Abstract
Scotland once had the largest herring fishery globally, generating local income, identity, and societal change. Following historic stock collapse, in spring 2018/2019 large herring shoals were observed on the west coast for the first time in decades, at a formerly important spawning ground. This highlights the urgency of maintaining historic (and contemporary) benthic spawning habitat, which these fish rely upon, in good condition. However, information on exact location, characteristics, and status of historic and contemporary spawning grounds, if existing, is not easily accessible. We searched over 1190 literature sources, dating back to 1884, using scientific databases and web-based searches, and ran a query for automated search of comprehensive historic reports. We present current knowledge on Scottish herring spawning grounds, retrieved through these searches and fisher interviews, maps showing historic and contemporary spawning grounds, and discuss challenges arising from the methods used to recognize these grounds. Knowledge gaps regarding location and environmental status of past and current spawning grounds, particularly relevant for Scotland’s west coast, are identified. Based on the importance of specific environmental and physical variables for herring reproductive success, we advocate the inclusion of essential spawning grounds into herring management plans. This will require additional data on spawning grounds, including historic local ecological knowledge rarely considered. An inclusive ecosystem-based approach to herring management would allow more targeted actions to conserve (and potentially restore) essential spawning habitat. More effective management strategies will also call for reversing the (global) issue of the disconnect between different stakeholder groups.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atlantic herring, Clupea harengus, are distributed widely across continental shelf waters of the North Atlantic and have provided economic and cultural benefits to coastal communities for centuries (Gullestad et al. 2020). In Scotland, the rich history of herring fishing dates back to Neolithic times (Harland and Parks 2008), and subsistence and commercial fisheries have been commonplace along the mainland coast and isles ever since (Coull 1986; Rorke 2005; Harland and Parks 2008). By the early twentieth century, Scotland’s herring fishery was the largest in the world, employing over 35,000 people—14,000 of them women—contributing significantly to rural and island communities (Coull 1986; Kumpulainen 2001). Herring populations are naturally prone to boom and busts, resulting in notoriously unpredictable fisheries through the centuries (Blaxter and Hunter 1982; Toresen and Østvedt 2008; Toresen et al. 2019; Trochta et al. 2020). Modern intensive fishing pressures and poor management during times of environmental variability have led to past stock collapses (Dickey-Collas et al. 2010) and resulted in longer recovery intervals for some herring populations in Scottish waters, compared to former times (Blaxter and Hunter 1982; Payne et al. 2009; Thurstan and Roberts 2010; Trochta et al. 2020).
Herring form dense shoals that migrate between feeding, spawning and overwintering grounds, maintaining similar migration patterns year after year (Stobo 1982). They exhibit fidelity to spawning season and grounds (Brophy et al. 2006; Berg et al. 2017), and thus management focusses on the timing of spawning, primarily spring and autumn in Scotland, and broad geographic region where spawning occurs (Fig. 1; Geffen 2009; Geffen et al. 2011). In Scottish waters, autumn-spawning mainly takes place offshore, while spring-spawning herring spawn inshore; although, there is some overlap in seasonal spawning ground locations (Heath 1993; Hay et al. 2001; ICES 2020b, a). Whilst it has been assumed (for stock assessment purposes) that herring aggregate in a series of discrete stocks within ICES (International Council for the Exploration of the Seas) areas (Fig. 1), significant mixing of stocks that spawn in different seasons occurs in the waters surrounding the British Isles and Ireland (Molloy et al. 1993; Geffen et al. 2011; Farrell et al. 2020), creating complex population assemblages that are difficult to account for in management and fisheries. Population discrimination is based on spawning season and location as well as biological parameters such as fecundity, egg size, growth rates, maximum size and age, otolith characteristics, and more recently, genetic analyses (Hempel and Blaxter 1967; Haegele and Schweigert 1985; Clausen et al. 2007; Geffen et al. 2011; Han et al. 2020b; Berg et al. 2021). Spring-spawned herring tend to live longer, reach a larger maximum size and produce fewer, heavier eggs that hatch into larger larvae when compared to autumn-spawned fish that hatch during less-favourable environmental conditions and slowly progress through developmental stages over the winter months (Parrish and Saville 1965; Gamble et al. 1985; Haegele and Schweigert 1985; Hunter et al. 2019). The observed differences in life-history characteristics likely reflect adaptations to specific environments and compensate for variability in larval mortality rates and environmental conditions between spawning seasons (Mcquinn 1997; Han et al. 2020a).
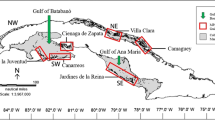
Stocks that are managed as a single unit often consist of several components that spawn in the same season, yet in different locations within the broad geographic region (Kerr et al. 2017; Farrell et al. 2020). For example, the North Sea autumn-spawning (NSAS) stock is a complex of different components that spawn in four locations in ICES subareas IVa and IVb, (Fig. 1; Table S1; Hay et al. 2001; Dickey-Collas et al. 2010 Farrell et al. 2020). The West of Scotland (WoS) stock composition changed throughout the twentieth century (Table S1). Previously a spring-spawning stock that spawned inshore along the west coast of Scotland and the Hebrides in ICES subarea VIa (Fig. 1), following collapse in the 1950s, it changed to its current composition as a primarily autumn-spawning stock that spawns offshore in the same ICES area (Coull et al. 1998; Farrell et al. 2020; ICES 2020b). However, since the 1970s the [autumn-spawning] WoS population has been in steady decline. In spring 2018 and 2019, for the first time in over 50 years, large shoals of spring-spawning WoS herring were observed on known former spawning grounds, sparking considerable public interest and debate on protected areas (BBC 2019).
Herring differ from most marine fish through their reliance on specific benthic spawning habitat. Females deposit sticky benthic eggs and males follow closely, releasing milt (sperm) that sinks over the eggs and fertilises them (Aneer et al. 1983). Herring spawn in multiple waves, with older fish spawning before recruits and subsequently migrating to feeding grounds (Jones 1968; Lambert 1987; Skaret et al. 2003). Suitable spawning grounds for egg deposition are vital for resilient herring stocks, yet such habitat is often adversely impacted by environmental and anthropogenic pressures (Thurstan and Roberts 2010; Moll et al. 2018; Moffat et al. 2020). Eggs layers create dense carpets within highly localized areas (Fig. 2), which increases vulnerability, particularly in shallower inshore spawning grounds, to mortality from storm damage, predation, toxins and bottom-towed gear (Rankine and Morrison 1989; Morrison et al. 1991; Ryan and Bailey 2012; Moll et al. 2018). Understanding the environmental and anthropogenic factors influencing herring reproductive success is crucial. Furthermore, effective conservation and management of herring populations will require information on the exact location, characteristics, and status of spawning grounds.
Based on a systematic literature review (see Supplementary Information for full details on the methodology; Tables S2, S3), we present the current knowledge on past and present herring spawning grounds in Scottish waters, including information on location and habitat characteristics and potential environmental and anthropogenic stressors affecting spawning, as well as the challenges associated with identifying herring spawning beds (Table S1). We highlight prominent examples of spawning ground abandonment and recolonization to put the recent spawning events in WoS into context. Further, we emphasize the importance of historical records and local ecological knowledge of fishers for understanding the environmental and geographical features essential for herring spawning. Finally, we identify critical gaps in current knowledge hindering the understanding of the status and geographic distribution of herring spawning grounds and, thus, the implementation of sustainable management strategies.
Spawning ground identification
Herring spawn in high energy and/or structurally complex environments, attaching adhesive eggs to coarse seabed substrates or aquatic vegetation in some nearshore habitats (e.g., Baltic herring and some spring-spawning WoS populations; Fig. 2; Table 1; Runnström 1941; Parrish and Saville 1965; Moll et al. 2018; von Nordheim et al. 2018). The offshore-banks where NSAS and WoS herring spawn typically have strong currents where the water column is more stratified (Dickey-Collas et al. 2009), while the shallower nearshore environments where spring-spawning stocks spawn are subject to stronger wave or tidal action or are lower energy environments with aquatic vegetation (Parrish and Saville 1965; Haegele and Schweigert 1985; Heath 1993; Hay et al. 2001). Spawning in high energy environments can prohibit the build-up of sediment that could smother developing eggs, as well as improve circulation and oxygenation over spawn (Hempel 1971; Drapeau (1973) in De Groot 1979).
Herring spawning ground locations (see Table S1 for descriptions of spawning beds and grounds) have been described using several different types of available information. These include direct visual observations or sampling of spawn from the seabed, the position of recently hatched or young larvae, records of catch locations of “ripe” herring in spawning condition (Table S1), and records of catch locations of fish that have recently preyed on herring spawn (Table S1). As stated previously by many authors (e.g., Postuma et al. 1975; Rankine 1986; Bauer et al. 2014), the only way to identify a herring spawning bed is by observing or sampling spawn in situ on the seabed. However, such direct observations are scarce in British waters, outside the few well-known spawning grounds in the Firth of Clyde, Scotland (Ewart 1884; Marshall et al. 1937; Parrish et al. 1959; Saville et al. 1974; Stratoudakis et al. 1998), the Black Water Estuary, England (Dempsey and Bamber 1983), the Irish Sea (Bowers 1969), and the English Channel (Bolster and Bridger 1957), as well as the recent spawning events in Wester Ross filmed by local scallop divers and the BBC. Other notable examples of herring spawn sampled from the seabed in the Northeast Atlantic are from the Baltic Sea (Aneer 1985, 1989; Kääriä et al. 1997; Moll et al. 2018), Norway (Runnström 1941; Axelsen et al. 2000; Skaret et al. 2003; Skaret and Slotte 2017), and Iceland (Fridriksson and Timmermann 1951).
Due to the paucity of direct egg observations, most spawning grounds have been described by the presence of young larvae, catch data of herring in spawning condition and fish predating on herring eggs (Rankine 1986; Ellis et al. 2012). These methods are merely indicators that eggs have been laid somewhere nearby where the larvae or fish were sampled, but do not explicitly identify spawning grounds, and with it, the important physical and environmental features of spawning habitat. Spawning grounds derived from larvae or adult presence appear as vast diffusive areas that likely encompass individual (point) herring spawning beds (Fig. 3; ICES 1994; Bauer et al. 2014). Spawning ground descriptions using catch locations of ripe herring can also be confounded by the fact that herring congregates near spawning locations in the months prior to spawning, in environmental conditions similar to those they will later spawn in, before moving to a specific spawning bed (Maravelias et al. 2000). Although these indirect methods are less accurate at determining the precise location of herring spawning beds, the consistency within and between methods as well as the long timeseries available for this type of data do provide some information on the extent of spawning grounds (Postuma et al. 1975). Identification of preferred spawning substrate within the wider areas derived by these methods can further narrow down the location of spawning grounds; however, other key habitat variables may only occur in isolated patches, complicating reliable extrapolations from substrate alone (Fig. S2). Finding the exact location of herring spawning beds requires intensive sampling in small geographical areas (Bowers 1980).
