Abstract
Global biodiversitytargets require us to identify species at risk of extinction and quantify status and trends of biodiversity. The Red List Index (RLI) tracks trends in the conservation status of entire species groups over time by monitoring changes in categories assigned to species. Here, we calculate this index for the world’s fishes in 2010, using a sampled approach to the RLI based on a randomly selected sample of 1,500 species, and also present RLI splits for freshwater and marine systems separately. We further compare specific traits of a worldwide fish list to our sample to assess its representativeness. Overall, 15.1% of species in the sample were estimated to be threatened with extinction, resulting in a sampled RLI of 0.914 for all species, 0.968 in marine and 0.862 in freshwater ecosystems. Our sample showed fishing as the principal threat for marine species, and pollution by agricultural and forestry effluents for freshwater fishes. The sampled list provides a robust representation for tracking trends in the conservation status of the world’s fishes, including disaggregated sampled indices for marine and freshwater fish. Reassessment and backcasting of this index is urgent to check the achievement of the commitments proposed in global biodiversity targets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2020, the UN Decade of Biodiversity came to its culmination, requiring a stocktake of the world’s progress towards the Aichi Targets, set by the Convention on Biological Diversity in 2010. Following our failure to reach the previous 2010 target to achieve a significant reduction in the rate of biodiversity loss by 2010 (Butchart et al. 2010), none of the Aichi Targets were fully met at the global level (Secretariat of the Convention on Biological Diversity 2020). This includes Aichi Target 12 which stipulated that “by 2020, the extinction of known threatened species has been prevented and their conservation status, particularly of those most in decline, has been improved and sustained” (Secretariat of the Convention on Biological Diversity 2020).
Monitoring global extinction risk of biodiversity (Butchart et al. 2004; Baillie et al. 2008) presents one way to track progress towards Aichi Target 12.Threats continue to increase, resulting in declines in the abundance and distribution of species (Tilman et al. 2017; WWF 2020). A recent global assessment of the state of biodiversity by the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES) estimated that up to one million species are at risk of extinction (Ruckelshaus et al. 2020). This estimate relies on inferences from the extinction risk of species assessed by the International Union for Conservation of Nature (IUCN) Red List of Threatened Species, arguably the most comprehensive inventory on species’ extinction risk worldwide (Rodrigues et al. 2006). The IUCN Red List splits the continuous scale of species’ extinction risk into seven categories, ranging from Least Concern (LC) and Near Threatened (NT) to the threatened categories (Vulnerable VU, Endangered EN and Critically Endangered CR) to Extinct in the Wild (EW) and Extinct (EX); an additional category of Data Deficient (DD) is used for species with insufficient data to evaluate their extinction risk (IUCN 2012). Specifically, the IUCN Red List uses a set of five criteria to assign species to one of the three threatened categories (VU, EN, CR). In 2019, the IUCN Red List reached the major milestone of 100,000 species assessed, the culmination of more than ten years of increased investment in species assessments through various targeted projects and a taxonomic and spatial expansion of the IUCN SSC Species Specialist Group network (IUCN 2020).
While the extinction risk of nearly all terrestrial vertebrates has been assessed (Butchart et al. 2004; Hoffmann et al. 2010; Cox et al. 2022) there are still substantial data gaps in other species groups (Collen et al. 2014; Miqueleiz et al. 2020). To address this situation, the sampled Red List Index (sRLI) was devised to expand taxonomic coverage of the IUCN Red List in a way that also allows aggregation of under-represented taxonomic groups into global biodiversity indicators (Baillie et al. 2008). A random sample of 900 non-Data Deficient species (i.e. species with sufficient data to estimate extinction risk) from a highly speciose taxonomic group (i.e. a class or order or family) was originally shown sufficiently large to accurately estimate the direction of trends in extinction risk (e.g. for birds (Baillie et al. 2008)). Subsequently, this approach has been applied to reptiles (Böhm et al. 2013), dragonflies (Clausnitzer et al. 2009), plants (Brummitt et al. 2015) and freshwater molluscs (Böhm et al. 2020). However, while providing a ‘shortcut’ for the inclusion of highly species-rich groups into the RLI, there have so far only been single baseline assessments completed, and no repeated assessments to show temporal trends are so far available (Henriques et al. 2020). Initial sampled assessments for other species groups are still work in progress (e.g., butterflies (Lewis and Senior 2011)). There is an urgent need to deliver the findings of these assessments and devise a future sustainable approach for sRLI reassessments, given that new targets for biodiversity will be set at the 15th Meeting of the Conference of the Parties to the Convention on Biological Diversity in 2022.
Fishes are by far the most species-rich group of vertebrates, with 36,105 species currently recognised (Fricke et al. 2022), approximately the same number of species as that of all non-fish vertebrates combined. Covering freshwater and marine habitats, assessing the status of the world’s fish is of utmost importance to ecosystem function and human wellbeing. For example, marine fish provide a vital income and food source for coastal communities around the world, and are of particular importance in tackling micronutrient deficiencies in humans (Hutchings 2000).
The IUCN Global Species Programme started the Global Marine Species Assessment in 2005, with the target to assess the extinction risk of 20,000 marine species for the IUCN Red List, of which approximately 17,000 are marine fishes. Currently, extinction risk for more than 16,000 marine species has been evaluated9, including reef-building corals (Carpenter et al. 2008), mangroves (Polidoro et al. 2010), seagrasses (Short et al. 2011), sea snakes (Eifes et al. 2013), sea cucumbers (Purcell et al. 2014), and cone snails (Peters et al. 2013). Most assessments have been for fishes (~ 11,700 species (IUCN 2020)), often spearheaded by taxon-specific IUCN Species Survival Commission (SSC) Specialist Groups, including hagfishes (Knapp et al. 2011), tunas and billfishes (Collette et al. 2011), parrotfishes and surgeonfishes (Comeros-Raynal et al. 2012), groupers (Sadovy de Mitcheson et al. 2013), sharks and rays (Dulvy et al. 2014), tarpons, ladyfishes and bonefishes (Adams et al. 2014), anguillid eels (Jacoby et al. 2015), porgies (Comeros-Raynal et al. 2016), and pufferfishes (Stump et al. 2018). Many additional marine fish assessments have come from regional initiatives, further broadening the taxonomic coverage of the Red List (IUCN 2012). These include nearly all nearshore and many deep sea marine fishes from the Mediterranean (Abdul Malak et al. 2011), Eastern Central Pacific (Polidoro et al. 2012), Western Central Atlantic and Gulf of Mexico (Linardich et al. 2019; Strongin et al. 2020), Oceania (Pippard et al. 2017), European waters (Fernandes et al. 2017), Eastern Central Atlantic (Polidoro et al. 2017), and Persian Gulf (Buchanan et al. 2019).
