolt Atlantic salmon, Salmo salar. Aquaculture 270:390–404
Millane M, Walsh L, Roche WK, Gargan PG (2019) Unprecedented widespread occurrence of Pink Salmon Oncorhynchus gorbuscha in Ireland in 2017. J Fish Biol 95(2):651–654
Mills D (2000) The ocean life of Atlantic salmon: environmental and biological factors influencing survival. Fishing news books. Blackwell Science, Oxford
Mills KE, Pershing AJ, Sheehan TF, Mountain D (2013) Climate and ecosystem linkages explain widespread declines in North American Atlantic salmon populations. Glob Chang Biol 19:3046–3061
Milner NJ, Elliott JM, Armstrong JD, Gardiner R, Welton JS, Ladle M (2003) The natural control of salmon and trout populations in streams. Fish Res 62:111–125
Milner NJ, Evans R (2003) The incidence of escaped Irish farmed salmon in English and Welsh rivers. Fish Manag Ecol 10:403–406
Milner N, Russell I, Aprahamian M, Inverarity R, Shelley J, Rippon P (2008) The role of stocking in the recovery of the River Tyne salmon fishery. Environment Agency Fisheries Technical Report No. 2004/1
Minkoff D, Putman NF, Atema J, Ardren WR (2020) Nonanadromous and anadromous Atlantic salmon differ in orientation responses to magnetic displacements. Can J Fish Aquat Sci 77(11):1846–1852
Mo TA, Thorstad EB, Sandlund OT, Berntsen HH, Fiske P, Uglem I (2018) The pink salmon invasion: a Norwegian perspective. J Fish Biol 93(1):5–7
Mobley KB, Granroth-Wilding H, Ellmen M, Orell P, Erkinaro J, Primmer CR (2020) Time spent in distinct life history stages has sex-specific effects on reproductive fitness in wild Atlantic salmon. Mol Eco 29(6):1173–1184
Molnar JL, Gamboa RL, Revenga C, Spalding MD (2008) Assessing the global threat of invasive species to marine biodiversity. Front Ecol Environ 6(9):485–492
Mooney HA, Cleland EE (2001) The evolutionary impact of invasive species. Proc Natl Acad Sci USA 98(10):5446–5451
Moore A, Bendall B (2011) River Tyne crossing salmon and sea trout tracking programme. Cefas Report, p 62
Moore A, Cotter D, Rogan G, Quayle V, Lower N, Privitera L (2008) The impact of a pesticide on the physiology and behaviour of hatchery reared salmon smolts during the transition from the freshwater to marine environment. Fish Manag Ecol 15:339–345
Moore A, Freake SM, Thomas IM (1990) Magnetic particles in the lateral line of the Atlantic salmon (Salmo salar L.). Philos Trans R Soc B 329:11–15
Moore A, Lower N, Mayer I, Greenwood L (2007) The impact of a pesticide on migratory activity and olfactory function in Atlantic salmon (Salmo salar L.) smolts. Aquaculture 273:350–359
Moore A, Potter ECE (2014) Provisional assessment of the River Tees barrage fish passage. Centre for Environment, Fisheries and Aquaculture Science, Lowestoft, UK. https://canalrivertrust.org.uk/media/library/7183-cefas-report-river-tees.pdf. Accessed 10 August 2019
Moore A, Scott AP, Lower N, Katsiadaki I, Greenwood L (2003) The effects of 4-nonylphenol and atrazine on Atlantic salmon (Salmo salar L) smolts. Aquaculture 222:253–263
Moore A, Stonehewer RO, Kell LT, Challiss MJ, Ives M, Russell IC, Riley WD, Mee DM (1996) The movements of emigrating salmonid smolts in relation to the Tawe Barrage, Swansea. In: Burt N, Watts J (eds) Barrages: engineering design and environmental impacts. International Conference, 10–13/09/1996 Cardiff, UK. John Wiley and Sons Ltd, UK
Mork KA, Gilbey J, Hansen LP, Jensen AJ, Jacobsen JA, Holm M, Holst JC, Maoiléidigh ÓN, Vikebø F, McGinnity P, Melle W (2012) Modelling the migration of post-smolt Atlantic salmon (Salmo salar) in the Northeast Atlantic. ICES J Mar Sci 69:1616–1624
Nedwell J, Langworthy J, Howell D (2003) Assessment of sub-sea acoustic noise and vibration from offshore wind turbines and its impact on marine wildlife; initial measurements of underwater noise during construction of offshore windfarms, and comparison with background noise. Subacoustech Report Ref: 544R0423. Subacoustech Ltd, p 62. http://users.ece.utexas.edu/~ling/2A_EU1.pdf. Accessed 12 May 2021