Spatial data on herring reproduction, spawning grounds and larval occurrences for ICES areas IV, VI and VII (including Isle of Man) (see Table S3 for original sources). Polygons are displayed based on the different survey techniques applied, i.e., manual collection of herring eggs from the seabed or grab sampling, location of recently hatched and/or young larvae, fisheries catch locations of ripe or running herring (see Table S1 for definition of terms), location of “spawny” haddock with herring roe found in stomach contents, or a combination of methods that included all available information. Some publications did not specify how spawning ground distributions were derived. Note: herring eggs have only been sampled in situ in the Firth of Clyde (from 1884 to 1998) and filmed in 2018/2019 off the coast of Wester Ross. For the latter, spatially explicit data of bed locations are not available and therefore point locations were plotted instead. Point locations are also given for historic data on the catch locations of ripe or running herring and historic spawning grounds mentioned by fishers
Information from ICES herring larval surveys has been used to infer the contemporary location of spawning grounds in areas with a high density of yolk-sac or young larvae (e.g., Wood 1971; Corten 1988; Coull et al. 1998; Ellis et al. 2012; Anonymous 2019). The ICES International Herring Larval Survey program was implemented in 1967 to provide a relative index of change in spawning stock biomass (SSB; Table S1) based on estimates of herring larval abundance. However, these larval surveys have not sampled the west coast of Scotland since 1994 (ICES 2010). Ellis et al. (2012) noted that the distribution of survey stations influences the “apparent” distribution of herring larvae, and thus spawning grounds (Fig. 3). This is particularly problematic on the west coast of Scotland where herring surveys target offshore summer feeding aggregations of mixed spawning stocks (ICES 2019a), and consequently do not encounter spring spawning shoals. Herring spawning aggregations are dense but the events are brief, which makes identifying and observing them challenging (ICES 2015a). In addition, the fact that hyper-aggregation of ripe herring often occurs close to the seabed in sheltered, shallow waters or banks make it difficult to survey inshore waters with larger governmental vessels. For many decades, large shoals of spring-spawning herring have been rarely observed on the Scottish west coast, so research efforts have focussed primarily on autumn-spawning herring stocks in easily accessible offshore grounds.
Temporal-spatial occurrence of herring spawning in Scotland
Spawning ground locations
Throughout their range in Scottish waters, herring have spawned at some point in time along most of the mainland and island coastlines as well as over offshore banks (Fig. 4). Spawning grounds on the west coast of Scotland were most recently reviewed in Rankine (1986), ICES (1994, 2010), Coull et al. (1998), Ellis et al. (2012) and Aires et al. (2014), based on survey data now decades old and/or targeting autumn spawning activity in the North Sea and west of the Hebrides only. Aires et al. (2014) attempted to model herring larvae aggregations to update spawning ground distributions in British waters but identified a critical lack of environmental data for coastal areas—most notable on the Scottish west coast. Furthermore, they commented on the unknown timespan between the act of spawning and larvae surveying that resulted in low predictive strength and uncertainty in their models. The authors cautioned against using maps of herring larvae aggregations as a proxy for spawning grounds when sampling coverage is poor and environmental data are limited, as is the case for western Scotland.
Original spatio-temporal data on herring reproduction, spawning grounds and larval occurrences reported in different seasons between 1880 and 2019 for ICES areas IV, VI and VII (including Isle of Man; Table S3). a Occurrence of ripe or running herring catch locations and “known” spawning grounds based on fisher interviews; b Occurrence of spawning herring and ‘spawny’ haddock (haddock feeding upon herring spawn, ‘Spring’ and ‘Autumn’) and herring eggs (W-coast Clyde area, ‘Spring’); c Occurrence of spawning herring (‘Spring’), herring eggs (W-coast Clyde and Wester Ross areas, ‘Spring’) and herring larvae (‘Autumn’ and ‘Season not specified’); d Occurrence of spawning herring (‘Spring’ and ‘Autumn’), herring eggs (W-coast Clyde area, ‘Spring’) and herring larvae (‘Spring’, ‘Summer’, ‘Autumn’ and ‘Season not specified’). Point locations are also included for historic data on the catch locations of ripe or running herring, historic spawning grounds mentioned by fishers, and spawning grounds filmed off Wester Ross in 2018/2019
The historic distribution of fisheries along the mainland coast and Hebrides reflects the former location of herring spawning grounds, particularly in the Minch, Firth of Clyde and Firth of Forth (Fig. 1; Scottish Government 1884; Coull 1986). Prior to their disappearance, spring-spawning WoS herring dominated catches in the Minch, where fisheries regularly took place on spawning grounds in the sea lochs from Cape Wrath, in the north, to Wester Ross, in the south of the Minch (Fig. 4; Munro 1883; Anonymous 1924; Wood 1930; Baxter 1958; Rankine 1986). The exact locations of the autumn-spawning grounds are mostly unknown (Baxter 1958; Saville et al. 1966). Spring-spawning herring fisheries were also commonplace further south, in the Firth of Clyde, dating back centuries (Fig. 4; Table S1; Marshall et al. 1937; Wood 1960; Funk et al. 2000). The Clyde also supported a small autumn-spawning population, although it was much less abundant and spawning activity was unpredictable (Scottish Government 1884; Scottish Home Department 1967). The Firth of Clyde was one of the last remaining spring-spawning grounds on the west coast of Scotland, but habitat degradation and high pollution levels are thought to have led to an “ecological meltdown” (Thurstan and Roberts 2010), and herring no longer spawn there in large numbers (ICES 2019b).
Spatial and temporal shifts in NSAS herring spawning grounds have also been observed during the last century (Fig. 4). On the Shetland/Orkney, Buchan, and Banks spawning grounds contemporary spawning occurs during September and October (Fig. 1; ICES 2020b). However, herring historically spawned in the spring to the northwest and west of Shetland and Orkney, while autumn-spawning took place primarily to the east of the Northern Isles (Fulton 1890; Wood 1930; Hodgson 1951; Rankine 1986). Spatial and temporal changes to the spawning ground distribution surrounding Shetland and Orkney likely occurred after the stock collapsed in the 1970s and subsequent recovery of the NSAS herring stock (Rankine 1986). Active spawning grounds are now considered limited to the autumn months when there is an abundance of yolk-sac larvae between Shetland and Orkney (Bartsch et al. 1989; Coull et al. 1998; Corten 2013).
Natal homing and seasonal straying behaviour
Herring philopatry to spawning grounds and season is well-documented (e.g., Mitchell 1864; Sinclair and Iles 1985; Geffen et al. 2011). It has, however, been questioned whether homing to natal spawning grounds is mediated by imprinting during the larval stage or by recruits learning from repeat spawners (Sinclair and Tremblay 1983; Mcquinn 1997; Brophy et al. 2006). Homing to natal spawning grounds has been evidenced through external tagging studies in the NW Atlantic (Stobo 1982; Stephenson et al. 2009; Wheeler and Winters 2011). However, tagging experiments on spawning grounds in the NE Atlantic revealed low recapture rates (Wood et al. 1954), or showed no clear evidence of homing to natal spawning grounds (Eggers et al. 2014). Biological markers, most notably otolith structure and microchemistry, have provided more empirical evidence that herring exhibit fidelity to spawning grounds and season in the Celtic Sea, west of the British Isles and Norway (Geffen et al. 2011; Deschepper et al. 2020; Berg et al. 2021). Straying between spring and autumn spawning seasons has been documented on both sides of the Atlantic, likely occurring at low but steady enough rates to facilitate gene flow (Brophy et al. 2006; Kerr et al. 2019; Berg et al. 2021). Herring spawning season is likely regulated by a combination of environmental and genetic variables (Han et al. 2020a).
Adaptation to specific environments
Recent molecular analyses revealed that herring have a “genomic toolbox” enabling them to adapt to specific ecological conditions, such as spawning season, temperature, salinity, and photoperiodic regulation, all likely related to life history parameters and environmental conditions experienced during larval development (Barrio et al. 2016; Bekkevold et al. 2016; Han et al. 2020a). The significant genomic differentiation between spring and autumn-spawning stocks suggests that spawning season has an underlying genetic-basis related to the biological responses to environmental variability between spawning season and the mechanisms that control reproductive timing (Kerr et al. 2019). Genetically different populations have been detected between spring and autumn-spawning herring in the waters surrounding the British Isles and Ireland (Farrell 2019). Autumn-spawning WoS and NSAS herring are genetically similar, while the spring-spawning WoS and Clyde stocks are genetically distinct from all herring populations in ICES Area VIa. Spring-spawning WoS herring are genetically similar to Norwegian spring-spawning (NSS; Table S1) fjord herring that spawn in comparable environmental conditions (Han et al. 2020a). Herring that spawn off the coast of Ireland represent several genetically differentiated populations, likely due to recent temporal shifts in spawning (Farrell 2019).
Spawning ground habitat characteristics
High-energy or structurally complex environments
High structural complexity of spawning substrate allows more egg surface area to encounter seawater, increasing oxygen supply and metabolic waste disposal, significantly improving development rates and egg survival on spawning grounds that are not subject to strong tides and currents (Hempel 1971; Woods and Podolsky 2007; Phillips and Moran 2015; von Nordheim et al. 2018). Spawning in multiple waves likely hedges against the uncertainty of spawning during poor weather in spring and autumn (Lambert 1987), but results in a build-up of egg masses at varying stages of development (Fig. 2; Parrish et al. 1959; Skaret et al. 2003; Skaret and Slotte 2017). Poor oxygen conditions can slow embryo development and increase mortality in benthic egg masses, particularly for embryos positioned centrally in the mass where oxygen concentrations are lowest (Cohen and Strathmann 1996; von Nordheim et al. 2018). WoS spring-spawning grounds were often recorded on or near maerl (Fig. 2; Table 1; Fig. S1; Morrison et al. 1991; Neervoort 2013), a hard “coralline” red algae that creates a complex three-dimensional bed of living maerl over dead maerl gravel, supporting a diverse community of plants and animals (Grall et al. 2006). Minch fishers believe herring preferentially selected maerl beds, referred to locally as “coral”, or seaweed/kelp to spawn on (Neervoort 2013). Interestingly, on the east coast of Scotland and the Norwegian shelf, herring used spawning beds covered with the Hydroida polyp phase of sea firs (reported in 3.3% of the examined literature sources). Half of the literature sources we reviewed here reported spawning grounds with macroalgal coverage (Table 1). Most sources (20%) described spawning beds covered in non-coralline macroalgae (to genus level), maerl was predominantly recorded on the western coast of Scotland and adjacent seas (13%), as well as by a single study in Norway, while herring eggs on kelp are only known from Scottish waters (albeit this is the preferred spawning substrate of Pacific herring (C. pallasii); reported in 16% of sources; Table 1).