More than 30,000 freshwater species have now been assessed for the IUCN Red List (IUCN 2012), including comprehensive assessments for freshwater crabs, freshwater shrimps and crayfish, and sampled assessments for freshwater molluscs (Cumberlidge et al. 2009; Richman et al. 2015; Böhm et al. 2020). The Freshwater Biodiversity Unit, within the IUCN Global Species Programme, has been central to the assessment effort for freshwater species, including fishes, generally carried out on a region-by-region basis (IUCN 2020). There have been several regional assessments that include freshwater fishes, for instance for the Eastern Himalaya (Allen et al. 2010), Western Ghats (Molur et al. 2011), Africa (Darwall et al. 2011), Europe (Freyhof and Brooks 2011), Indo-Burma (Allen et al. 2012), Madagascar and the Indian Ocean islands (Máiz-Tomé et al. 2018), and Mexico (Contreras-MacBeath et al. 2020), which have started to fill the assessment gap for freshwater fishes, and other freshwater species, on the IUCN Red List. Currently, the IUCN-Toyota Red List Partnership (https://www.iucn.org/theme/species/our-work/iucn-red-list-threatened-species/iucn-toyota-partnership) aims to complete the global comprehensive assessment of freshwater fishes.
Still, only 61% of fish have been assessed by the IUCN Red List (currently just over 20,000 species), compared to 91% of mammals, 100% of birds, 87% of amphibians and 87% of reptiles (IUCN 2021). A sampled assessment of 1,500 fishes was initially completed in 2010, as part of the development of the sRLI as a global biodiversity indicator (Baillie et al. 2008, 2010). Here, we provide the long-overdue presentation and analysis of the results, updated to include the latest knowledge on species status. We pursue two goals: firstly, we present the first global assessment of extinction risk for a random sample of fishes, highlighting major threats impacting fishes in marine and freshwater environments, and put the findings in the context of the conservation status of other non-fish species groups, including others assessed under the sRLI approach. Secondly, despite providing a ‘shortcut’ to achieve RLIs for species-rich groups, the sRLI approach has so far only produced single status points rather than trends over time. Here, we investigate the representativeness of the sRLI sample in terms of taxonomic, biological and ecological traits, to ensure that going forward, the sRLI for fishes presents a robust indicator which adequately reflects the high trait and ecological diversity of this species group. Additionally, we investigate whether the current fish sRLI can form the basis for disaggregated indices for freshwater and marine fish, as a workable solution to standalone indicators without imposing a new workload on already overburdened Red List assessors. This global assessment and definition of a way forward are of particular importance since the sRLI for fishes is now due for reassessment to evaluate the trends in the conservation status of the world’s fishes.
Methods
A sampled Red List Index for the world’s fish
In 2009, a random sample of 1,500 species (the Sampled List) was drawn from Eschmeyer’s Catalog of fishes (Baillie et al. 2010) to evaluate the status and trends of fishes, according to the sRLI protocol (Baillie et al. 2008). A sample size of 900 non-Data Deficient species was considered sufficiently large to detect the correct trend direction in the extinction risk of a group, while a sample of 1,500 species was deemed to be large enough to also account for up to 40% data deficiency in under-studied groups (Baillie et al. 2008). Extinction risk of every species in the sample was assessed according to the IUCN Red List Categories and Criteria (IUCN 2012) through consultation with experts of relevant IUCN SSC Specialist Groups and other species experts. Categories were assigned based on quantitative thresholds relating to population reductions (criterion A), restricted geographic distribution and decline or fragmentation (criterion B), population size and decline (criterion C), extremely small population size or restricted distribution (criterion D) and/or quantitative analyses (criterion E) (IUCN 2012). Initial assessment was completed in 2010.
Several species (167) have been subsequently reassessed as part of other assessment processes (e.g. by the Freshwater Biodiversity Unit or the Global Marine Species Assessment). In reassessments, species may change Red List category due to actual improvements or deteriorations of their conservation status (termed “genuine change”) or due to improved knowledge on the species or changed taxonomy (termed “nongenuine change” (IUCN Standards and Petitions Committee 2019)). We summarised the number of reassessments already carried out, and the number of category changes due to genuine and nongenuine changes, based on the latest status of species on the IUCN Red List v. 2020–1. Since only genuine changes are reflected in the sRLI, nongenuine changes from reassessments have to be accounted for by amending the original erroneous category through a process of “backcasting” (IUCN Standards and Petitions Committee 2019). Here, we integrated improved knowledge from recent reassessments by adopting the reassessed categories for species undergoing nongenuine changes as the 2010 Red List category. A full backcasting of all nongenuine changes will be carried out following a complete reassessment of the Sampled List in the near future.
We calculated proportions of threatened species in our sample by assuming that DD species will fall into threatened categories in the same proportion as non-DD species, as per previous studies on other species groups (Hoffmann et al. 2010; Richman et al. 2015; Böhm et al. 2020):
where N is the total number of species in the sample, CR, EN and VU are the numbers of threatened species, and DD and EX are the numbers of species in the DD and EX categories, respectively. To incorporate uncertainty introduced by DD species, we calculated upper and lower bounds of threat proportions by assuming that (a) no DD species were threatened [lower margin: Propthr = (CR + EN + VU)/(N-EX)], and (b) all DD species were threatened [upper margin; Propthr = (CR + EN + VU + DD)/(N-EX)]. We also estimated the sampled Red List Index in 2010, following the modified formula of Butchart et al. (2007).
where W is the category weight (category weights increase from 0 for LC in equal steps to 5 for EX and EW) for species S at time t; and N is the total number of assessed species, excluding those considered DD. Thus, RLI values can vary from 0 (all species are EX) to 1 (all species are LC). In this way, we produced an sRLI for all fish. A recent re-visit of the sRLI sample size, analysing data for a broader set of species than in the original sRLI paper by Baillie et al. (2008), suggested that 200 to 400 non-DD species are sufficient to accurately detect trend in RLI (Henriques et al. 2020). Thus, we also produced sRLI values for freshwater and marine fish separately, given that for both individually, sample size exceeded 400 non-DD species.
The threats impacting each species were recorded during the Red List assessments, following the IUCN's unified threats classification scheme (Salafsky et al. 2008). We summarised the frequency of threats for threatened (VU, EN, CR) and non-threatened species (LC and NT). We also analysed species population trends, which are recorded as unknown, decreasing, stable and increasing populations on the IUCN Red List (IUCN 2012).
Species distribution was mapped – where possible – for all assessed species for which the distribution could be mapped (n = 1,484). For some species, specifically DD species, distribution data was too uncertain to allow mapping. To visualise the distribution pattern of our Sampled List, we selected only those parts of a species’ distribution map where the species was considered extant or probably extant, resident, and native or reintroduced70, resulting in 1,473 species remaining. We mapped species richness, threatened species richness and DD species richness of our sample by overlaying a grid with 1° grid cells onto the respective aggregated species’ distribution and summing the number of species occurring in each grid cell. We normalised species richness relative to the richest cell to derive a synthetic pattern of species richness ranging from zero (no species present) to one (highest species richness), as described in Collen et al. (2014). We created richness maps for freshwater (n = 714) and marine species (n = 799), separately. All maps were created in R Studio v. 1.2.1335 and R Studio v. 3.6.0 (R Development Core Team 2019).