Newcombe CP, Jensen JO (1996) Channel suspended sediment and fisheries: a synthesis for quantitative assessment of risk and impact. N Am J Fish Manag 16:693–727
Nicola GG, Elvira B, Jonsson B, Ayllón D, Almodóvar A (2018) Local and global climatic drivers of Atlantic salmon decline in southern Europe. Fish Res 198:78–85
North Atlantic Salmon Conservation Organisation (NASCO) (2014) The management approach to the West Greenland salmon fishery – Fairness and balance in the management of distant-water fisheries. NASCO. https://nasco.int/wp-content/uploads/2020/02/CNL_14_44.pdf. Accessed 1 August 2020
North Atlantic Salmon Conservation Organisation (NASCO) (2016) Addressing impacts of salmon farming on wild Atlantic salmon: Challenges to, and developments supporting, achievement of NASCO’s international goals. Report of a Theme-based Special Session of the Council of NASCO. Document No. CNL(16)60. 196 pp. https://nasco.int/wp-content/uploads/2020/02/2016ThemeBasedSession.pdf. Accessed 12 October 2021
North Atlantic Salmon Conservation Organisation (NASCO) (2020) NASCO Implementation Plan for Atlantic Salmon Management in the Faroe Islands. Ad Hoc Review Group, IP(07)22 FINAL. Implementation Plan, Denmark (in respect of the Faroe Islands and Greenland)—Faroe Islands, 10 pp. https://nasco.int/wp-content/uploads/2020/02/IP_Faroes.pdf. Accessed 17 May 2021
North Atlantic Salmon Conservation Organisation (NASCO) (2021) Report of the thirty-eighth annual meeting of the Council of the North Atlantic Salmon Conservation Organization. Document No. CNL(21)62. NASCO, Edinburgh 128 pp. https://nasco.int/wp-content/uploads/2021/09/CNL2162_Report-of-the-Thirty-Eighth-Annual-Meeting-of-the-Council.pdf. Accessed 12 October 2021
Norwegian Institute for Natural Research (NINA) (2021) Pink salmon. https://www.nina.no/english/Biodiversity/Alien-Species/Pink-salmon. Accessed 12 November 2021
Norwegian Ministry of Trade, Industry and Fisheries (2015) Meld. St. 16 (2014–2015) Forutsigbar og miljømessig bærekraftig vekst i norsk lakse- og ørretoppdrett. https://www.regjeringen.no/no/dokumenter/meld.-st.-16-2014-2015/id2401865/. Accessed 29 April 2021
Nuttall PM, Purves JB (1974) Numerical indices applied to the results of a survey of the macro-invertebrate fauna of the Tamar catchment (southwest England). Freshw Biol 4(3):213–222
O’Connor JJ, Fobert EK, Besson M, Jacob H, Lecchini D (2019) Live fast, die young: behavioural and physiological impacts of light pollution on a marine fish during larval recruitment. Mar Pollut Bull 146:908–914
Öhman MC, Sigray P, Westerberg H (2007) Offshore windmills and the effects of electromagnetic fields on fish. Ambio 36:630–633
Olmos M, Massiot-Granier F, Prévost E, Chaput G, Bradbury IR, Nevoux M, Rivot E (2019) Evidence for spatial coherence in time trends of marine life history traits of Atlantic salmon in the North Atlantic. Fish Fish 20(2):322–342
Olmos M, Payne MR, Nevoux M, Prévost E, Chaput G, Du Pontavice H, Guitton J, Sheehan T, Mills K, Rivot E (2020) Spatial synchrony in the response of a long range migratory species (Salmo salar) to climate change in the North Atlantic Ocean. Glob Chang Biol 26:1319–1337
Ordnance Survey (2021a) Boundary-Line [shapefile geospatial data]. Scale 1:10 000. https://www.ordnancesurvey.co.uk/business-government/products/boundaryline. Accessed 1 August 2020. Using: ArcMap GIS. Version 10.5. Redlands, CA: Environmental Systems Research Institute, Inc., 1999–2016. All content is available under the Open Government licence v3.0. © Crown Copyright 2021a
Ordnance Survey (2021b) GB Overview Maps [shapefile geospatial data]. Scale 1:5,000,000. https://www.ordnancesurvey.co.uk/business-government/products/gb-overview. Accessed 1 August 2020. Using: ArcMap GIS. Version 10.5. Redlands, CA: Environmental Systems Research Institute, Inc., 1999–2016. All content is available under the Open Government licence v3.0. © Crown Copyright 2021b