Herring that spawn in higher energy (e.g., strong currents or tidally active) environments still select structurally complex environments and deposit eggs over “coarse” substrates (e.g., gravel, small rocks, shingle, or coarse sand). The occurrence of coarse seabed substrate in herring spawning grounds was reported by most sources reviewed (92%) and has been widely documented throughout the Northeast Atlantic (e.g., De Groot 1979; Table 1), including the well-known spawning grounds at Ballantrae Bank in the Firth of Clyde (e.g., Ewart 1884; Parrish et al. 1959; Morrison et al. 1991; Stratoudakis et al. 1998). Several (8%) studies also described spawning beds consisting of broken mollusc shells. Although NSAS spawning grounds have been determined by capture of herring in spawning condition and distribution of young larvae (Fig. 3), the consistency with which these sightings have been recorded over gravel deposits increases confidence that spawning likely takes place on coarse substrate (Fig. S2; Parrish and Saville 1965; Postuma et al. 1975; Schmidt et al. 2009). Furthermore, aquaria experiments of ripe herring demonstrated that, when given the choice of different spawning substrata, herring choose complex textures and patterns (i.e., gravel or macrophyte), to deposit spawn on (Holliday 1958; Scottish Home Department 1960). However, the full extent of potentially suitable spawning habitat (e.g., coarse substrate) is often far larger than the actual areas used for spawning (O’Sullivan et al. 2013; Šaškov et al. 2014). Whether this is due to an overabundance of suitable spawning habitat or to a lack of the right combination of required (multiple) habitat characteristics is not known.
Strong winds and tidal forcing likely impact deposited herring spawn, particularly in shallow or nearshore spawning grounds, where storm events cause significant mortality of eggs (Moll et al. 2018). Spring-spawning WoS herring did not spawn on the mainland side of the Minch during periods of strong easterly winds and would leave spawning grounds if winds became unfavourable (Scottish Government 1884; Neervoort 2013). Wind and tidal forcing integrated into biophysical models of larvae transport from Celtic Sea spawning grounds affected the outcomes, suggesting that these parameters impact when and where herring spawn (Deschepper et al. 2020). There is no empirical evidence suggesting that herring spawning activity is influenced by lunar cycles in the Northeast Atlantic (see Lambert 1987 and references therein).
Geomorphology and salinity
Whilst herring appear to seek structurally complex substrates to deposit spawn on, geomorphology and salinity also seem to be important habitat cues for the selection of spawning beds. For example, seabed geomorphology was a far more significant determinant than substrate for the location of spawning beds of Baltic herring on the Lithuanian coast, who only used one third of presumed suitable spawning habitat (Šaškov et al. 2014). Similarly, spring-spawning Clyde herring deposited eggs almost exclusively on ridges at Ballantrae Banks and off the coast of Arran, with very few egg observations in the hollows (Scottish Home Department 1966; Morrison et al. 1991; Stratoudakis et al. 1998). In the southern English Channel, geomorphology as well as hydrography seem to be important for herring that used to spawn on a narrow strip of seabed only, in line with the direction of tidal flow (Bolster and Bridger 1957).
Regarding the salinity of their spawning grounds, herring show a high plasticity. Whilst most spawning locations in Scottish waters are characterized by fully marine salinities, some spring-spawning was reported in the upper reaches of sea lochs, which are subject to freshwater input from seasonal rainfall and snowmelt resulting in salinity fronts (i.e., ~ 20–34.75 PSU) (Matthews 1885; English 2000; Neervoort 2013; Scanlon et al. 2021). Off the Isle of Mull, herring spawned near a productive fresh water convergence zone close to the coastal current (Macleod et al. 2004a). Similarly, herring in the Firth of Forth, on the Scottish East Coast, used to spawn in brackish water (Scottish Government 1886), and in Irish waters, many herring spawning grounds are located at the mouth of large rivers (O’Sullivan et al. 2013). In Norwegian fjords, where NSS herring spawn, freshwater runoff regularly modifies the hydrography (Johannessen et al. 1995; Sætre et al. 2002; Berg et al. 2017).
Water temperature and depth
Water temperature of spawning beds is highly variable, depending on spawning season (spring versus autumn/winter spawning), proximity to shore and habitat type. The Fishery Board for Scotland, the branch of the Scottish Government that monitored fisheries in the 19th and early twentieth century, assumed that the disappearance of herring from their usual spring-spawning grounds was temperature related and that herring would not spawn when bottom temperatures were too low (Scottish Government 1884). In fact, exceptionally cold winter temperatures can delay WoS herring maturation, and thus delay the onset of spawning to some extent (De Silva 1973). Low temperature at spawning beds during reproduction impacts recruitment and subsequent year-class strength through increased embryo mortality (Blaxter 1956; Postuma 1971). Blaxter (1956) reported preferred spawning temperatures of Scottish herring of 5–14 °C (Table 2), but embryo mortality did not occur until temperatures fell below 1.3 °C or went above 22 °C (Blaxter 1956, 1960). Skaret et al. (2003) suggested that NSS herring can modify their spawning time with prevailing conditions to optimise reproduction and survival of their offspring. Similarly, Icelandic spring-spawning, which occurs over a constant 30 day period year-to-year, can be delayed by up to 10 days when ocean temperatures are colder than average (Óskarsson and Taggart 2009). The temporal spawning patterns of Baltic herring (Table S1) is plastic, coinciding with temperatures reaching 6 °C, regardless of when that temperature is reached (Šaškov et al. 2014), allowing these populations to mitigate some of the effects of variability in spring temperatures (Dodson et al. 2019). However, in the shallow Western Baltic, the delayed seasonal onset of cold periods and corresponding elongation of the interval over which larvae hatch has reduced contemporary reproductive success of spring-spawning herring (Polte et al. 2021). Spawning phenology shifting as seas warm could severely impact the reproductive timing and success of herring in Scottish waters because of short daylength limiting larval growth and match-mismatch dynamics of larvae and zooplankton prey (Hufnagl and Peck 2011).
Water depth of NE Atlantic herring spawning grounds also varies considerably. Spawning beds have been observed between 1 and 250 m for spring-spawners (Table 2). For example, Baltic herring populations spawn almost exclusively in the littoral or sublittoral zones as shallow as ~ 1 m (Geffen 2009; Kanstinger et al. 2018), while NSS populations have spawned in depths up to 250 m (Slotte 2001). However, the majority of spring-spawning grounds (apart from the Baltic Sea and Blackwater Estuary) occur at depths less than 80 m (Wood 1930; Runnström 1941; Fridriksson and Timmermann 1951; Parrish et al. 1959; De Groot 1979). The depth of autumn-spawning grounds appears less variable (~ 35–150 m; Table 2) than spring-spawning grounds, but there is much less precise information on autumn-spawning grounds, due to the lack of direct observations of offshore spawning activity.
Larval advection/retention
Whether herring choose spawning sites to retain larvae near the area or to facilitate transport of the hatch to more productive nursery grounds is contradictorily discussed. Changes in hydrography can significantly impact larval development and survival (Røttingen 1990). Herring larvae can adjust their swimming behaviour in response to environmental variables (e.g., light and water turbidity) and prey availability (Munk and Kierboe 1985; MacKenzie and Kiørboe 1995; Folkvord et al. 2009). Although, the swimming ability of small (i.e., 8-11 mm) larvae is largely limited to vertical movements (Rosenthal 1968), often making them more reliant on current velocity for movement in high energy environments (Henri et al. 1985), larger larvae can actively influence their position within the water column (Stephenson and Power 1988; Fortier and Leggett 2011). Larvae in the shallow Baltic Sea, not exposed to strong tidal forcing or current regimes, were able to actively control their distribution within the’retention area’, suggesting that behavioural mechanisms might be important for larval dispersal in the absence of significant hydrographic features (Polte et al. 2017).
It has been suggested that the location of herring spawning grounds in the North Atlantic is a function of the number, location, and geographic extent of larval retention areas (Iles and Sinclair 1982). A review on herring larval drift and transport supports the hypothesis that spawning locations of Atlantic herring populations are actively chosen to promote larval retention near spawning grounds, although some dispersal is unavoidable (Sinclair and Power 2015). For example, the larvae of WoS herring spawning inshore remain in those areas for long periods resulting from lower flushing rates of waters in the Minch, Inner Hebrides, and along the northern coast of Scotland (Heath et al. 1987; Heath 1989). Conversely, larvae hatched west of the Hebrides are transported by currents from shelf waters to the north coast of Scotland (Heath and Maclachlan 1987; Heath et al. 1987). Dooley and McKay (1975) examined herring larval transport from west of the Hebrides to the North Sea, via the Fair Isle current, and observed that the youngest larvae were confined to areas between the current and northern coast of mainland Scotland, whilst older ones occurred within the core of the current. They concluded that the current was too narrow to allow the transport of a significant number of herring larvae. High recruitment of Downs (English Channel) herring has been linked to longer retention times near spawning grounds (Dickey-Collas et al. 2009). NSS spawning grounds are often located in areas of reduced vertical water stratification compared to surrounding areas, favouring larval retention. High wind-turbulence during springtime likely increases encounter rates between herring and their food source in retention areas, thus contributing to higher survival rates (Sætre et al. 2002). Biophysical models simulating dispersal of young larvae from spawning grounds in the Celtic Sea suggest that retention and transport are influenced by a combination of larval behaviour, tides, and wind (Deschepper et al. 2020).
Spawning ground abandonment and recolonization: prominent examples
Scotland
When herring abundance declines, the geographic distribution of spawning sites often contracts (Patterson 1998; Ivshina 2001; Rottingen and Slotte 2001; Overholtz and Friedland 2002; Schmidt et al. 2009). Spawning grounds used by NSAS herring changed throughout the twentieth century (Schmidt et al. 2009), and the stock collapse in the 1970s led to a decrease in the area used for spawning, but the process involved in the recolonization of spawning grounds is not well understood (Schmidt et al. 2009; Dickey-Collas et al. 2010). Prior to collapse of the stock, the Buchan grounds, off the northeast coast of Scotland, were a historically important fishing ground for pre-spawning (Table S1) and spawning NSAS herring (Scottish Home Department 1960; Parrish and Saville 1965). The grounds were abandoned from the late 1960s to early 1980s, which coincided with a period of low plankton biomass in the area but ended in 1981. During the time of low plankton abundance, spawning in the North Sea was restricted entirely to the more productive waters surrounding Orkney and Shetland (Corten 1988, 1999). The reappearance of spawning herring at Buchan Banks in 1983 was not ascribed to increased herring abundance in the Northern Isles that would cause spill-over spawning, but instead attributed to a strong Atlantic inflow of warm water that resulted in a southern displacement of feeding and pre-spawning herring (Corten 1999). However, “core” spawning grounds in Shetland and Orkney were maintained throughout the 1960s–1980s, and Schmidt et al. (2009) contended that the recolonization of southern spawning grounds resulted from a change in the core sites that eventually spread to areas with lower herring abundance. Regardless of the mechanism behind recolonization, herring are particular in where they choose to spawn, and subtle changes in environmental variables can potentially alter their preference for spawning in certain grounds that meet habitat requirements, offering their offspring the best chance of survival (Corten 1988). Hence, the herrings’ plasticity in spawning ground usage can buffer against temporary variations in the environment (Schmidt et al. 2009), improving stock resilience.