Taxonomic, ecological, and biological trait data
To obtain a full picture of trait and ecology of the world’s fishes, we extracted information on taxonomy, distribution, preferred habitat and biological traits for the full list of 33,112 fish species from the FishBase online database (Froese and Pauly 2016). The Sampled List was taxonomically matched to FishBase taxonomy (Froese and Pauly 2016). We determined the number of species in each order according to the FishBase taxonomy and obtained the number of species for marine and inland waters per FAO Major Fishing Areas (http://www.fao.org/fishery/area/search/). We extracted the following habitat information (particular habitat preferred by each species, adapted from Holthus and Maragos 1995): pelagic, benthopelagic, demersal, reef-associated, bathypelagic and bathydemersal, according to the glossary of FishBase (Froese and Pauly 2016). We summarised the number of species in each habitat type.
We collected the following biological traits from FishBase (Froese and Pauly 2016): life span, generation time, trophic level and vulnerability index. Life span is the approximate maximum age individuals of a given species are estimated to reach, and generation time is the average age of parents within the cohort. Trophic level is the position of species in the food chain, determined by the number of energy-transfer steps to that level (Froese and Pauly 2016). Trophic levels reported in FishBase are derived from Ecopath (Pauly et al. 2000). The index of intrinsic vulnerability to fisheries presented in FishBase is calculated via an expert system developed for fishes that integrates life history and ecological characteristics (Cheung et al. 2005). These biological traits collected from FishBase are not based on direct observations, but are obtained from algorithmic relationships of other variables (e.g. total length or maximum reported age).
Subsequently, we compared the taxonomic, geographic, ecological, and biological representativeness of the full list of fish against traits of our Sampled List, to assess whether the randomly Sampled List adequately represents taxonomic, spatial, and biological trait diversity of global fishes. Furthermore, we included separate analyses for DD species, testing their representativeness; in addition to showing us whether fishes with any particular traits or from any particular regions are understudied, any patterns of bias in DD species would also be reflected in the non-DD species which make up the sRLI. Full and Sampled lists of fish species were tested for differences in the number of species among taxonomic orders, among FAO areas and among habitat types, using chi-square tests (R Development Core Team 2019). To assess the representativeness of biological traits, we used non-parametric analyses because the normal distribution assumption was not met in these data sets, even after data transformation. First, two-tailed (Wilcoxon) Mann–Whitney U test was used to examine whether the medians of the two samples were different. Second, Kolmogorov–Smirnov tests were used to assess whether the distributions were equal, independently of differences in other descriptive parameters as mean or variance (R Development Core Team 2019). We tested power of the analyses using package pwr package (version 1.3–0) in R.
Results
Status of fishes
In the 1,500-species sample, 178 species were assessed as threatened with extinction in 2010: 105 were Vulnerable, 40 Endangered and 33 Critically Endangered (Fig. 1a). Another 320 species (21.3%) were assessed as Data Deficient in 2010, 961 as Least Concern, 41 as Near Threatened, and no species were assessed as Extinct or Extinct in the Wild. Since 2010, 441 species have been reassessed; of these, 272 retained their Red List category and 169 changed status, 157 due to non-genuine reasons. Of these 157 non-genuine status changes, 54 species changed to a non-Data Deficient category, 18 moved to a Data Deficient category, 40 led to increased extinction risk and 45 to reduced extinction risk. Taking these non-genuine changes into account, the 1,500-species sample contained 177 threatened species (85 Vulnerable, 59 Endangered, 33 Critically Endangered), 39 Near Threatened, 998 Least Concern and 285 Data Deficient species in 2010. One species was listed as Extinct (Barbodes herrei (Fowler 1934)).
Status of the world’s fishes, based on a random sample of 1,500 species: A) IUCN Red List Categories for the full sample, freshwater and marine species; B) Percentage of species assigned via the different IUCN Red List Criteria; C) Red List Indices for birds, mammals, amphibians and corals (source: IUCN), crayfish (Richman et al. 2015), freshwater crabs (Cumberlidge et al. 2009), freshwater shrimps (De Grave et al. 2015), and estimated Red List Indices based on a sampled approach for dragonflies and damselflies (Clausnitzer et al. 2009), reptiles (Böhm et al. 2013), plants (Brummitt et al. 2015) and fishes, distinguishing marine (M) and freshwater (FW) species (black circle, this study). Taxa not yet reassessed appear as a single point
Thus, we estimated 14.6% of species threatened with extinction in 2010, with a lower threat estimate of 11.8%, and an upper threat estimate of 30.1%. Over half of our threatened species (58%) were assessed under restricted geographical range size (criterion B) and 30% under population reduction (criterion A). Fifteen percent of threatened species were classified based on criterion D2 (only used for VU species, listed based on restricted area of occupancy or number of locations with a plausible future threat that could drive the taxon to CR or EX in a very short time), while only three and four species were classified under criterion D/D1 (very small population size) and criterion C (small, declining populations), respectively (Fig. 1b). Recorded population trends of sampled species were mainly unknown (66%), although 12% showed decreasing populations, 21% were stable, and only six species showed increasing populations.
In our sample, 805 species were marine and 733 freshwater (40 species were found in both marine and freshwater). The percentage of species threatened with extinction varies from 5.7% threatened in marine fishes (lower: 4.5%; upper 26.2%) to 24.8% in freshwater fishes (lower: 20.7%; upper 37.2%, Fig. 1a). The sRLI in 2010 was 0.914 (N = 1,214 species) for the world’s fishes, 0.968 for marine fishes (N = 630) and 0.862 for freshwater species (N = 616; Fig. 1c).
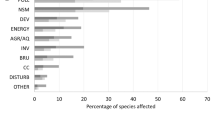
The most commonly stated threat to fishes is exploitation (34.9%), especially for marine fishes (44%). However, pollution is the most prominent threat to fish species (49.4% of the 176 threatened species), particularly from agricultural or forestry effluent, domestic and urban wastewater and industrial effluents, and is particularly prominent for freshwater species (63%) (Fig. 2). Natural system modifications such as dams and channelling in freshwater ecosystems (9%), invasive species (6.5%), and habitat loss for urban development (4.9%) were also affecting species, especially threatened freshwater species (Fig. 2).