OSPAR (2015) 2015 update of the inventory of oil and gas offshore installations in the OSPAR maritime area. OSPAR Commission. https://oap.ospar.org/en/ospar-assessments/committee-assessments/offshore-industry/offshore-installations/2015-update-inventory-offshore-installations/ Accessed 1 July 2019
Otero J, Jensen AJ, L’Abée-Lund JH, Stenseth NC, Storvik GO, Vøllestad LA (2012) Contemporary ocean warming and freshwater conditions are related to later sea age at maturity in Atlantic salmon spawning in Norwegian rivers. Ecol Evol 2:2192–2203
Otero J, L’Abée-Lund JH, Castro-Santos T, Leonardsson K, Storvik GO, Jonsson B, Dempson B, Russell IC, Jensen AJ, Baglinière JL, Dionne M (2014) Basin-scale phenology and effects of climate variability on global timing of initial seaward migration of Atlantic salmon (Salmo salar). Glob Chang Biol 20:61–75
Ou M, Hamilton TJ, Eom J, Lyall EM, Gallup J, Jiang A, Lee J, Close DA, Yun SS, Brauner CJ (2015) Responses of pink salmon to CO2-induced aquatic acidification. Nat Clim 5(10):950–955
Pankhurst NW, King HR (2010) Temperature and salmonid reproduction: implications for aquaculture. J Fish Biol 76:69–85
Parrish DL, Behnke RJ, Gephard SR, McCormick SD, Reeves GH (1998) Why aren’t there more Atlantic salmon (Salmo salar)? Can J Fish Aquat Sci 55:281–287
Pawson MG, Eaton DR (1999) The influence of a power station on the survival of juvenile sea bass in an estuarine nursery area. J Fish Biol 54(3):1143–1160
Peyronnet A, Friedland KD, Maoileidigh NO, Manning M, Poole WR (2007) Links between patterns of marine growth and survival of Atlantic salmon Salmo salar, L. J Fish Biol 71:684–700
Pinder AC, Riley WD, Ibbotson AT, Beaumont WRC (2007) Evidence for an autumn downstream migration and the subsequent estuarine residence of 0+ year juvenile Atlantic salmon Salmo salar L., in England. J Fish Biol 71:260–264
Popper AN, Fay RR (1999) The auditory periphery in fishes. Comparative hearing: fish and amphibians. Springer, New York, pp 43–100
Popper AN, Hawkins AD, Thomsen F (2020) Taking the animals’ perspective regarding anthropogenic underwater sound. Trends Ecol Evol 35:787–794
Potter ECE, Crozier WW (2000) A perspective on the marine survival of Atlantic salmon. In: Mills D (ed) The ocean life of Atlantic salmon: environmental and biological factors influencing survival. Fishing News Books, Oxford, pp 19–36
Priede IG, Solbe JF, Nott JE, O’Grady KT, Cragg-Hine D (1988) Behaviour of adult Atlantic salmon, Salmo salar L., in the estuary of the river Ribble in relation to variations in dissolved oxygen and tidal flow. J Fish Biol 33:133–139
Pulgar J, Zeballos D, Vargas J, Aldana M, Manriquez PH, Manriquez K, Quijón PA, Widdicombe S, Anguita C, Quintanilla D, Duarte C (2019) Endogenous cycles, activity patterns and energy expenditure of an intertidal fish is modified by artificial light pollution at night (ALAN). Environ Pollut 244:361–366
Putland RL, Montgomery JC, Radford CA (2019) Ecology of fish hearing. J Fish Biol 95:39–52
Putman NF, Lohmann KJ, Putman EM, Quinn TP, Klimley AP, Noakes DL (2013) Evidence for geomagnetic imprinting as a homing mechanism in Pacific salmon. Curr Biol 23(4):312–316
Ramírez F, Coll M, Navarro J, Bustamante J, Green AJ (2018) Spatial congruence between multiple stressors in the Mediterranean Sea may reduce its resilience to climate impacts. Sci Rep 8:14871. https://doi.org/10.1038/s41598-018-33237-w
Richardson NE, McCleave JD, Albert EH (1976) Effect of extremely low frequency electric and magnetic fields on locomotor activity rhythms of Atlantic salmon (Salmo salar) and American eels (Anguilla rostrata). Environ Pollut 10:65–76
Rikardsen AH, Dempson JB (2011) Dietary life-support: the food and feeding of Atlantic salmon at sea. In: Aas Ø, Einum S, Klemetsen A, Skurdal J (eds) Atlantic salmon ecology. Blackwell Publishing Ltd, Oxford, pp 115–143