Norway
In Norway, the formerly productive herring spring-spawning stock underwent a significant decline in the 1950s and 60 s, during which time there was high fisheries mortality, a lack of fishing regulations, and an inflow of cold Atlantic water masses (Rottingen and Slotte 2001; Toresen and Østvedt 2008). The reduction of the stock led to a range contraction that altered migration patterns and resulted in a northward shift of spawning activity from previously south of the 60°N parallel during the early twentieth century, to spawning occurring north of the 62°N parallel only at the beginning of the rebuilding phase in the late 1960s (Rottingen and Slotte 2001). Herring began recolonizing historically important southern spawning grounds after the recruitment of strong year classes in 1983 and 1989 (Høines et al. 1998). Management measures were then introduced to protect spawning grounds, allocating [low] fishing quotas to small inshore vessels only, in an effort to increase SSB by extending the geographic extent of available spawning habitat and reducing density-dependent mortality of eggs and larvae (Patterson 1998; Rottingen and Slotte 2001; Slotte 2001). During the 1990s, the SSB of the NSS stock recovered to similar levels as in the early twentieth century (Toresen and Østvedt 2008), driving the recolonization of southern spawning grounds (Rottingen and Slotte 2001). In 2009, significant spawning in some southern spawning grounds led to recruitment of a strong year-class that continued using the former grounds (Eggers et al. 2014; ICES 2020c). However, a lack of recruitment success elsewhere in Norwegian waters has caused a substantial reduction in the overall SSB of the NSS stock (ICES 2020c; Tiedemann et al. 2020). Although the exact mechanisms behind the abandonment and recolonization of herring spawning grounds is not implicit, it remains a common feature among herring populations.
Effects of primary productivity and climate on early life stages
Primary production in the North Sea has been in decline since the late 1980s, due to warming sea temperatures and reduction of riverine runoff (Capuzzo et al. 2018). This has resulted in a bottom-up effect on higher tropic levels, mediated through a decline in copepod abundance, leading to recruitment depressions in herring and other important commercial fish stocks. Unfortunately, due to the paucity of data on plankton and larval abundance from the west and northwest coasts of Scotland, similar assessments cannot be made for this region. However, a short time-series of recent data from a fixed-point plankton monitoring site in Loch Ewe (Minch) showed a negative trend in the abundance of all zooplankton lifeforms between 2003 and 2017 (Moffat et al. 2020). Climate variability has also been proposed as a mechanism driving density-dependent cannibalism, negatively affecting herring recruitment, for example, when prey availability (e.g., copepods and small fish) is low and adult herring numbers are high (Holst 1992; Gröger et al. 2010), or when changes in the distribution of herring stocks increase contact between adults and larvae (Corten 2013). Herring stock dynamics appear to be influenced by the dynamics of multiple trophic levels in the North Sea (Segers et al. 2007). Akimova et al. (2016) suggested that herring SSB is more strongly influenced by fluctuations in the Atlantic inflow, which has a significant impact on zooplankton abundance and composition, than by temperature (Akimova et al. 2016). Temperature in the Barents Sea was a poor predictor of NSS herring recruitment (Bogstad et al. 2013), and modelled simulations of NSS larvae survival in this area was more closely associated with rapid displacement of larvae to nursery grounds than with temperature (Vikebø et al. 2010). When temperature data used in recruitment models is not representative of the full range of temperatures experienced by the early life stages of a stock, regime shifts in the plankton community can provide a good temporal indicator of changes in recruitment (Payne et al. 2009, 2013; Capuzzo et al. 2018; Estrella-Martínez et al. 2019).
In the North Atlantic, herring recruitment depression during the late 1960s and 1970s coincided with an abrupt drop in sea surface temperature (SST) in the Northern Hemisphere, when an influx of freshwater entered the North Atlantic (Thompson et al. 2010). Herring stocks would likely have naturally experienced lower levels of recruitment, but fisheries continued to expand, likely contributing to stock collapses in the North Atlantic during that decade (Corten 1988; Toresen and Østvedt 2008). The population dynamics of NSS herring have been linked to warming and cooling phases of the Atlantic Multidecadal Oscillation (AMO; Table S1), with higher recruitment and catches occurring in warm AMO phases during the 19th and early twentieth centuries (Gröger et al. 2010; Alheit et al. 2014; Tiedemann et al. 2020). A warm AMO phase began in the 1990s, presumably facilitating recruitment of strong-year classes at its beginning, but was more recently characterized by higher SST in the Celtic and Nordic Seas, likely outside the optimal thermal conditions for survival of early life-history stages of herring (Alheit et al. 2014; Toresen et al. 2019; Tiedemann et al. 2020). This could explain the lack of abundant year-classes since 2005. In Garcia et al.'s (2021) appraisal of the drivers of NSS herring recruitment, the authors tested 30 different hypotheses from previous studies to explain variation in recruitment success. Only 2 hypotheses were supported: (i) top-down control of larvae by predation and (ii) a positive relationship between recruitment and warmer temperatures (~ 3.5 °C), until the 2000s, when higher temperatures (reaching ~ 4.4 °C) started to negatively affect recruitment. NSAS herring also experienced lower recruitment in the early 2000s, which was attributed to decreased growth and larval survival rates caused by a regime shift in the plankton community arising from contemporary warming of the North Sea (Payne et al. 2009, 2013).
The optimum temperatures for the early life stages of spring-spawning Clyde and NSAS herring is ~ 8–9 °C (Overnell 1997), but viable hatching and larval development of both populations has been observed in temperatures ranging from 3.5 to 17 °C (Blaxter 1956; Overnell 1997). Year-class strength in NSAS herring is associated with winter bottom temperatures, likely reflecting the physiological effect of temperature on growth and development of young larvae (Nash and Dickey-Collas 2005). The abundance of early life-history stages of spring-spawning Baltic herring near shallow spawning grounds exhibits a dome-shaped relationship with SST, and survival rates have been adversely affected by increasing summer temperatures, presumably exceeding the optimal thermal window for eggs and larvae (Arula et al. 2016; Dodson et al. 2019). Local or regional environmental changes can alter the likelihood of recruitment success for spawning in either spring or autumn, often resulting in fluctuations in stock dominance between spring or autumn-spawners, which can hedge against risks associated with changing environmental conditions (Melvin et al. 2009). However, climate-driven changes will presumably affect spring and autumn-spawning herring differently. While autumn-spawned herring are unlikely to avoid unfavourable conditions by delaying spawning time, or by shifting to a more northern geographic range (due to their long development period and short daylength), early spring or late summer-spawned larvae will be tightly coupled with zooplankton production dynamics in the NE Atlantic (Hufnagl and Peck 2011).
Contemporary warming trends in the North Atlantic have seemingly favoured the predominance of autumn-spawning herring, except in distributional extremes where stocks are restricted to a single spawning season, and changing environmental conditions have resulted in recruitment suppression and northward movements of some stocks (Melvin et al. 2009; Alheit et al. 2014; Tiedemann et al. 2020). However, following unusually harsh winters in 2013–2015, the North Atlantic entered a cooler phase, likely to persist for some time (Frajka-Williams et al. 2017). This may have helped to facilitate the WoS spring-spawning events in 2018 and 2019, either directly or indirectly as a proxy for other environmental or oceanographic mechanisms. Evidence (e.g., fidelity to spawning season and grounds with low rates of straying) suggests that WoS spring-spawning herring have likely gone undetected in the Minch for some time. Herring in a spawning state have periodically been recorded in the spring (Peter Cunningham, Wester Ross Fisheries Trust, Pers. Comm.), but survey efforts have primarily focussed on more profitable species (e.g., salmon and trout). Recruitment of a particularly strong year class (or several), when environmental conditions became favourable, has previously facilitated rebuilding of stocks and extant spawning populations (see examples above) and could be responsible for the recent re-emergence of WoS spring-spawning herring.
Management and conservation of spawning grounds
Intense fishing pressure, environmental variability and insufficient management measures during the nineteenth and twentieth centuries led to herring stock collapses and widespread ecosystem change of near-shore habitats, with significant ecological and economic consequences (Dickey-Collas et al. 2010; Thurstan et al. 2014; Jones et al. 2016). Historic habitat use by fish is typically not accounted for in contemporary management plans. Local extirpations and geographic range shifts may gradually become accepted as the new normal, the so-called “shifting baseline syndrome” (Pauly 1995; Plumeridge and Roberts 2017), leading to unambitious conservation and recovery targets for priority species and habitats (Plumeridge and Roberts 2017). On the other hand, distribution shifts driven by climate change could lead to unattainable population rebuilding goals. Areas where fish were once abundant may no longer provide the species’ climate niche. For herring, which are reliant on specific habitat for the survival of their early life stages, historic temporal and spatial fluctuations in the use of spawning grounds should be recognized and considered. This would ensure that limited availability of suitable spawning habitat does not restrict recolonization and proper rebuilding plans are established (Ellis et al. 2012). Effective herring management strategies should, but currently do not in Scotland, incorporate the species’ spawning grounds, particularly in shallower inshore areas that are more vulnerable to anthropogenic and environmental impacts (Olsen et al. 2010).
Scientific advice recommends that activities negatively impacting herring spawning grounds should not occur (ICES 2020a). Yet Scotland’s recent Marine Assessment (Moffat et al. 2020) identified pressure from bottom-contacting gear (e.g., trawling and dredging) as one of the most widespread and direct pressures across all Scottish marine regions. These practices can alter the physical and biological characteristics of seabed habitats, posing an immediate threat to essential herring spawning grounds (Watling and Norse 1998; Ryan and Bailey 2012). While ~ 7% of Scottish inshore waters are included in a network of Marine Protected Areas (MPAs), bottom-towed gear is only prohibited in 2.5% of the inshore MPA network (Langton et al. 2020). In addition, maerl, a seemingly important spawning substrate for spring-spawning herring in Scottish waters, is extremely vulnerable to physical disturbance and environmental change (Donnan and Moore 2003). Maerl could undergo spatial declines of up to 84% under projected climate change scenarios (Simon-Nutbrown et al. 2020), indicating that its protection in Scottish waters may be crucial in order to maintain [maerl] refuge populations. A more holistic ecosystem-based approach, encompassing the needs of all herring life stages, and their essential habitats, is therefore key for effective management (Zhou et al. 2010; Link et al. 2019). This would likely require conserving more of Scotland’s seabed from damaging anthropogenic activities (e.g., bottom-contacting gear) as advised by the scientific community (ICES 2020b; Moffat et al. 2020). The planning and implementation of an ecosystem-based approach should involve industry stakeholders to assure their ‘buy-in’, as successfully achieved elsewhere (e.g., in New Zealand) (Mackinson and Middleton 2018).