Number of species threatened (dark grey) and not threatened (light grey) classified to each of the main threat categories for the Sampled Red List of fishes of the IUCN Red List, distinguishing marine and freshwater species. Threats are classified according to the Threats Classification Scheme (Version 3.2) of the IUCN Red List. Diagrams in the right show threat subcategories for biological resource use and pollution. 5.1: Hunting & collecting terrestrial animals, 5.2: Gathering terrestrial plants, 5.3: Logging & wood harvesting, 5.4: Fishing & harvesting aquatic resources, 9.1: Domestic & urban waste water, 9.2: Industrial & military effluents, 9.3: Agricultural & forestry effluents, 9.4: Garbage & solid waste, 9.5: Air-borne pollutants
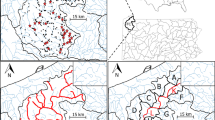
Normalised species richness in the sample is shown in Fig. 3. Highest species richness and threatened species richness for marine fishes in our sample are located in Southeast Asia, and secondarily around tropical islands in the Caribbean Ocean. Data Deficient species richness of our sample was highest in Southeast Asian waters and along the north and western Australian coast. Freshwater species richness in our sample was highest in Southeast Asia and the Amazon Basin, whereas threatened species are concentrated mainly in Southeast Asia. Data Deficient freshwater species richness in our sample was highest in parts of Southeast and Eastern Asia.
Species richness of the sampled assessment, showing normalised species richness per grid cell: A) all freshwater fish (n = 714 species); B) all marine fish (n = 799); C) Data Deficient (DD) freshwater fish (n = 114); D) DD marine fish (n = 173); E) threatened freshwater fish (CR, EN, VU; n = 133); F) threatened marine fish (n = 34)
Representativeness of the Sampled List
In both the full and the Sampled List of fishes, the largest order is the Perciformes, which includes both freshwater and marine species. The next largest orders are represented primarily or exclusively by freshwater species belonging to the Cypriniformes, Siluriformes and Characiformes (Fig. 4). In terms of spatial distribution, both lists show that species richness is highest in the Western Central Pacific and Northwest Pacific. In inland waters, tropical areas of Asia and South America are the most species-rich. In terms of habitat preference, most fish species prefer pelagic and benthopelagic habitats, followed by reef-associated ecosystems, in both the full and sampled lists (Fig. 4). Chi-square tests showed no significant differences in the proportion of species among orders, FAO areas and preferred habitats between the full and the Sampled List (Table 1). Similarly, there are no significant differences in estimated biological traits (life span, generation time, trophic level and vulnerability index) comparing full and Sampled Lists (Table 2). The Sampled List is also representative of DD species, with no significant differences found between DD species in the Sampled List and the full list of fish species except for the generation time, which is higher for DD species in the Sampled List (Tables 1 and 2).
Comparison among Global List of fishes (blue) and Sampled list (brown) used to estimate the Sampled Red List indices for all fishes, and marine and freshwater species separately. A) Number of species belonging to the 15 more extensive orders; B) number of species distributed by FAO Major Fishing Areas; C) percentage of species habitat preferences, according to FishBase (adapted from Holthus and Maragos 1995)
Discussion
The first assessment of the Sampled List presented here provides a baseline of the extinction risk of the world’s fish, against which to track future trends. Overall, we show that around 15% of fish species in our sample are estimated to be threatened with extinction and that threat is higher in freshwaters than marine systems. Our results confirm previous findings on the alarming conservation status of freshwater fishes (Pelayo-Villamil et al. 2015; Arthington et al. 2016) in the same way that other freshwater biodiversity is highly threatened with extinction (Collen et al. 2014; Richman et al. 2015; Böhm et al. 2020).
The overall sRLI for fishes is similar to the Red List Index for birds (Hoffmann et al. 2010), and dragonflies and damselflies (Clausnitzer et al. 2009), and higher than for the remaining evaluated taxonomic groups (Fig. 1). Overall, the sRLI for marine fishes is the highest of the Red List indices calculated so far (bar the historical index estimated for reef-building corals pre-1998 (Carpenter et al. 2008)). Our estimate of 5.7% of marine fish threatened with extinction is lower than threat levels found in other studies on extinction risk of marine fishes: regional shorefishes (5–9.4% Buchanan et al. 2019; Linardich et al. 2019)), sharks and rays (32% (Dulvy et al. 2021)), hagfishes (12% (Knapp et al. 2011)), groupers (12% (Sadovy de Mitcheson et al. 2013)), tarpons, ladyfishes and bonefishes (12.5% (Adams et al. 2014)), porgies (8.6% (Comeros-Raynal et al. 2016)), and pufferfishes (7.9% (Stump et al. 2018)). This is unsurprising as threat tends to be greatest in shallower waters because of likely greater interaction with fishing gears and higher fishing pressure and a random sample of marine fishes will include more deepwater species than the assessment processes. The sRLI calculated for freshwater fishes is much lower and similar to the RLI for mammals and plants (Brummitt et al. 2015). It is slightly higher than RLIs for other freshwater groups, reflecting a lower estimated threat level than crayfishes (Richman et al. 2015), freshwater crabs (Cumberlidge et al. 2009), freshwater molluscs (Böhm et al. 2020) and freshwater shrimps (De Grave et al. 2015). However, note that the sRLI protocol was not developed to accurately estimate the proportion of threatened species in a taxonomic group, but to accurately detect extinction risk trends in a species group over time (Baillie et al. 2008). Thus, our threat estimates of 14.5% of fish species threatened with extinction, derived from our sample, should be treated with caution and may only be broadly indicative of overall levels of threat within fishes. However, ongoing work to test the accuracy of the sRLI in establishing proportions of threat in taxonomic groups suggests that such estimates fall within acceptable margins of error and give a good representation of the relative threat level of a taxonomic group (Henriques, unpublished data).
High levels of data deficiency introduce uncertainty into assessments of threat levels of taxonomic groups and the resulting RLIs (Butchart and Bird 2010). With improved knowledge of species, reassessments have led to reassignment of DD species to non-DD categories (e.g. in birds (Butchart and Bird 2010) and reptiles (Bland and Böhm 2016)). With close to one third of species in the Sampled List for fishes having undergone reassessment already (160 marine and 305 freshwater species), there are already 34 fewer species listed as DD; these changes to non-DD status have been mostly attributed to the marine realm. Conversely, designation of species to the DD category has also occurred as uncertainty was not previously accurately acknowledged in some assessments (18 species). Overall, the level of data deficiency in our Sampled List of fishes is comparable to that found in other species groups such as crayfish (Richman et al. 2015) and reptiles (Böhm et al. 2013), and lower than that observed in freshwater crabs (Cumberlidge et al. 2009) and freshwater molluscs (Böhm et al. 2020). While DD species should be considered as potentially threatened until their status can be properly assessed (IUCN 2012), they cannot contribute to the Red List Index (Butchart et al. 2004) unless we improve our knowledge on these species or their status can be meaningfully predicted, e.g. using trait-based methods (Bland et al. 2015). Reducing data deficiency is thus important to produce more robust extinction risk patterns and RLI values in future (Bland and Böhm 2016). With data deficiency in our sample highest in parts of Southeast Asia, this region would make a logical place to target to reduce DD, specifically for marine fish for which DD currently produces wide margins of uncertainty around estimated threat levels.