Riley WD, Bendall B, Ives MJ, Edmonds NJ, Maxwell DL (2012) Street lighting disrupts the diel migratory pattern of wild Atlantic salmon, Salmo salar L., smolts leaving their natal stream. Aquaculture 330–333:74–81
Riley WD, Davison PI, Maxwell DL, Bendall B (2013) Street lighting delays and disrupts the dispersal of Atlantic salmon (Salmo salar) fry. Biol Conserv 158:140–146
Riley WD, Davison PI, Maxwell DL, Newman RC, Ives MJ (2015) A laboratory study to determine the dispersal response of Atlantic salmon (Salmo salar) fry to street light intensity. Freshw Biol 60:1016–1028
Riley WD, Ibbotson AT, Beaumont WRC (2009) Adult returns from Atlantic salmon, Salmo salar, parr autumn migrants. Fish Manag Ecol 16:75–76
Riley WD, Ibbotson AT, Beaumont WR, Pawson MG, Cook AC, Davison PI (2011) Predation of the juvenile stages of diadromous fish by sea bass (Dicentrarchus labrax) in the tidal reaches of an English chalk stream. Aquat Conserv Mar Freshw Ecosyst 21(3):307–312
Riley WD, Potter EC, Biggs J, Collins AL, Jarvie HP, Jones JI, Kelly-Quinn M, Ormerod SJ, Sear DA, Wilby RL, Broadmeadow S (2018) Small water bodies in Great Britain and Ireland: ecosystem function, human-generated degradation, and options for restorative action. Sci Total Environ 645:1598–1616
Robertson MJ, Scruton DA, Clarke KD (2007) Seasonal effects of suspended sediment on the behavior of juvenile Atlantic salmon. Trans Am Fish Soc 136:822–828
Roche RC, Walker-Springett K, Robins PE, Jones J, Veneruso G, Whitton TA, Piano M, Ward SL, Duce CE, Waggitt JJ, Walker-Springett GR, (2016) Research priorities for assessing potential impacts of emerging marine renewable energy technologies: insights from developments in Wales (UK). Renew Energy 99:1327–1341
Rose GA (2005) On distributional responses of North Atlantic fish to climate change. ICES J Mar Sci 62:1360–1374
Rosten C, Horsfield R, Anderson K, Turnpenny A (2010) Influences of environmental variables and stocking on Atlantic salmon upstream migrations in the River Thames, UK. In: Kemp P (ed) Salmonid fisheries: freshwater habitat management. Blackwell Publishing Ltd, Oxford, pp 296–306
Ruggerone GT (1986) Consumption of migrating juvenile salmonids by gulls foraging below a Columbia River dam. Trans Am Fish Soc 115:736–742
Russell IC, Aprahamian MW, Barry J, Davidson IC, Fiske P, Ibbotson AT, Kennedy RJ, Maclean JC, Moore A, Otero J, Potter T (2012a) The influence of the freshwater environment and the biological characteristics of Atlantic salmon smolts on their subsequent marine survival. ICES J Mar Sci 69(9):1563–1573
Russell IC, Broughton B, Keller T, Carss DN (2012b) The INTERCAFE cormorant management toolbox: methods for reducing cormorant problems at European fisheries. Report for EU under COST Action (635)—INTERCAFE - “Conserving biodiversity: interdisciplinary initiative to reduce pan-European cormorant-fishery conflicts”. https://www.researchgate.net/publication/265783908_The_INTERCAFE_Cormorant_Management_Toolbox_methods_for_reducing_Cormorant_problems_at_European_fisheries. Accessed 20 March 2021
Russell IC, Dare PJ, Eaton DR, Armstrong JD (1996) Assessment of the problem of fish-eating birds in inland fisheries in England and Wales. Report to the Ministry of Agriculture, Fisheries and Food (MAFF project VC0104). Directorate of Fisheries Research, Lowestoft
Russell IC, Moore A, Ives S, Kell LT, Ives MJ, Stonehewer RO (1998) The migratory behaviour of juvenile and adult salmonids in relation to an estuarine barrage. Hydrobiologia 371/372:321–334
Sandlund OT, Berntsen HH, Fiske P, Kuusela J, Muladal R, Niemelä E, Uglem I, Forseth T, Mo TA, Thorstad EB, Veselov AE (2019) Pink salmon in Norway: the reluctant invader. Biol Invasions 21:1033–1054
Saltveit SJ, Brittain JE, Bremnes T, Brabrand Å, Bækken T (2014) The return of Atlantic Salmon (Salmo salar L.) and improved water quality in urban rivers in Oslo. Norway River Res Appl 30(5):571–577