Gaps of knowledge and future study needs
Incorporating conservation of essential herring spawning habitat into management plans first requires identifying all historic and extant spawning grounds. Here we have shown that this information is often lacking, particularly for the west coast of Scotland and offshore regions (Haegele and Schweigert 1985; Aires et al. 2014). Key gaps of knowledge (GoK) we have identified refer to: (1) location, habitat type and status of current and historic herring spawning beds in Scottish waters; (2) presence and abundance of larvae on the west coast and inshore waters of Scotland; and (3) presence and abundance of spring-spawning herring on the west coast of Scotland. The first GoK is based on the lack of herring egg observations on the seabed and the reliance on “proxy” methods of spawning ground identification. The unpredictable nature of spawning events and inclement weather during herring spawning seasons has historically made sampling of eggs in situ difficult. However, modern survey techniques, such as ROVs and state-of-the-art dive and camera equipment, as well as industry and citizen-science monitoring projects could facilitate the detection and recording of herring spawning events.
Most important fished species in the NE Atlantic recruit from pelagic eggs and larvae that move towards coastal habitats as juveniles, but herring stocks represent a reversed pattern with dispersal towards pelagic zones. This has resulted in larval surveys focussing on offshore regions, neglecting inshore habitats despite their significant ecological roles (Polte et al. 2017). To tackle this and help fill GoK 2, surveys should include sampling of larvae and (spawning) adult herring in inshore waters on the west coast of Scotland, where habitat status and biodiversity information is limited. Locating ‘hidden’ aggregations of smaller extant spawning populations and detecting the early life stages of different herring populations is difficult (e.g., Skaret et al. 2003). However, conducting environmental DNA (eDNA) analyses from water samples could help determine if and when herring are present in inshore waters by detecting their DNA shed in the water column (Ratcliffe et al. 2021). This could potentially provide a rapid, non-destructive and widely accessible approach to monitoring spawning grounds. Trained citizen scientists could conduct community-based eDNA sampling and help filling the above GoKs.
Engaging with communities and the fishing sector is also important to tap into their valuable local ecological knowledge (LEK) regarding herring spawning ecology (helping to fill GoK 1–3), which is largely unexplored to date. Past industry and community engagement gave considerable insight into the location of spawning grounds (Fulton 1890; Baxter 1963; Neervoort 2013), but historic LEK from the last herring boom, i.e., prior to the 1970s, is on the brink of being lost forever. O’Sullivan et al. (2013) highlighted that the exact location of herring spawning beds in Irish waters was not known outside the fishing industry, and their inventory of herring spawning ground locations exemplifies what can be achieved through collaboration across sectors.
Conclusion
The precise locations of herring spawning grounds in Scottish waters have not been identified, aside from a few sites, and have never been incorporated into management plans (Fulton 1890; English 2000; Neervoort 2013). Yet, documented recolonization of formerly abandoned spawning grounds on the west coast of Scotland, the North Sea and Norway highlight the importance of maintaining these essential habitats to increase stock resilience (Patterson 1998; Corten 1999; Rottingen and Slotte 2001; Schmidt et al. 2009). Contemporary locations of herring spawning grounds determined by “proxies”, rather than by direct sampling of eggs, are insufficient for spatial management and conservation of herring stocks adapted to spawn under specific environmental conditions differing across populations (Bauer et al. 2014; Barrio et al. 2016; Han et al. 2020b). Furthermore, the use of contemporary data alone for spatial management underestimates the significant alterations marine ecosystems have experienced (Plumeridge and Roberts 2017). The recent observation of WoS spring-spawning herring at their historical spawning grounds, in a location without spatial management, demonstrates why historical data need to be included in conservation decisions.
We advocate the incorporation of essential spawning habitat into marine management plans, without which, herring stock recovery and expansion is likely to be jeopardized. Bridging historic and contemporary knowledge of herring spawning grounds in Scottish waters would allow to conserve, and potentially restore, such essential spawning habitat while accounting for natural variability in stock dynamics and potential future range shifts under climate change scenarios.
Data availability
Relevant data will be made available in supplementary material.
Code availability
Not applicable.
References
Aires C, Gonzalez-Irusta JM, Watret R (2014) Updating fisheries sensitivity maps in British waters. Scottish Mar Freshw Sci 5:1–89
Akimova A, Núñez-Riboni I, Kempf A, Taylor MH (2016) Spatially-resolved influence of temperature and salinity on stock and recruitment variability of commercially important fishes in the North sea. PLoS ONE 11:e0161917. https://doi.org/10.1371/journal.pone.0161917
Alheit J, Licandro P, Coombs S et al (2014) Atlantic Multidecadal Oscillation (AMO) modulates dynamics of small pelagic fishes and ecosystem regime shifts in the eastern North and Central Atlantic. J Mar Syst 131:21–35. https://doi.org/10.1016/j.jmarsys.2013.11.002
Aneer G (1985) Some speculations about the Baltic herring (Clupea harengus membras) in connection with the eutrophication of the Baltic Sea. Can J Fish Aquat Sci 42:83–90. https://doi.org/10.1139/f85-264
Aneer G (1989) Herring (Clupea harengus L.) spawning and spawning ground characteristics in the Baltic Sea. Fish Res 8:169–195. https://doi.org/10.1016/0165-7836(89)90030-1
Aneer G, Florell G, Kautsky U et al (1983) In-situ observations of Baltic herring (Clupea harengus membras) spawning behaviour in the Askö-Landsort area, northern Baltic proper. Mar Biol 74:105–110. https://doi.org/10.1007/BF00413912
Anonymous (1924) Scientific Investigations. Herring: The Spring and Autumn Broods. In: Forty-Third Annual Report of the Fishery Board for Scotland 1924, Cmd. 2479. H.M. Stationary Office, Edinburgh, p 92
Anonymous (2019) Scottish Sea Fisheries Statistics 2019. Marine Scotland. Edinburgh
Arula T, Raid T, Simm M, Ojaveer H (2016) Temperature-driven changes in early life-history stages influence the Gulf of Riga spring spawning herring (Clupea harengus m.) recruitment abundance. Hydrobiologia 767:125–135. https://doi.org/10.1007/s10750-015-2486-8
Axelsen BE, Nottestad L, Ferno A et al (2000) “Await” in the pelagic: dynamic trade-off between reproduction and survival within a herring school splitting vertically during spawning. Mar Ecol Prog Ser 205:259–269. https://doi.org/10.3354/meps205259
BBC (2019) BBC One—Blue Planet UK, Series 1, Episode 5, Herring have not been seen off UK coasts for many years...until now. https://www.bbc.co.uk/programmes/p074qrg7. Accessed 31 May 2021
Barrio AM, Lamichhaney S, Fan G et al (2016) The genetic basis for ecological adaptation of the Atlantic herring revealed by genome sequencing. Elife 5:1–33. https://doi.org/10.7554/eLife.12081
Bartsch J, Brander K, Heath M et al (1989) Modelling the advection of herring larvae in the North Sea. Nature 340:632–636. https://doi.org/10.1038/340632a0
Bauer RK, Grawe U, Stepputtis D et al (2014) Identifying the location and importance of spawning sites of Western Baltic herring using a particle backtracking model. ICES J Mar Sci 71:499–509. https://doi.org/10.1038/278097a0
Bauer RK, Stepputtis D, Gräwe U et al (2013) Wind-induced variability in coastal larval retention areas: a case study on Western Baltic spring-spawning herring. Fish Oceanogr 22:388–399. https://doi.org/10.1111/fog.12029
Baxter IG (1958) The composition of the Minch herring stocks. Rapp P-v Réun Cons Perm Int Explor Mer 143:81–94
Baxter IG (1963) Features of the Minch herring fisheries in pre- and post-war years. Cons Perm Int Pour L’exploration La Mer 154:227–235
Bekkevold D, Gross R, Arula T et al (2016) Outlier loci detect intraspecific biodiversity amongst spring and autumn spawning herring across local scales. PLoS ONE. https://doi.org/10.1371/journal.pone.0148499
Berg F, Husebø Å, Godiksen JA et al (2017) Spawning time of Atlantic herring (Clupea harengus) populations within a restricted area reflects their otolith growth at the larval stage. Fish Res 194:68–75. https://doi.org/10.1016/j.fishres.2017.05.009
Berg F, Østgaard HD, Slotte A et al (2021) A combination of genetic and phenotypic characterization of spring- and autumn-spawning herring suggests gene flow between populations. ICES J Mar Sci 78:694–703. https://doi.org/10.1093/ICESJMS/FSAA046
Blaxter JHS (1956) Herring rearing II: the effect of temperature and other factors on development. Mar Res 5:1–19
Blaxter JHS (1960) The effect of extremes of temperature on herring larvae. J Mar Biol Ass UK 39:605–608
Blaxter JHS, Hunter JR (1982) The biology of the clupeoid fishes. Adv Mar Biol 20:1–223. https://doi.org/10.1016/S0065-2881(08)60140-6
Bogstad B, Dingsør GE, Ingvaldsen RB, Gjøsæter H (2013) Changes in the relationship between sea temperature and recruitment of cod, haddock and herring in the Barents Sea. Mar Biol Res 9:895–907. https://doi.org/10.1080/17451000.2013.775451
Bolster GC, Bridger JP (1957) Nature of the spawning area of herrings. Nature 179:638
Bowers AB (1969) Spawning beds of Manx autumn herrings. J Fish Biol 1:355–359. https://doi.org/10.1111/j.1095-8649.1969.tb03883.x
Bowers AB (1980) Characteristics of herring spawning grounds. ICES C.M. 1980/H:13. Port Erin, Isle of Man
Brophy D, Danilowicz BS, King PA (2006) Spawning season fidelity in sympatric populations of Atlantic herring (Clupea harengus). Can J Fish Aquat Sci 63:607–616. https://doi.org/10.1139/f05-235