Population trends were lacking for many marine fishes beyond coastal areas, as most of our knowledge on the marine realm comes from coastal, intertidal or neritic habitats: for example, 73% of marine fish species assessed on the IUCN Red List occur in these habitats (IUCN 2020). This is especially problematic since marine fishes were predominantly assessed as threatened under criterion A (Fig. 1B), i.e. because of a population reduction over ten years or three generations. Results offered by the Living Planet Index, a measure of the trends of global biodiversity based on population trends of vertebrate species from around the world (McRae et al. 2017), showed an average decline of around 52% for monitored marine vertebrate populations since 1970 (WWF 2015), compared to 84% for freshwater vertebrate populations (WWF 2020). This suggests that the risk of population declines for those species with unknown populations trends in our sample should not be underestimated, and that we need to push efforts towards better monitoring and estimating populations.
Fishes are among the most diverse classes of vertebrates with significant differences between marine and freshwater environmental realms. Despite differences between realms, our results consistently show exploitation and pollution are the main threats to both marine and freshwater fishes (Fig. 2). In the marine realm, overexploitation is overwhelmingly prominent in assessments of nearshore and epipelagic fishes (Knapp et al. 2011; Dulvy et al. 2014; Stump et al. 2018). Despite low overall threat levels of marine fishes in our study, in 2015 only 7% of globally assessed stocks were underfished according to the FAO (FAO 2018), and increases in exploitation pressure in future may lead to further declines in species. Safeguarding marine fish diversity needs the urgent engagement of different stakeholders to ensure the sustainability of this resource while also addressing the United Nations Sustainable Development Goals, e.g. such as SDG2 on combatting hunger and malnutrition and SDG14 on preserving life below water. IUCN Red List evaluations for marine fishes are important to achieve their survival and that of human communities which rely on them for food. Despite the difficulties of integrating fisheries data into the IUCN Red List evaluation process (Davies and Baum 2012; Millar and Dickey-Collas 2018; Miqueleiz et al. 2022), we consider that current threat levels require the commitment of all parties involved to ensure marine fish conservation.
The impact of human settlements and cities around aquatic ecosystems and increasing water demand have led to the degradation of freshwater biodiversity (Pelayo-Villamil et al. 2015; Arthington et al. 2016), especially through water pollution, dams and water extraction, river fragmentation, habitat loss, and establishment of non-native species (Arthington et al. 2016), all threats which were prominently recorded in the sRLI assessments. Rivers are highly connected linear structures (Hermoso et al. 2017): they are collectors of terrestrial impacts of the landscapes they drain, conducting them downstream. Management plans therefore need to consider the unique characteristics of freshwater systems and their high connectivity (Hermoso et al. 2016, 2017).
Our study provides the first in-depth test of representativeness of the sRLI — including the separate disaggregated indices obtained for marine, freshwater, and also DD species — in terms of geographic, ecological and trait diversity. This is particularly important since the sRLI method at present randomly draws species from the species list; stratification of the sample according to key measures was originally considered, but was rejected as a workable strategy due to the general lack of knowledge on any of these factors prior to the assessment process (Baillie et al. 2008). Thus far, tests have only been carried out to show that the recommended sRLI sample sizes are large enough to accurately reflect species group attributes regarding biogeographic realm, ecosystem types and taxonomy (Baillie et al. 2008; Henriques et al. 2020). Representativeness is important since, for example, marine fishes that are restricted to the continental shelf, and especially those that occupy shallow habitats of less than 50 m depth, have a significantly higher proportion of threatened species compared to marine fishes that occur in waters deeper than 300 m (Polidoro et al. 2017). Similarly, deep sea fishes are often assumed to be LC because of a lack of intense fishing pressure on these fishes, although low growth rates, late maturity, low fecundity and long lifespans of many deep sea fishes make them particularly vulnerable to any level of exploitation (Baker et al. 2009). Here, we again showed that there were no significant differences in the proportions among taxonomic groups, geographic regions and habitat types between the Sampled and full list of fishes, while also showing representativeness of other biological traits (life span, generation time, trophic level and vulnerability index), both in the sRLI for fish and its disaggregates into freshwater and marine fishes, respectively. The Sampled List seems to not only be sufficiently large to accurately detect trend direction in the extinction risk of the world’s fishes (Baillie et al. 2008; Henriques et al. 2020), but also to be representative of the world’s fish taxonomic, trait and ecological diversity. In terms of spatial representativeness, the data generated by the global freshwater fish assessment— once completed — can be used to re-evaluate the representativeness of our sRLI sample of freshwater fishes spatially (Fig. 3).
In this study, we calculated the baseline sRLI for 2010, the year in which the assessments of the selected 1,500 species were concluded, updated based on recent knowledge gathered through reassessments which have resulted in non-genuine changes of Red List status of 157 species. The index results published here provide the baseline towards monitoring global extinction risk in this highly species-rich group, allowing us to track future changes and trends in the conservation status of the world’s fishes. Specifically, through a future full reassessment of the sample, this index enables us to track improvements or deteriorations in the status of the world’s fishes. However, previous sRLI assessments have so far mainly achieved a single status point, and are still struggling to present trends in extinction risk over time, mainly due to Red Listing requiring considerable time and resources (Juffe-Bignoli et al. 2016). Recent work has shown that where reassessments occur every ten years, randomly drawn samples of 400 non-DD species from the full species list may be sufficient to accurately show direction of RLI trend of a group (Henriques et al. 2020), thus reducing some of the time and resource constraints from the reassessment process. Applying this reduced sample size to our sRLI for fishes, the respective sample sizes for marine (631 non-DD species) and freshwater fishes (617 non-DD species) are large enough to function as stand-alone, disaggregated indices.
A reassessment would allow us to check how fishes fared against Aichi Target 12, and provide a starting point for better conservation action and management for these vital aquatic resources. Many of the original assessments have already undergone (20,878 fish species) or are in the process of reassessment of their IUCN Red List status. As such, as a first step for reassessment of the sRLI, we have already collated recent reassessments and produced preliminary updates of the 2010 status of those species which had undergone non-genuine changes in their assessment status in recent years (i.e. changes because of improved data rather than actual improvements or deteriorations in extinction risk status). We also identified thirteen genuine status changes from 441 reassessments carried out so far. Based on this preliminary work, we next need to prioritise reassessments of those species which were in threatened or Near Threatened categories in 2010. As in other assessment processes, Least Concern species may be fast-tracked more rapidly through the assessment process (Red List Technical Working Group 2018). Finally, application of retrospective assessments to assess past extinction risk status from a present perspective (Hoffmann et al. 2011; Di Marco et al. 2014; Kyne et al. 2020) should be considered to derive long-term trends in extinction risk over time.