Sarkar DK (2015) Thermal power plant—Design and operation. Elsevier, Amsterdam
Saunders RL, Sprague JB (1967) Effects of copper-zinc mining pollution on a spawning migration of Atlantic salmon. Water Res 1:419–432
Scanlan MM, Putman NF, Pollock AM, Noakes DL (2018) Magnetic map in nonanadromous Atlantic salmon. Proc Natl Acad Sci USA 115:10995–10999
Scheffer M, Barrett S, Carpenter SR, Folke C, Green AJ, Holmgren M, Hughes TP, Kosten S, Van de Leemput IA, Nepstad DC, van Nes EH (2015) Creating a safe operating space for iconic ecosystems. Science 347(6228):1317–1319
Scotland Food and Drink (2016) Aquaculture Growth to 2030: A strategic plan for farming Scotland's seas. https://www.scotlandfoodanddrink.org/resources/sector-strategies/aquaculture-growth-to-2030-a-strategic-plan-for-farming-scotland-s-seas/. Accessed 29 April 2021
Serra-Llinares RM, Bjørn PA, Finstad B, Nilsen R, Harbitz A, Berg M, Asplin L (2014) Sea lice infection on wild salmonids in marine protected areas: an evaluation of the Norwegian “National Salmon Fjords.” Aquac Environ Interact 5:1–16
Shelton RGJ, Turrell WR, Macdonald A, McLaren IS, Nicoll NT (1997) Records of post-smolt Atlantic salmon, Salmo salar L., in the Faroe-Shetland Channel in June 1996. Fish Res 31:159–162
Shephard S, Gargan P (2020) Wild Atlantic salmon exposed to sea lice from aquaculture show reduced marine survival and modified response to ocean climate. ICES J Mar Sci 78:368–376
Simmons OM, Gregory SD, Gillingham PK, Riley WD, Scott LJ, Britton JR (2021) Biological and environmental influences on the migration phenology of Atlantic salmon Salmo salar smolts in a chalk stream in southern England. Freshw Biol 66:1581–1594
Sitharam TG, Yang S-Q, Falconer R, Sivakumar M, Jones B, Kolathayar S, Sinpoh L (2020) Sustainable water resource development using coastal reservoirs, 1st edn. Butterworth-Heinemann, Oxford
Skilbrei OT, Finstad B, Urdal K, Bakke G, Kroglund F, Strand R (2013) Impact of early salmon louse, Lepeophtheirus salmonis, infestation and differences in survival and marine growth of sea-ranched Atlantic salmon, Salmo salar L., smolts 1997–2009. J Fish Dis 36:249–260
Smialek N, Pander J, Geist J (2021) Environmental threats and conservation implications for Atlantic salmon and brown trout during their critical freshwater phases of spawning, egg development and juvenile emergence. Fish Manag Ecol 28(5):437–467
Sobocinski KL, Greene CM, Schmidt MW (2018) Using a qualitative model to explore the impacts of ecosystem and anthropogenic drivers upon declining marine survival in Pacific salmon. Environ Conserv 45(3):278–290
Solomon DJ, Sambrook HT (2004) Effects of hot dry summers on the loss of Atlantic salmon, Salmo salar, from estuaries in South West England. Fish Manag Ecol 11(5):353–363
Soto DX, Trueman CN, Samways KM, Dadswell MJ, Cunjak RA (2018) Ocean warming cannot explain synchronous declines in North American Atlantic salmon populations. Mar Ecol Prog Ser 601:203–213
Strøm JF, Rikardsen AH, Campana SE, Righton D, Carr J, Aarestrup K, Stokesbury MJW, Gargan P, Javierre PC, Thorstad EB (2019) Ocean predation and mortality of adult Atlantic salmon. Sci Rep 9:7890. https://doi.org/10.1038/s41598-019-44041-5
Strøm JF, Thorstad EB, Hedger RD, Rikardsen AH (2018) Revealing the full ocean migration of individual Atlantic salmon. Anim Biotelem 6:2. https://doi.org/10.1186/s40317-018-0146-2
Summers DW (1995) Long-term changes in the sea-age at maturity and seasonal time of return of salmon, Salmo salar L., to Scottish rivers. Fish Manag Ecol 2(2):147–156
Sumner K (2015) Review of protection measures for Atlantic salmon and sea trout in inshore waters. Environment Agency Evidence Report. https://secure.toolkitfiles.co.uk/clients/25364/sitedata/files/Net-Environment-Agency-Paper.pdf. Accessed 1 May 2019
Suttle KB, Power ME, Levine JM, McNeely C (2004) How fine sediment in riverbeds impairs growth and survival of juvenile salmonids. Ecol Appl 14:969–974