Capuzzo E, Lynam CP, Barry J et al (2018) A decline in primary production in the North Sea over 25 years, associated with reductions in zooplankton abundance and fish stock recruitment. Glob Chang Biol 24:e352–e364. https://doi.org/10.1111/gcb.13916
Clausen LAW, Bekkevold D, Hatfield EMC, Mosegaard H (2007) Application and validation of otolith microstructure as a stock identification method in mixed Atlantic herring (Clupea harengus) stocks in the North Sea and western Baltic. ICES J Mar Sci 64:377–385. https://doi.org/10.1093/ICESJMS/FSL036
Cohen CS, Strathmann RR (1996) Embryos at the edge of tolerance: effects of environment and structure of egg masses on supply of oxygen to embryos. Biol Bull 190:8–15. https://doi.org/10.2307/1542671
Corten A (1999) The reappearance of spawning Atlantic herring (Clupea harengus) on Aberdeen Bank (North Sea) in 1983 and its relationship to environmental conditions. Can J Fish Aquat Sci 56:2051–2061
Corten A (2013) Recruitment depressions in North Sea herring. ICES J Mar Sci 70:1–15. https://doi.org/10.1038/278097a0
Corten A (1988) Shifts In Herring Spawning Areas In The Northwestern North Sea In Relation To Environmental Changes. ICES C.M. 1988/H:22. IJmuiden
Coull JR (1986) The Scottish herring fishery 1800–1914: development and intensification of a pattern of resource use. Scott Geogr Mag 102:4–17. https://doi.org/10.1080/00369228618736643
Coull K, Johnstone R, Rogers S (1998) Fisheries Sensitivity Maps in British Waters, 1st edn. UKOOA Ltd
Cunningham P (2018) Spring spawning herring rediscovered to the west of Gairloch. Wester Ross Fisheries Trust. Wester Ross
Cunningham P (2019) Wester Ross spring spawning herring recorded on video to the west of Red Point, near Gairloch. Wester Ross Fisheries Trust. Wester Ross
De Silva SS (1973) Clupeid populations of inshore waters of the west coast of Scotland. Dissertation, University of Stirling
De Groot SJ (1979) The consequence of marine gravel extraction for the spawning of herring. ICES C.M. 1979/ E: 5. IJmuiden
Dempsey CH, Bamber RN (1983) Spawning of herring (Clupea harengus L.) in the Blackwater Estuary, spring 1979. ICES J Mar Sci 41:85–92. https://doi.org/10.1093/icesjms/41.1.85
Deschepper I, Lyons K, Lyashevska O, Brophy D (2020) Biophysical models reveal the role of tides, wind, and larval behaviour in early transport and retention of Atlantic herring (Clupea harengus) in the Celtic Sea. Can J Fish Aquat Sci 77:90–107. https://doi.org/10.1139/cjfas-2018-0491
Dickey-Collas M, Bolle LJ, Van Beek JKL, Erftemeijer PLA (2009) Variability in transport of fish eggs and larvae. II. Effects of hydrodynamics on the transport of downs herring larvae. Mar Ecol Prog Ser 390:183–194. https://doi.org/10.3354/meps08172
Dickey-Collas M, Nash RDM, Brunel T et al (2010) Lessons learned from stock collapse and recovery of North Sea herring: a review. ICES J Mar Sci 67:1875–1886
Dodson JJ, Daigle G, Hammer C et al (2019) Environmental determinants of larval herring (Clupea harengus) abundance and distribution in the western Baltic Sea. Limnol Oceanogr 64:317–329. https://doi.org/10.1002/lno.11042
Donnan DW, Moore PG (2003) Conclusions. Aquat Conserv Mar Freshw Ecosyst 13:77–78. https://doi.org/10.1002/aqc.570
Dooley H, McKay D (1975) Herring larvae and currents west of the Orkneys. ICES CM 1975/H:43. Aberdeen
von Dorrien C, Hammer C, Zimmermann C et al (2013) A review on herring, Clupea harengus (Actinopterygii: Clupeiformes: Clupeidae) recruitment and early life stage ecology in the western Baltic sea. Acta Ichthyol Piscat 43:169–182. https://doi.org/10.3750/AIP2013.43.3.01
Eggers F, Slotte A, Libungan LA et al (2014) Seasonal dynamics of Atlantic herring (Clupea harengus L.) populations spawning in the vicinity of marginal habitats. PLoS ONE. https://doi.org/10.1371/journal.pone.0111985
Ellis JR, Milligan SP, Readdy L et al (2012) Spawning and nursery grounds of selected fish species in UK waters. Sci Ser Tech Rep 147:56
English PR (2000) Arnisdale and Loch Hourn: v. 1: The Clachans, People, Memories and the Future. Arnisdale and Loch Hourn Community Association, Arnisdale
Estrella-Martínez J, Schöne BR, Thurstan RH et al (2019) Reconstruction of Atlantic herring (Clupea harengus) recruitment in the North Sea for the past 455 years based on the δ13C from annual shell increments of the ocean quahog (Arctica islandica). Fish Fish 20:537–551. https://doi.org/10.1111/faf.12362
Ewart JC (1884) Natural history of the herring. In: Annual report for the Fishery Board for Scotland 1884. Appendix F, IV. Edinburgh, pp 61–73
Farrell ED, Campbell N, Carlsson J, et al (2020) Herring in Divisions Assessment of the Identity of the Southern and Northern Stocks through Genetic and Morphometric Analysis. Final Report. EASME/EMFF/2017/1.3.2.1/SI2.767459. European Commission. Luxembourg
Farrell E (2019) Annex 6: Summaries of presentations from Stock ID mini symposium A6.1 Genetic Stock Identification of 6a/7bc Herring
Folkvord A, Høie H, Johannessen A, Solbakken T (2009) Effects of prey concentration, light regime, and parental origin on growth and survival of herring larvae under controlled experimental conditions. ICES J Mar Sci 66:1702–1709. https://doi.org/10.1093/icesjms/fsp072
Fortier L, Leggett WC (2011) Fickian transport and the dispersal of fish larvae in estuaries. Can J Fish Aquat Sci 39:1150–1163. https://doi.org/10.1139/F82-153
Frajka-Williams E, Beaulieu C, Duchez A (2017) Emerging negative Atlantic Multidecadal Oscillation index in spite of warm subtropics. Sci Rep 7:1–8. https://doi.org/10.1038/s41598-017-11046-x
Fridriksson A, Timmermann G (1951) Herring spawning grounds off the south coast of Iceland during spring 1950. ICES J Mar Sci 17:172–180. https://doi.org/10.1093/icesjms/17.2.172
Fulton TW (1890) The chief fishing grounds on the east coast of Scotland with charts showing their position and extent. Ninth Annual Report of the Fishery Board for Scotland 1890:397–411
Funk FC, Blackburn JC, Hay DC, et al (2000) Herring: expectations for a new millennium. Proceedings of the symposium, Anchorage, Alaska, February 23–26 2000. Anchorage
Gamble JC, Maclachlan P, Seaton DD (1985) Comparative growth and development of autumn and spring spawned Atlantic herring larvae reared in large enclosed ecosystems. Source Mar Ecol Prog Ser 26:19–33
Garcia T, Planque B, Arneberg P et al (2021) An appraisal of the drivers of Norwegian spring-spawning herring (Clupea harengus) recruitment. Fish Oceanogr 30:159–173. https://doi.org/10.1111/fog.12510
Geffen AJ (2009) Advances in herring biology: from simple to complex, coping with plasticity and adaptability. ICES J Mar Sci 66:1688–1695
Geffen AJ, Nash RDM, Dickey-Collas M (2011) Characterization of herring populations west of the British Isles: an investigation of mixing based on otolith microchemistry. ICES J Mar Sci 68:1447–1458. https://doi.org/10.1093/icesjms/fsr051
Gell FG, Hanley LJ (2013) Manx Marine Environmental Assessment. In: Hanley L, Gell FG, Kennington K et al (eds) Isle of Man Marine Plan. Douglas, Isle of Man Government, p 45
Grall J, Le Loc’h F, Guyonnet B, Riera P (2006) Community structure and food web based on stable isotopes (δ15N and δ13C) analysis of a North Eastern Atlantic maerl bed. J Exp Mar Bio Ecol 338:1–15. https://doi.org/10.1016/j.jembe.2006.06.013
Gröger JP, Kruse GH, Rohlf N (2010) Slave to the rhythm: How large-scale climate cycles trigger herring (Clupea harengus) regeneration in the North Sea. ICES J Mar Sci 67:454–465. https://doi.org/10.1093/icesjms/fsp259
Gullestad P, Sundby S, Kjesbu OS (2020) Management of transboundary and straddling fish stocks in the Northeast Atlantic in view of climate-induced shifts in spatial distribution. Fish Fish 21:1008–1026. https://doi.org/10.1111/faf.12485
Haegele CW, Schweigert JF (1985) Distribution and characteristics of herring spawning grounds and description of spawning behavior. Can J Fish Aquat Sci 42:39–55. https://doi.org/10.1139/f85-261
Han F, Jamsandekar M, Pettersson M et al (2020a) The genetic architecture underlying ecological adaptation in Atlantic herring is not consistent with the infinitesimal model. bioRxiv. https://doi.org/10.1101/2020.07.15.204214
Han F, Jamsandekar M, Pettersson ME et al (2020b) Ecological adaptation in Atlantic herring is associated with large shifts in allele frequencies at hundreds of LOCI. Elife 9:1–20. https://doi.org/10.7554/ELIFE.61076
Harland J, Parks R (2008) Technical Report: The fish remains from the Holm of Papa Westray North, a Neolithic chambered tomb. Reports from the Centre for Human Palaeoecology. Report 2008/14. University of York
Hay DE, Toresen R, Stephenson R et al (2001) Taking stock: an inventory and review of world herring stocks in 2000. In: Funk FC, Blackburn JC, Hay DC et al (eds) Herring: expectations for a new millennium. University of Alaska Sea Grant, AK-SG-01-04, Fairbanks, pp 381–454
Heath M (1989) Transport of larval herring (Clupea harengus L.) by the Scottish coastal current. Cons Int Explor Mer 191:85–91
Heath M (1993) An evaluation and review of the ICES herring larval surveys in The North Sea and adjacent waters. Bull Mar Sci 53:795–817
Heath M, MacLachlan P, Martin J (1987) Inshore circulation and transport of herring larvae off the north coast of Scotland. Mar Ecol Prog Ser 40:11–23. https://doi.org/10.3354/meps040011
Heath M, Maclachlan P (1987) Dispersion and mortality of yolk-sac herring (Clupea harengus L.) larvae from a spawning ground to the west of the Outer Hebrides. J Plankton Res 9:613–630. https://doi.org/10.1093/plankt/9.4.613
Hemmings CC (1965) Underwater observations on a patch of herring spawn. Scottish Fish Bull 23:21–22
Hempel G (1971) An estimate of mortality in eggs of North Sea herring (Clupea harengus L.). Rapp P-v Réun Cons Perm Int Explor Mer 160:8–11
Hempel G, Blaxter JHS (1967) Egg weight in Atlantic herring (Clupea harengus L.). ICES J Mar Sci 31:170–195. https://doi.org/10.1093/icesjms/31.2.170
Henri M, Dodson JJ, Powles H (1985) Spatial configurations of young herring (Clupea harengus) larvae in the St. Lawrence Estuary: importance of biological and physical factors. Can J Fish Aquat Sci 42:91–104. https://doi.org/10.1139/f85-265
Hodgson WC (1951) The herring Atlas. ICES J Mar Sci 17:196–196. https://doi.org/10.1093/icesjms/17.2.196