Aichi Target 12 for biodiversity has not been met (Secretariat of the Convention on Biological Diversity 2020). Considering the existing priorities and limited conservation resources to establish an efficient reassessment of larger samples, the selected subset of species can inform current and future policy targets about trends on fish species conservation and help to allocate efforts and resources. Given that to date, fishes have been largely neglected in large-scale conservation analyses, likely due to an apathetic public perception of these animals (Darwall et al. 2009; Pino-del-Carpio et al. 2014) and a comparatively low level of research compared to other vertebrate groups, not only in non-commercial (Reynolds et al. 2005), but also charismatic species of fish (McClenachan et al. 2012), development and upkeep of an effective tool to communicate fish trends is urgently required. Current and updated assessments are necessary in the scenario of a changing world where threats and conservation status of biodiversity are constantly changing (Tilman et al. 2017; Reid et al. 2019). For example, climate change is an emerging threat of freshwater ecosystems (Pelayo-Villamil et al. 2015; Reid et al. 2019), though still features less prominently in IUCN Red List assessments. Other IUCN assessments for freshwater species have already noted climate change as a major future threat (Böhm et al. 2020) and it is likely to become a more important threat in future reassessments of our sample.
Availability of data and material
All data were obtained from public and open-access repositories.
Code availability
All code used in the study is explained in the manuscript.
References
Abdul Malak D, Livingstone SR, Pollard D, et al (2011) Overview of the conservation status of the marine fishes of the mediterranean sea. IUCN, Gland, Switzerland and Malaga, Spain
Adams AJ, Horodysky AZ, Mcbride RS et al (2014) Global conservation status and research needs for tarpons (Megalopidae), ladyfishes (Elopidae) and bonefishes (Albulidae). Fish Fish 15:280–311. https://doi.org/10.1111/faf.12017
Allen DJ, Molur S, Daniel BA (2010) The status and distribution of freshwater biodiversity in the eastern Himalaya. Cambridge, UK and Gland, Switzerland: IUCN, and Coimbatore, India: Zoo Outreach Organisation
Allen DJ, Smith KG, Darwall WRT, Thomas R (2012) The status and distribution of freshwater biodiversity in Indo-Burma. IUCN, Cambridge, UK and Gland, Switzerland, UK and Gland, Switzerland
Arthington AH, Dulvy NK, Gladstone W, Winfield IJ (2016) Fish conservation in freshwater and marine realms: status, threats and management. Aquat Conserv Mar Freshw Ecosyst 26:838–857
Baillie JEM, Collen B, Amin R et al (2008) Toward monitoring global biodiversity. Conserv Lett 1:18–26. https://doi.org/10.1111/j.1755-263X.2008.00009.x
Baillie JEM, Griffiths J, Turvey ST et al (2010) Evolution Lost: Status and Trends of the World’s Vertebrates. Zoological Society of London, London
Baker KD, Devine JA, Haedrich RL (2009) Deep-sea fishes in Canada’s Atlantic: Population declines and predicted recovery times. Environ Biol Fishes 85:79–88. https://doi.org/10.1007/s10641-009-9465-8
Bland LM, Böhm M (2016) Overcoming data deficiency in reptiles. Biol Conserv 204A:16–22
Bland LM, Collen B, Orme CDL, Bielby J (2015) Predicting the conservation status of data-deficient species. Conserv Biol 29:250–259. https://doi.org/10.1111/cobi.12372
Böhm M, Collen B, Baillie JEMM et al (2013) The conservation status of the world’s reptiles. Biol Conserv 157:372–385. https://doi.org/10.1016/j.biocon.2012.07.015
Böhm M, Dewhurst-Richman NI, Seddon M, et al (2020) The conservation status of the world’s freshwater molluscs. Hydrobiologia 0123456789: https://doi.org/10.1007/s10750-020-04385-w
Brummitt NA, Bachman SP, Griffiths-Lee J et al (2015) Green plants in the red: A baseline global assessment for the IUCN Sampled Red List Index for Plants. PLoS ONE 10:1–22. https://doi.org/10.1371/journal.pone.0135152
Buchanan JR, Ralph GM, Krupp F et al (2019) Regional extinction risks for marine bony fishes occurring in the Persian/Arabian Gulf. Biol Conserv 230:10–19. https://doi.org/10.1016/j.biocon.2018.11.027
Butchart SHM, Bird JP (2010) Data Deficient birds on the IUCN Red List: What don’t we know and why does it matter? Biol Conserv 143:239–247. https://doi.org/10.1016/j.biocon.2009.10.008
Butchart SHM, Stattersfield AJ, Bennun La et al (2004) Measuring global trends in the status of biodiversity: red list indices for birds. PLoS Biol 2:2294–2304. https://doi.org/10.1371/journal.pbio.0020383
Butchart SHM, Walpole M, Collen B et al (2010) Global biodiversity: Indicators of recent declines. Science 80-(328):1164–1169. https://doi.org/10.1126/science.1187512
Butchart SHM, Akçakaya HR, Chanson J, et al (2007) Improvements to the Red List Index. PLoS One 2. https://doi.org/10.1371/journal.pone.0000140
Carpenter KE, Abrar M, Aeby G et al (2008) One-Third of Reef-Building Corals Face Elevated Extinction Risk from Climate Change and Local Impacts. Science 80-(321):560–563. https://doi.org/10.1126/science.1159196
Cheung WWL, Pitcher TJ, Pauly D (2005) A fuzzy logic expert system to estimate intrinsic extinction vulnerabilities of marine fishes to fishing. Biol Conserv 124:97–111
Clausnitzer V, Kalkman VJ, Ram M et al (2009) Odonata enter the biodiversity crisis debate: The first global assessment of an insect group. Biol Conserv 142:1864–1869. https://doi.org/10.1016/j.biocon.2009.03.028
Collen B, Whitton F, Dyer EE et al (2014) Global patterns of freshwater species diversity, threat and endemism. Glob Ecol Biogeogr 23:40–51
Collette BB, Carpenter KE, Polidoro B a et al (2011) High Value and Long Life—Double Jeopardy for Tunas and Billfishes. Science 80-(333):291–292. https://doi.org/10.1126/science.1208730
Comeros-Raynal MT, Polidoro BA, Broatch J et al (2016) Key predictors of extinction risk in sea breams and porgies (Family: Sparidae). Biol Conserv 202:88–98. https://doi.org/10.1016/j.biocon.2016.08.027
Comeros-Raynal MT, Choat JH, Polidoro BA, et al (2012) The likelihood of extinction of iconic and dominant herbivores and detritivores of coral reefs: The parrotfishes and surgeonfishes. PLoS One 7. https://doi.org/10.1371/journal.pone.0039825