Taranger GL, Hansen T (1993) Ovulation and egg survival following exposure of Atlantic salmon, Salmo salar L., broodstock to different water temperatures. Aquac Fish Manage 24:151–156
Teichert N, Borja A, Chust G, Uriarte A, Lepage M (2016) Restoring fish ecological quality in estuaries: Implication of interactive and cumulative effects among anthropogenic stressors. Sci Total Environ 542:383–393
Tett P, Benjamins S, Coulson M, Davidson K, Fernandes T, Fox C, Hicks N, Hunter D-C, Nickell T, Risch D, Tocher D, Vespoor E, Wilson B, Wittich A, Hart M, Vare L (2018) Review of the environmental impacts of salmon farming in Scotland. Report for the Environment, Climate Change and Land Reform (ECCLR) Committee. The Scottish Parliament, p 196. https://archive2021.parliament.scot/S5_Environment/General%20Documents/20180125_SAMS_Review_of_Environmental_Impact_of_Salmon_Farming_-_Report.pdf. Accessed 27 Oct 2021
Thorstad EB, Bliss D, Breau C, Damon-Randall K, Sundt-Hansen LE, Hatfield EMC, Horsburgh G, Hansen H, Maoiléidigh ÓN, Sheehan T, Sutton SG (2021) Atlantic salmon in a rapidly changing environment - Facing the challenges of reduced marine survival and climate change. Aquat Conserv Mar Freshw Ecosyst 31(9):2654–2665
Thorstad EB, Økland F, Aarestrup K, Heggberget TG (2008) Factors affecting the within-river spawning migration of Atlantic salmon, with emphasis on human impacts. Rev Fish Biol Fish 18(4):345–371
Thorstad EB, Uglem I, Finstad B, Chittenden CM, Nilsen R, Økland F, Bjørn PA (2012b) Stocking location and predation by marine fishes affect survival of hatchery-reared Atlantic salmon smolts. Fish Manag Ecol 19:400–409
Thorstad EB, Uglem I, Finstad B, Kroglund F, Einarsdottir IE, Kristensen T, Diserud O, Arechavala-Lopez P, Mayer I, Moore A, Nilsen R (2013) Reduced marine survival of hatchery-reared Atlantic salmon post-smolts exposed to aluminium and moderate acidification in freshwater. Estuar Coast Shelf Sci 124:34–43
Thorstad EB, Whoriskey F, Uglem I, Moore A, Rikardsen AH, Finstad B (2012a) A critical life stage of the Atlantic salmon Salmo salar: behaviour and survival during the smolt and initial post-smolt migration. J Fish Biol 81:500–542
Tidbury H, Taylor N, Copp G, Garancho E, Stebbing P (2014) Introduction of Marine Non-Indigenous Species into Great Britain and Ireland: Hotspots of Introduction and the Merit of Risk Based Monitoring. Cefas contract report C5955 (Objective 1). Centre for Environment, Fisheries & Aquaculture Science. http://randd.defra.gov.uk/Document.aspx?Document=13891_ME5215Objective1-Hotspots.pdf. Accessed 10 February 2019
Tiselius P, Møller LF (2017) Community cascades in a marine pelagic food web controlled by the non-visual apex predator Mnemiopsis leidyi. J Plankton Res 39(2):271–279
Todd CD, Friedland KD, MacLean JC, Hazon N, Jensen AJ (2011) Getting into hot water? Atlantic salmon responses to climate change in freshwater and marine environments. In: Aas Ø, Klemetsen A, Einum S, Skurdal J (eds) Atlantic salmon ecology. Blackwell Publishing Ltd, Oxford, pp 409–443
Todd CD, Hughes SL, Marshall CT, Maclean JC, Lonergan ME, Biuw EM (2008) Detrimental effects of recent ocean surface warming on growth condition of Atlantic salmon. Glob Chang Biol 14:958–970
Toft JD, Munsch SH, Cordell JR, Siitari K, Hare VC, Holycross BM, DeBruyckere LA, Greene CM, Hughes BB (2018) Impact of multiple stressors on juvenile fish in estuaries of the northeast Pacific. Glob Chang Biol 24(5):2008–2020
Torrissen O, Jones S, Asche F, Guttormsen A, Skilbrei OT, Nilsen F, Horsberg TE, Jackson D (2013) Sea lice - Impact on wild salmonids and salmon aquaculture. J Fish Dis 36:171–194
Tréhin C, Rivot E, Lamireau L, Meslier L, Besnard AL, Gregory SD, Nevoux M (2021) Growth during the first summer at sea modulates sex-specific maturation schedule in Atlantic salmon. Can J Fish Aquat Sci 78(6):659–669