Holliday FGT (1958) The spawning of herring. Scottish Fish Bull 10:11–13
Holst JC (1992) Cannibalism as a factor regulating year class strength in the Norwegian spring-spawning herring stock. ICES C.M.1992/H:14. Bergen-Nordnes
Hopkins P, Morrison J (1991) Evaluation of geostatistical methods for the estimation of total egg numbers in a herring spawning bed. ICES C.M. 1991/11
Hufnagl M, Peck MA (2011) Physiological individual-based modelling of larval Atlantic herring (Clupea harengus) foraging and growth: insights on climate-driven life-history scheduling. ICES J Mar Sci 68:1170–1188. https://doi.org/10.1093/ICESJMS/FSR078
Hunter A, Speirs DC, Heath MR (2019) Population density and temperature correlate with long-term trends in somatic growth rates and maturation schedules of herring and sprat. PLoS ONE 14:e0212176. https://doi.org/10.1371/journal.pone.0212176
Høines ÅS, Bergstad OA, Albert OT (1998) The structure and temporal stability of the fish community on a coastal bank utilized as a spawning ground by herring. ICES J Mar Sci 55:271–288. https://doi.org/10.1006/jmsc.1997.0268
ICES (2019b) Herring assessment working group for the area south of 62° N (HAWG). ICES Scientific Reports, Copenhagen
ICES (1994) Report of The Study Group on herring assessment and biology in the Irish Sea and adjacent waters. ICES C.M. 1994/H:5. Belfast
ICES (2010) Report of the Study Group on the evaluation of assessment and management strategies of the western herring stocks (SGHERWAY). ICES CM 2010/SSGSUE:08. 194. Dublin
ICES (2015a) Second Interim Report of the Working Group on maritime systems (WGMARS). ICES C.M. 2014/SSGSUE:08. Copenhagen
ICES (2015b) ICES Stock Annex: Herring (Clupea harengus) in divisions 6.a and 7.b–c (West of Scotland, West of Ireland). Benchmark Workshop on West of Scotland Herring (WKWEST)
ICES (2019a) Herring (Clupea harengus) in divisions 6.a (combined) and 7.b–c. Herring Assessment Working Group. ICES Scientific Reports 1:2
ICES (2020a) Herring (Clupea harengus) in Subarea 4 and divisions 3.a and 7.d, autumn spawners (North Sea, Skagerrak and Kattegat, eastern English Channel). ICES Advice on fishing opportunities, catch, and effort Greater North Sea ecoregion
ICES (2020b) Herring (Clupea harengus) in divisions 6.a and 7.b–c (West of Scotland, West of Ireland). ICES Advice on fishing opportunities, catch, and effort Celtic Seas ecoregion
ICES (2020c) Herring (Clupea harengus) in subareas 1, 2, and 5, and in divisions 4.a and 14.a, Norwegian spring-spawning herring (the Northeast Atlantic and the Arctic Ocean). ICES Advice on fishing opportunities, catch, and effort Ecoregions in the Northeast Atlantic
Iles TD, Sinclair M (1982) Atlantic herring: stock discreteness and abundance. Science 215:627–633
Ivshina ER (2001) Decline of the Sakhalin-Hokkaido herring spawning grounds near the Sakhalin Coast. In: Funk FC, Blackburn JC, Hay DC et al (eds) Herring: expectations for a new millennium. University of Alaska Sea Grant, AK-SG-01-04, Fairbanks, pp 245–254
Johannessen A, Blom G, Folkvord A, Svendsen H (1995) The effect of local wind on the distribution of Norwegian spring spawning herring (Clupea harengus L.) larvae. In: HR S, C H, KE E, HP L (eds) Ecology of Fjords and Coastal Waters. Elsevier Science Ltd, Tromso, Norway, pp 365–384
Jones HFR (1968) Fish migration. Edward Arnold Publishers Ltd., London
Jones P, Cathcart A, Speirs DC (2016) Early evidence of the impact of preindustrial fishing on fish stocks from the mid-west and southeast coastal fisheries of Scotland in the 19th century. ICES J Mar Sci 73:1404–1414. https://doi.org/10.1093/icesjms/fsv189
Kanstinger P, Beher J, Grenzdörffer G et al (2018) What is left? Macrophyte meadows and Atlantic herring (Clupea harengus) spawning sites in the Greifswalder Bodden, Baltic Sea. ECSS 201:72–81. https://doi.org/10.1016/J.ECSS.2016.03.004
Kerr Q, Fuentes-Pardo AP, Kho J et al (2019) Temporal stability and assignment power of adaptively divergent genomic regions between herring (Clupea harengus) seasonal spawning aggregations. Ecol Evol 9:500–510. https://doi.org/10.1002/ECE3.4768
Kerr LA, Hintzen NT, Cadrin SX et al (2017) Lessons learned from practical approaches to reconcile mismatches between biological population structure and stock units of marine fish. ICES J Mar Sci 74:1708–1722
Kumpulainen R (2001) Timber and herring: modernisation and mobility in Finnish Lapland and The Western Islands of Scotland, 1770–1970
Kääriä J, Rajasilta M, Kurkilahti M, Soikkeli M (1997) Spawning bed selection by the Baltic herring (Clupea harengus membras) in the Archipelago of SW Finland. ICES J Mar Sci 54:917–923. https://doi.org/10.1006/jmsc.1996.0204
Lambert T (1987) Duration and intensity of spawning in herring Clupea harengus as related to the age structure of the mature population. Mar Ecol Prog Ser 39:209–220. https://doi.org/10.3354/meps039209
Langton R, Stirling DA, Boulcott P, Wright PJ (2020) Are MPAs effective in removing fishing pressure from benthic species and habitats? Biol Conserv 247:108511. https://doi.org/10.1016/j.biocon.2020.108511
Link JS, Dickey-Collas M, Rudd M et al (2019) Food for thought: clarifying mandates for marine ecosystem-based management. ICES J Mar Sci 76:41–44. https://doi.org/10.1093/icesjms/fsy169
Little Green Island Films (2018) Local scallop divers discovered a vast area of herring spawn on maerl beds near Gairloch. https://vimeo.com/271000785. Accessed 1 Feb 21
MacKenzie BR, Kiørboe T (1995) Encounter rates and swimming behavior of pause-travel and cruise larval fish predators in calm and turbulent laboratory environments. Limnol Oceanogr 40:1278–1289. https://doi.org/10.4319/lo.1995.40.7.1278
Mackinson S, Middleton DAJ (2018) Evolving the ecosystem approach in European fisheries: transferable lessons from New Zealand’s experience in strengthening stakeholder involvement. Mar Policy 90:194–202. https://doi.org/10.1016/j.marpol.2017.12.001
Macleod K, Fairbairns R, Gill A et al (2004a) Seasonal distribution of minke whales Balaenoptera acutorostrata in relation to physiography and prey off the Isle of Mull, Scotland. Mar Ecol Prog Ser 277:263–274. https://doi.org/10.3354/meps277263
Macleod K, Macleod K, Fairbairns R et al (2004b) Seasonal distribution of minke whales. Mar Ecol Prog Ser 277:263–274
Maravelias CD (2001) Habitat associations of Atlantic herring in the Shetland area: influence of spatial scale and geographic segmentation. Fish Oceanogr 10:259–267. https://doi.org/10.1046/j.1365-2419.2001.00172.x
Maravelias CD, Reid DG, Swartzman G (2000) Seabed substrate, water depth and zooplankton as determinants of the prespawning spatial aggregation of North Atlantic herring. Mar Ecol Prog Ser 195:249–259. https://doi.org/10.3354/meps195249
Marshall SM, Nicholls AG, Orr AP (1937) On the growth and feeding of the larval and post-larval stages of the Clyde herring. J Mar Biol Assoc UK 22:245–267. https://doi.org/10.1017/S002531540001198X
Matthews JD (1885) Report as to the variety among the herrings on the Scottish coasts. Annual report for the Fishery Board for Scotland 1885. Appendix F No IV. Edinburgh
Mcquinn IH (1997) Metapopulations and the Atlantic herring. Rev Fish Biol Fish 7:297–329
Melvin GD, Stephenson RL, Power MJ (2009) Oscillating reproductive strategies of herring in the western Atlantic in response to changing environmental conditions. ICES J Mar Sci 66:1784–1792. https://doi.org/10.1093/icesjms/fsp173
Mitchell JM (1864) The Herring: Its Natural History and National Importance. Edinburgh
Moffat C, Baxter J, Berx B, et al (2020) Scotland’s marine assessment 2020. Scottish Government. http://marine.gov.scot/sma/. Accessed 18 Mar 21
Moll D, Kotterba P, von Nordheim L, Polte P (2018) Storm-induced Atlantic Herring (Clupea harengus) egg mortality in Baltic Sea inshore spawning areas. Estuaries Coasts 41:1–12. https://doi.org/10.1007/s12237-017-0259-5
Molloy J, Barnwall E, Morrison J (1993) Herring tagging experiments around Ireland, 1991. Republic of Ireland Fisheries Research Centre Fisheries Leaflet 15: 1–7, Dublin
Morrison JA, Napier IR, Gamble JC (1991) Mass mortality of herring eggs associated with a sedimenting diatom bloom. ICES J Mar Sci 48:237–245. https://doi.org/10.1093/icesjms/48.2.237
Morrison JA, Gamble JC, Shand C (1990) Mass mortality of spring spawning herring eggs in Scottish waters. ICES C.M. 1990/L:I06. Aberdeen
Munk P, Kierboe T (1985) Feeding behaviour and swimming activity of larval herring (Clupea harengus) in relation to density of copepod nauplii. Mar Biol Res 24:15–21
Munro RJ (1883) The herring fisheries. International fisheries exhibition London. William Clowes and Sons Limited, London
Nash RDM, Dickey-Collas M (2005) The influence of life history dynamics and environment on the determination of year class strength in North Sea herring (Clupea harengus L.). Fish Oceanogr 14:279–291. https://doi.org/10.1111/j.1365-2419.2005.00336.x
Neervoort R (2013) The fish that did not get away—Tales from herring fishers about the decline of the Wester Ross herring fishery. Dissertation, Vrije Universiteit Amsterdam
von Nordheim L, Kotterba P, Moll D, Polte P (2018) Impact of spawning substrate complexity on egg survival of Atlantic herring (Clupea harengus, L.) in the Baltic Sea. Estuaries Coasts 41:549–559. https://doi.org/10.1007/s12237-017-0283-5
von Nordheim L, Kotterba P, Moll D, Polte P (2020) Lethal effect of filamentous algal blooms on Atlantic herring (Clupea harengus) eggs in the Baltic Sea. Aquat Conserv Mar Freshw Ecosyst 30:1362–1372. https://doi.org/10.1002/aqc.3329
Olsen E, Aanes S, Mehl S et al (2010) Cod, haddock, saithe, herring, and capelin in the Barents Sea and adjacent waters: a review of the biological value of the area. ICES J Mar Sci 67:87–101. https://doi.org/10.1093/icesjms/fsp229
Overholtz WJ, Friedland KD (2002) Recovery of the Gulf of Maine-Georges Bank Atlantic herring (Clupea harengus) complex: perspectives based on bottom trawl survey data. Fish Bull 100:593–608
Overnell J (1997) Temperature and efficiency of development during endogenous feeding in herring embryos and yolk-sac larvae. J Fish Biol 50:358–365. https://doi.org/10.1006/jfbi.1996.0298
O’Sullivan D, Eimear O’keeffe S, Berry A et al (2013) An inventory of Irish herring spawning grounds. Irish Fish Bull 42:1–31