Contreras-MacBeath T, Hendrickson DADA, Arroyave J, et al (2020) The status and distribution of freshwater fishes in Mexico. IUCN and ABQ BioPark, Cambridge, UK and Albuquerque, New Mexico, USA, UK and Albuquerque, New Mexico, USA
Cox NA, Young BE, Bowles P, et al (2022) Global reptile assessment shows commonality of tetrapod conservation needs. Nature In press:
Cumberlidge N, Ng PKL, Yeo DCJ et al (2009) Freshwater crabs and the biodiversity crisis: Importance, threats, status, and conservation challenges. Biol Conserv 142:1665–1673. https://doi.org/10.1016/j.biocon.2009.02.038
Darwall WRT, Smith KG, Allen D, et al (2009) Freshwater biodiversity: a hidden resource under threat. In: Vié J-C, Hilton-Taylor C, Stuart SN (eds) Wildlife in a Changing World–An Analysis of the 2008 IUCN Red List of Threatened Species. IUCN, Gland, Switzerland and Cambridge, UK., p 43
Darwall WRT, Smith KG, Allen DJ, et al (2011) The diversity of life in African freshwaters: Underwater, under threat. An analysis of the status and distribution of freshwater species throughout mainland Africa. IUCN, Cambridge, United Kingdom and Gland, Switzerland, UK and Gland, Switzerland
Davies TD, Baum JK (2012) Extinction risk and overfishing: Reconciling conservation and fisheries perspectives on the status of marine fishes. Sci Rep 2:1–9. https://doi.org/10.1038/srep00561
De Grave S, Smith KG, Adeler NA et al (2015) Dead shrimp blues: A global assessment of extinction risk in freshwater shrimps (Crustacea: Decapoda: Caridea). PLoS ONE 10:1–14. https://doi.org/10.1371/journal.pone.0120198
Di Marco M, Boitani L, Mallon D et al (2014) A Retrospective evaluation of the global decline of carnivores and ungulates. Conserv Biol 28:1109–1118. https://doi.org/10.1111/cobi.12249
Dulvy NK, Fowler SL, Musick JA et al (2014) Extinction risk and conservation of the world’s sharks and rays. Elife 3:e00590
Dulvy NK, Pacoureau N, Rigby CL et al (2021) Overfishing drives over one-third of all sharks and rays toward a global extinction crisis. Curr Biol 31:4773–4787.e8. https://doi.org/10.1016/J.CUB.2021.08.062
Eifes CT, Livingstone SR, Lane A et al (2013) Fascinating and forgotten: The conservation status of marine elapid snakes. Herpetol Conserv Biol 8:37–52
FAO (2018) The state of world fisheries and aquaculture 2018. Meeting the sustainable development goals. Rome, Italy
Fernandes PG, Ralph GM, Nieto A et al (2017) Coherent assessments of Europe’s marine fishes show regional divergence and megafauna loss. Nat Ecol Evol 1:0170. https://doi.org/10.1038/s41559-017-0170
Freyhof J, Brooks E (2011) European Red List of Freshwater Fishes. Publications Office of the European Union, Luxembourg
Fricke R, Eschmeyer WN, Van der Laan R (2022) Eschmeyer’s Catalog of Fishes: Genera, Species. References, San Francisco
Froese R, Pauly D (2016) FishBase. World Wide Web electronic publication. www.fishbase.org. Accessed 16 Sept 2016
Henriques S, Böhm M, Collen B et al (2020) Accelerating the monitoring of global biodiversity: revisiting the sampled approach to generating Red List Indices. Conserv Lett 13:1–9. https://doi.org/10.1111/conl.12703
Hermoso V, Abell R, Linke S, Boon P (2016) The role of protected areas for freshwater biodiversity conservation: challenges and opportunities in a rapidly changing world. Aquat Conserv Mar Freshw Ecosyst 26:3–11
Hermoso V, Filipe AF, Segurado P, Beja P (2017) Freshwater conservation in a fragmented world: Dealing with barriers in a systematic planning framework. Aquat Conserv Mar Freshw Ecosyst 28:1–9. https://doi.org/10.1002/aqc.2826
Hoffmann M, Hilton-taylor C, Angulo A et al (2010) The Impact of Conservation on the Status of the World ’s Vertebrates. Science 80-(330):1503–1509. https://doi.org/10.1126/science.1194442
Hoffmann M, Belant JL, Chanson JS et al (2011) The changing fates of the world’s mammals. Philos Trans R Soc B Biol Sci 366:2598–2610. https://doi.org/10.1098/rstb.2011.0116
Holthus PF, Maragos JE (1995) Marine ecosystem classification for the Tropical Indo-Pacific: Coast. Manage Trop Asia 5:15–18
Hutchings JA (2000) Collapse and recovery of marine fishe. Nature 406:882–885
IUCN Standards and Petitions Committee (2019) Guidelines for Using the IUCN Red List Categories and Criteria. Version 14
IUCN (2012) IUCN Red List Categories and Criteria. Version 3.1, 2nd edn. Gland, Switzerland and Cambridge, UK, Switzerland and Cambridge, UK
IUCN (2020) The IUCN Red List of Threatened Species. Version 2020–2. https://www.iucnredlist.org/. Accessed 23 June 2020
IUCN (2021) The IUCN Red List of Threatened Species. Version 2021–3
Jacoby DMP, Casselman JM, Crook V et al (2015) Synergistic patterns of threat and the challenges facing global anguillid eel conservation. Glob Ecol Conserv 4:321–333. https://doi.org/10.1016/j.gecco.2015.07.009
Juffe-Bignoli D, Brooks TM, Butchart SHM et al (2016) Assessing the Cost of Global Biodiversity and Conservation Knowledge. PLoS ONE 11:e0160640. https://doi.org/10.1371/journal.pone.0160640
Knapp L, Mincarone MM, Harwell H et al (2011) Conservation status of the world’s hagfish species and the loss of phylogenetic diversity and ecosystem function. Aquat Conserv Mar Freshw Ecosyst 21:401–411. https://doi.org/10.1002/aqc.1202
Kyne PM, Jabado RW, Rigby CL et al (2020) The thin edge of the wedge: Extremely high extinction risk in wedgefishes and giant guitarfishes. Aquat Conserv Mar Freshw Ecosyst 30:1337–1361. https://doi.org/10.1002/aqc.3331
Lewis OT, Senior MJM (2011) Assessing conservation status and trends for the world’s butterflies: The Sampled Red List Index approach. J Insect Conserv 15:121–128. https://doi.org/10.1007/s10841-010-9329-8
Linardich C, Ralph GM, Robertson DR et al (2019) Extinction risk and conservation of marine bony shorefishes of the Greater Caribbean and Gulf of Mexico. Aquat Conserv Mar Freshw Ecosyst 29:85–101. https://doi.org/10.1002/aqc.2959
Máiz-Tomé L, Sayer CA, Darwall WRT (2018) The status and distribution of freshwater biodiversity in Madagascar and the Indian Ocean islands hotspot. IUCN, Gland, Switzerland
McClenachan L, Cooper AB, Carpenter KE, Dulvy NK (2012) Extinction risk and bottlenecks in the conservation of charismatic marine species. Conserv Lett 5:73–80. https://doi.org/10.1111/j.1755-263X.2011.00206.x