Turnpenny AWH (1988) Fish impingement at estuarine power stations and its significance to commercial fishing. J Fish Biol 33:103–110
Turnpenny AWH, Coughlan J, Crews P, Bamber R, Rowles P (2010) Cooling water options for the new generation of nuclear power stations in the UK. Environment Agency, Bristol, UK. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/291077/scho0610bsot-e-e.pdf. Accessed 10 May 2021
United Nations Framework Convention on Climate Change (UNFCCC) (2015). Paris Agreement, Paris
University of Gdańsk (2021) Dr Michał Skóra with Marie Skłodowska-Curie actions scholarship. https://ug.edu.pl/news/en/817/dr-michal-skora-marie-sklodowska-curie-actions-scholarship. Accessed 23 May 2021
Utne KR, Thomas K, Jacobsen JA, Fall J, Maoiléidigh ÓNP, Broms C, Melle W (2020) Feeding interactions between Atlantic salmon (Salmo salar L.) post-smolts and other planktivorous fish in the Northeast Atlantic. Can J Fish Aquat Sci 78(3):255–268
Valiente AG, Juanes F, Garcia-Vazquez E (2011) Increasing regional temperatures associated with delays in Atlantic salmon sea-run timing at the southern edge of the European distribution. Trans Am Fish Soc 140:367–373
Vera LM, Davie A, Taylor JF, Migaud H (2010) Differential light intensity and spectral sensitivities of Atlantic salmon, European sea bass and cod pineal glands ex vivo. Gen Comp Endocrinol 165:25–33
Verspoor E, Stradmeyer L, Nielsen JL (2007) The Atlantic salmon: genetics, conservation and management. Blackwell Publishing Ltd, Oxford, p 520
Vikingstad E, Andersson E, Norberg B, Mayer I, Klenke U, Zohar Y, Stefansson SO, Taranger GL (2008) The combined effects of temperature and GnRHa treatment on the final stages of sexual maturation in Atlantic salmon (Salmo salar L.) females. Fish Physiol Biochem 34:289–298
Vollset KW, Dohoo I, Karlsen Ø, Halttunen E, Kvamme BO, Finstad B, Wennevik V, Diserud OH, Bateman A, Friedland KD, Mahlum S (2018) Disentangling the role of sea lice on the marine survival of Atlantic salmon. ICES J Mar Sci 75(1):50–60
Walker AM, Beveridge MC, Crozier W, Maoiléidigh ÓN, Milner N (2006) Monitoring the incidence of escaped farmed Atlantic salmon, Salmo salar L., in rivers and fisheries of the United Kingdom and Ireland: current progress and recommendations for future programmes. ICES J Mar Sci 63(7):1201–1210
Walker MM, Diebel CE, Haugh CV, Pankhurst PM, Montgomery JC, Green CR (1997) Structure and function of the vertebrate magnetic sense. Nature 390:371–376
Walker MM, Kirschvink JL, Dizon AE (1985) Magnetoreception and biomineralization of magnetite in fish. In: Kirschvink JL, Jones WJ, McFadden BJ (eds) Magnetite biomineralization in organisms. Plenum Press, New York, pp 417–497
Ward DM, Hvidsten NA (2011) Predation: compensation and context dependence. In: Aas Ø, Einum S, Klemetsen A, Skurdal J (eds) Atlantic salmon ecology. Wiley-Blackwell, Oxford, pp 199–220
Waring CP, Moore A (2004) The effect of atrazine on Atlantic salmon (Salmo salar) smolts in fresh water and after sea water transfer. Aquat Toxicol 66:93–104
Wells BK, Huff DD, Burke BJ, Brodeur RD, Santora JA, Field JC, Richerson K, Mantua NJ, Fresh KL, McClure MM, Satterthwaite WH (2020) Implementing ecosystem-based management principles in the design of a salmon ocean ecology program. Front Mar Sci 7:342
Williams CR, Dittman AH, McElhany P, Busch DS, Maher MT, Bammler TK, MacDonald JW, Gallagher EP (2019) Elevated CO2 impairs olfactory-mediated neural and behavioral responses and gene expression in ocean-phase coho salmon (Oncorhynchus kisutch). Glob Chang Biol 25(3):963–977
Wilson B, Batty RS, Daunt F, Carter C (2006) Collision risks between marine renewable energy devices and mammals, fish and diving birds: Report to the Scottish Executive. Scottish Association for Marine Science. https://tethys.pnnl.gov/sites/default/files/publications/Wilson-et-al-2007.pdf. Accessed 15 June 2020
Winterwerp JC, Kranenburg C (2002) Fine sediment dynamics in the marine environment In: Proceedings in Marine Science 5. Elsevier, Amsterdam
Wither A, Bamber R, Colclough S, Dyer K, Elliott M, Holmes P, Jenner H, Taylor C, Turnpenny A (2012) Setting new thermal standards for transitional and coastal (TraC) waters. Mar Pollut Bull 64(8):1564–1579
Wolf J, Walkington IA, Holt J, Burrows R (2009) Environmental impacts of tidal power schemes In: Proceedings of the Institution of Civil Engineers – Maritime Engineering 162(4):165–177
Worrall F, McIntyre P (1998) The Wansbeck barrage scheme: twenty-one years of environmental impact. Water Environ J 12:144–149
Wright RF, Couture RM, Christiansen AB, Guerrero JL, Kaste Ø, Barlaup BT (2017) Effects of multiple stresses hydropower, acid deposition and climate change on water chemistry and salmon populations in the River Otra, Norway. Sci Total Environ 574:128–138
Wringe BF, Jeffery NW, Stanley RR, Hamilton LC, Anderson EC, Fleming IA, Grant C, Dempson JB, Veinott G, Duffy SJ, Bradbury IR (2018) Extensive hybridization following a large escape of domesticated Atlantic salmon in the Northwest Atlantic. Commun Biol 1:1–9
Wyman MT, Klimley AP, Battleson RD, Agosta TV, Chapman ED, Haverkamp PJ, Pagel MD, Kavet R (2018) Behavioral responses by migrating juvenile salmonids to a subsea high-voltage DC power cable. Mar Biol 165(8):1–15
Xia J, Falconer RA, Lin B (2010) Impact of different tidal renewable energy projects on the hydrodynamic processes in the Severn Estuary, UK. Ocean Model 32:86–104
Yano A, Ogura M, Sato A, Sakaki Y, Shimizu Y, Baba N, Nagasawa K (1997) Effect of modified magnetic field on the ocean migration of maturing chum salmon, Oncorhynchus keta. Mar Biol 129(3):523–530
Yoon J-D, Kim J-H, Park S-H, Kim E, Jang M-H (2017) Impact of estuary barrage construction on fish assemblages in the lower part of a river and the role of fishways as a passage. Ocean Sci J 52(1):147–164
Acknowledgements
Thanks to the UK Government’s Department for Environment, Food, and Rural Affairs (Defra) for providing funding for this review. Gratitude must be expressed to England’s Environment Agency fisheries officers for providing expert opinion on the stressors impacting individual river stocks in England. The authors are indebted to A. Moore, G. Horsburgh, S. Watts, C. Wicker, G. Copp, P. Rippon, G. Peirson, S. Jennings, R. Faulkner, E. Bell, K. Jeffery, T. Ellis, and A. Gill for providing constructive comments on the development of this manuscript.
Funding
Both Cefas and Environment Agency contributions to this review were provided through core funding (Grant-in-Aid) by the Department for Environment, Food, and Rural Affairs (Defra) to the respective institutions.
Author information
Authors and Affiliations
Contributions
All authors have been involved throughout the project and contributed significantly to the writing. Specifically: Conceived and designed the study: IR, JG, TB; Analysed the data: JG, TB; Wrote the paper: JG, TB, IR, PD, WR, LT, AW.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to report.
Consent to participate
All the authors agree with the contents of the manuscript and give their consent to submit.
Consent for publication
This work is original research carried out by the authors and all of us agree with its submission in the present form to the journal. The manuscript is not currently under consideration in another journal. The consent has also been obtained from the responsible authorities at Cefas, where the work has been carried out (internal QA).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jonathan Gillson and Tea Bašić are joint first authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gillson, J.P., Bašić, T., Davison, P.I. et al. A review of marine stressors impacting Atlantic salmon Salmo salar, with an assessment of the major threats to English stocks. Rev Fish Biol Fisheries 32, 879–919 (2022). https://doi.org/10.1007/s11160-022-09714-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11160-022-09714-x