McRae L, Deinet S, Freeman R (2017) The diversity-weighted living planet index: Controlling for taxonomic bias in a global biodiversity indicator. PLoS ONE 12:1–20. https://doi.org/10.1371/journal.pone.0169156
Millar S, Dickey-Collas M (2018) Report on IUCN assessments and fisheries management approaches. Copenhagen, Denmark
Miqueleiz I, Bohm M, Ariño AH, Miranda R (2020) Assessment gaps and biases in knowledge of conservation status of fishes. Aquat Conserv Mar Freshw Ecosyst 30:225–236. https://doi.org/10.1002/aqc.3282
Miqueleiz I, Miranda R, Ariño AH, Ojea E (2022) Conservation-Status Gaps for Marine Top-Fished Commercial Species. Fishes 7:2. https://doi.org/10.3390/FISHES7010002
Molur S, Smith KG, Daniel BA, Darwall WRT (2011) The status and distribution of freshwater biodiversity in the Western Ghats, India. Cambridge, UK and Gland, Switzerland: IUCN, and Coimbatore, India: Zoo Outreach Organisation
Pauly D, Christensen V, Walters C (2000) Ecopath, Ecosim, and Ecospace as tools for evaluating ecosystem impact of fisheries. ICES J Mar Sci 57:697–706. https://doi.org/10.1006/jmsc.2000.0726
Pelayo-Villamil P, Guisande C, Vari RP et al (2015) Global diversity patterns of freshwater fishes - Potential victims of their own success. Divers Distrib 21:345–356. https://doi.org/10.1111/ddi.12271
Peters H, O’Leary BC, Hawkins JP et al (2013) Conus: First Comprehensive Conservation Red List Assessment of a Marine Gastropod Mollusc Genus. PLoS ONE 8:e83353. https://doi.org/10.1371/journal.pone.0083353
Pino-del-Carpio A, Ariño AH, Miranda R (2014) Data exchange gaps in knowledge of biodiversity: Implications for the management and conservation of Biosphere Reserves. Biodivers Conserv 23:2239–2258
Pippard H, Ralph G, Harvey M et al (2017) The conservation status of marine biodiversity of the Pacific Islands of Oceania. IUCN, Gland, Switzerland
Polidoro BA, Carpenter KE, Collins L et al (2010) The Loss of Species: Mangrove Extinction Risk and Geographic Areas of Global Concern. PLoS ONE 5:e10095. https://doi.org/10.1371/journal.pone.0010095
Polidoro BA, Brooks T, Carpenter KE et al (2012) Patterns of extinction risk and threat for marine vertebrates and habitat-forming species in the Tropical Eastern Pacific. Mar Ecol Prog Ser 448:93–104. https://doi.org/10.3354/meps09545
Polidoro BA, Ralph GM, Strongin K et al (2017) The status of marine biodiversity in the Eastern Central Atlantic (West and Central Africa). Aquat Conserv Mar Freshw Ecosyst 27:1021–1034. https://doi.org/10.1002/aqc.2744
Purcell SW, Polidoro BA, Hamel JF, et al (2014) The cost of being valuable: Predictors of extinction risk in marine invertebrates exploited as luxury seafood. Proc R Soc B Biol Sci 281. https://doi.org/10.1098/rspb.2013.3296
R Development Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Red List Technical Working Group (2018) Mapping standards and data quality for the IUCN Red List Categories and Criteria. Version 1.16. 1.16:30
Reid AJ, Carlson AK, Creed IF et al (2019) Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol Rev 94:849–873. https://doi.org/10.1111/brv.12480
Reynolds JD, Webb T, Hawkins L (2005) Life history and ecological correlates of extinction risk in European freshwater fishes. Can J Fish Aquat Sci 62:854–862. https://doi.org/10.1139/f05-066
Richman NI, Böhm M, Adams SB et al (2015) Multiple drivers of decline in the global status of freshwater crayfish (Decapoda: Astacidea). Philos Trans R Soc B Biol Sci 370:1–11. https://doi.org/10.1098/rstb.2014.0060
Rodrigues ASL, Pilgrim JD, Lamoreux JF et al (2006) The value of the IUCN Red List for conservation. Trends Ecol Evol 21:71–76
Ruckelshaus MH, Jackson ST, Mooney HA et al (2020) The IPBES Global Assessment: Pathways to Action. Trends Ecol Evol 35:407–414. https://doi.org/10.1016/j.tree.2020.01.009
Sadovy de Mitcheson Y, Craig MT, Bertoncini AA et al (2013) Fishing groupers towards extinction: A global assessment of threats and extinction risks in a billion dollar fishery. Fish Fish 14:119–136. https://doi.org/10.1111/j.1467-2979.2011.00455.x
Salafsky N, Salzer D, Stattersfield AJ et al (2008) A standard lexicon for biodiversity conservation: Unified classifications of threats and actions. Conserv Biol 22:897–911. https://doi.org/10.1111/j.1523-1739.2008.00937.x
Secretariat of the Convention on Biological Diversity (2020) Global Biodiversity Outlook 5 Summary. Montreal, Canada
Short FT, Polidoro B, Livingstone SR et al (2011) Extinction risk assessment of the world’s seagrass species. Biol Conserv 144:1961–1971. https://doi.org/10.1016/j.biocon.2011.04.010
Strongin K, Polidoro B, Linardich C et al (2020) Translating globally threatened marine species information into regional guidance for the Gulf of Mexico. Glob Ecol Conserv 23:e01010. https://doi.org/10.1016/j.gecco.2020.e01010
Stump E, Ralph GM, Comeros-Raynal MT et al (2018) Global conservation status of marine pufferfishes (Tetraodontiformes: Tetraodontidae). Glob Ecol Conserv 14:e00388. https://doi.org/10.1016/j.gecco.2018.e00388
Tilman D, Clark M, Williams DR et al (2017) Future threats to biodiversity and pathways to their prevention. Nature 546:73–81. https://doi.org/10.1038/nature22900
WWF (2015) Living Blue Planet Report: Species, habitats and human well-being. WWF, Gland, Switzerland
WWF (2020) Living Planet Report 2020 - Bending the curve of biodiversity loss. WWF, Gland, Switzerland
Acknowledgements
The Obra Social La Caixa and the Bancaria Caja Navarra Foundation grant co-author Rafael Miranda and Imanol Miqueleiz through an international mobility grant of the University of Navarra. Monika Böhm and the sRLI assessment process were supported by funding from the Rufford Foundation and Esmée Fairbairn Foundation. Co-author Ben Collen died on May 19th, 2018, aged 40 after he had been bravely battling bone cancer for 16 months. We want to acknowledge his friendship and affection. Rest in peace.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. I. Miqueleiz. was supported by the Association of Friends of the University of Navarra.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Miranda, R., Miqueleiz, I., Darwall, W. et al. Monitoring extinction risk and threats of the world’s fishes based on the Sampled Red List Index. Rev Fish Biol Fisheries 32, 975–991 (2022). https://doi.org/10.1007/s11160-022-09710-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11160-022-09710-1