Parrish BB, Saville A (1965) The biology of the north-east Atlantic herring populations. Oceanogr Mar Biol an Annu Rev 3:323–373
Parrish BB, Saville A, Craig RE et al (1959) Observations on herring spawning and larval distribution in the Firth of Clyde in 1958. J Mar Biol Assoc United Kingdom 38:445–453. https://doi.org/10.1017/S002531540000686X
Patterson KR (1998) Biological modelling of the Norwegian spring-spawning herring stock. Fisheries Research Services Report No 1/98. Aberdeen
Pauly D (1995) Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol Evol 10:430
Payne MR, Hatfield EMC, Dickey-Collas M et al (2009) Recruitment in a changing environment: the 2000s North Sea herring recruitment failure. ICES J Mar Sci 66:272–277. https://doi.org/10.1093/icesjms/fsn211
Payne MR, Ross SD, Clausen LW et al (2013) Recruitment decline in North Sea herring is accompanied by reduced larval growth rates. Mar Ecol Prog Ser 489:197–211. https://doi.org/10.3354/meps10392
Phillips NE, Moran AL (2015) Oxygen production from macrophytes decreases development time in benthic egg masses of a marine gastropod. Hydrobiologia 757:251–259. https://doi.org/10.1007/s10750-015-2256-7
Plumeridge AA, Roberts CM (2017) Conservation targets in Marine protected area management suffer from shifting baseline syndrome: a case study on the Dogger bank. Mar Pollut Bull. https://doi.org/10.1016/j.marpolbul.2017.01.012
Polte P, Gröhsler T, Kotterba P et al (2021) Reduced reproductive success of western Baltic herring (Clupea harengus) as a response to warming winters. Front Mar Sci 8:1–13. https://doi.org/10.3389/fmars.2021.589242
Polte P, Kotterba P, Moll D, Von Nordheim L (2017) Ontogenetic loops in habitat use highlight the importance of littoral habitats for early life-stages of oceanic fishes in temperate waters. Sci Rep 7:1–10. https://doi.org/10.1038/srep42709
Postuma KH (1971) The effect of temperature in the spawning and nursery areas on recruitment of autumn-spawning herring in the North Sea. Rapp P-v Réun Cons Int Explor Mer 160:173–183
Postuma KH, Saville A, Wood RJ (1975) Herring Spawning Grounds in the North Sea. ICES C.M.1975/H:46
Rankine PW, Morrison JA (1989) Predation on herring larvae and eggs by sand-eels Ammodytes marinus (rait) and Hyperoplus lanceolatus (lesauvage). J Mar Biol Assoc United Kingdom 69:493–498. https://doi.org/10.1017/S0025315400029556
Rankine PW (1986) Herring spawning grounds around the Scottish coast. ICES C.M. 1986/H:15
Ratcliffe FC, Uren Webster TM, Garcia de Leaniz C, Consuegra S (2021) A drop in the ocean: monitoring fish communities in spawning areas using environmental DNA. Environ DNA 3:43–54. https://doi.org/10.1002/edn3.87
Rorke M (2005) The Scottish herring trade, 1470–1600. Scott Hist Rev 84:149–165. https://doi.org/10.3366/shr.2005.84.2.149
Rosenthal H (1968) (1968) Schwimmverhalten und schwimmgeschwindigkeit bei den larven des herings Clupea harengus. Helgoländer Meeresun 184(18):453–486. https://doi.org/10.1007/BF01611680
Rottingen I, Slotte A (2001) The relevance of a former important spawning area in the present life history and management of Norwegian spring-spawning herring. In: Funk FC, Blackburn JC, Hay DC et al (eds) Herring: expectations for a new millennium. University of Alaska Sea Grant, AK-SG-01-04, Fairbanks, pp 297–313
Runnström S (1941) Quantitative investigations on herring spawning and its yearly fluctuations at the West Coast of Norway. Rep nor Fish Mar Investig 6:1–71
Ryan MR, Bailey DM (2012) Trawling and dredging in the Clyde sea area: history, impacts and prospects for Recovery University of Glasgow
Røttingen I (1990) A review of variability in the distribution and abundance of Norwegian spring spawning herring and Barents Sea capelin. Polar Res 8:33–42. https://doi.org/10.3402/polar.v8i1.6801
Samarra FIP, Miller PJO (2015) Prey-induced behavioural plasticity of herring-eating killer whales. Mar Biol 162:809–821. https://doi.org/10.1007/s00227-015-2626-8
Saville A, Baxter IG, McKay DW (1974) Relations between egg production, larval production and spawning stock size in Clyde herring. The early life history of fish. Springer, Berlin, pp 129–138
Saville A, Parrish BB, Baxter IG (1966) Review of Herring Fisheries and Stocks off the West Coast of Scotland. ICES C.M. 1966:H1
Scanlon T, Moreau J, Stickland M (2021) A Hydrodynamic Model of the West Coast of Scotland with Coupled Sea Lice Dispersion. TELEMAC-MASCARET User Conference—Lindner Hotel, Antwerp
Schmidt JO, van Damme CJG, Röckmann C, Dickey-Collas M (2009) Recolonisation of spawning grounds in a recovering fish stock: recent changes in North Sea herring. Sci Mar 73:153–157. https://doi.org/10.3989/scimar.2009.73s1153
Scottish Government (1884) Second Annual Report of the Fishery Board for Scotland. Edinburgh
Scottish Government (1886) Fourth Annual Report of the Fishery Board for Scotland. Cmd. 5097. Edinburgh
Scottish Government (1890) Ninth Annual Report of the Fishery Board for Scotland. Cmd. 6450. HMSO. Edinburgh
Scottish Government (1899) Eighteenth Annual Report of the Fishery Board for Scotland. Cmd. 149. Edinburgh
Scottish Government (1936) Fifty-fifth Annual Report of the Fishery Board for Scotland. Cmd. 5411. Edinburgh
Scottish Home Department (1959) Report on the Fisheries of Scotland 1958. Cmd. 704. HMSO. Edinburgh
Scottish Home Department (1960) Report on the Fisheries of Scotland 1959. Cmd. 1097. HMSO. Edinburgh
Scottish Home Department (1965) Fisheries of Scotland Report for 1964. Cmd. 2644. HMSO. Edinburgh
Scottish Home Department (1966) Fisheries of Scotland Report for 1965. Cmd. 2947. HMSO. Edinburgh
Scottish Home Department (1967) Fisheries of Scotland Report for 1966. Cmd. 3460. HMSO. Edinburgh
Segers FHID, Dickey-Collas M, Rijnsdorp AD (2007) Prey selection by North Sea herring (Clupea harengus), with special reference to fish eggs. ICES J Mar Sci 64:60–68. https://doi.org/10.1093/icesjms/fsl002
Simon-Nutbrown C, Hollingsworth PM, Fernandes TF et al (2020) Species distribution modelling predicts significant declines in coralline algae populations under projected climate change with implications for conservation policy. Front Mar Sci 7:1–14. https://doi.org/10.3389/fmars.2020.575825
Sinclair M, Iles TD (1985) Atlantic herring (Clupea harengus) distributions in the gulf of Maine—Scotian shelf area in relation to oceanographic features. Can J Fish Aquat Sci 42:880–887. https://doi.org/10.1139/F85-112
Sinclair M, Power M (2015) The role of “larval retention” in life-cycle closure of Atlantic herring (Clupea harengus) populations. Fish Res 172:401–414
Sinclair M, Tremblay MJ (1983) A New Hypothesis to Account for the Timing of Spawning of Herring populations. ICES C.M.1983/H:47. Halifax
Skaret G, Nøttestad L, Fernö A et al (2003) Spawning of herring: day or night, today or tomorrow? Aquat Living Resour 16:299–306. https://doi.org/10.1016/S0990-7440(03)00006-8
Skaret G, Slotte A (2017) Herring submesoscale dynamics through a major spawning wave: duration, abundance fluctuation, distribution, and schooling. ICES J Mar Sci 74:717–727. https://doi.org/10.1093/icesjms/fsw180
Slotte A (2001) Factors influencing location and time of spawning in Norwegian spring-spawning herring: an evaluation of different hypotheses. In: Funk FC, Blackburn JC, Hay DC et al (eds) Herring: expectations for a new millennium. University of Alaska Sea Grant, AK-SG-01-04, Fairbanks, pp 255–278
Stephenson RL, Melvin GD, Stephenson MJP et al (2009) Population integrity and connectivity in Northwest Atlantic herring: a review of assumptions and evidence. ICES J Mar Sci 66:1733–1739. https://doi.org/10.1093/ICESJMS/FSP189
Stephenson RL, Power MJ (1988) Semidiel vertical movements in Atlantic herring Clupea harengus larvae: a mechanism for larval retention? Source Mar Ecol Prog Ser 50:3–11
Stobo WT (1982) Tagging Studies on Scotian Shelf Herring. Northwest Atl Fish Organ Serial No. N617. NAFO SCR Doc. 82/IX/108
Stratoudakis Y, Gallego A, Morrison JA (1998) Spatial distribution of developmental egg ages within a herring Clupea harengus spawning ground. Mar Ecol Prog Ser 174:27–32. https://doi.org/10.3354/meps174027
Sætre R, Toresen R, Søiland H, Fossum P (2002) The Norwegian spring-spawning herring—spawning, larval drift and larval retention. Sarsia 87:167–178. https://doi.org/10.1080/003648202320205247
Thompson DWJ, Wallace JM, Kennedy JJ, Jones PD (2010) An abrupt drop in Northern Hemisphere sea surface temperature around 1970. Nature 467:444–447. https://doi.org/10.1038/nature09394
Thurstan RH, Hawkins JP, Roberts CM (2014) Origins of the bottom trawling controversy in the British Isles: 19th century witness testimonies reveal evidence of early fishery declines. Fish Fish 15:506–522. https://doi.org/10.1111/faf.12034
Thurstan RH, Roberts CM (2010) Ecological meltdown in the firth of Clyde, Scotland: two centuries of change in a coastal marine ecosystem. PLoS ONE 5:e11767. https://doi.org/10.1371/journal.pone.0011767
Tiedemann M, Nash RDM, Stenevik EK et al (2020) Environmental influences on Norwegian spring-spawning herring (Clupea harengus L.) larvae reveal recent constraints in recruitment success. ICES J Mar Sci. https://doi.org/10.1093/icesjms/fsaa072
Toresen R, Skjoldal HR, Vikebø F, Martinussen MB (2019) Sudden change in long-term ocean climate fluctuations corresponds with ecosystem alterations and reduced recruitment in Norwegian spring-spawning herring (Clupea harengus, Clupeidae). Fish Fish 20:686–696. https://doi.org/10.1111/faf.12369
Toresen R, Østvedt OJ (2008) Variation in abundance of Norwegian spring-spawning herring (Clupea harengus, Clupeidae) throughout the 20th century and the influence of climatic fluctuations. Fish Fish 1:231–256. https://doi.org/10.1111/j.1467-2979.2000.00022.x
Trochta JT, Branch TA, Shelton AO, Hay DE (2020) The highs and lows of herring: a meta-analysis of patterns and factors in herring collapse and recovery. Fish Fish 21:639–662. https://doi.org/10.1111/faf.12452
Vikebø FB, Husebø Å, Slotte A et al (2010) Effect of hatching date, vertical distribution, and interannual variation in physical forcing on northward displacement and temperature conditions of Norwegian spring-spawning herring larvae. ICES J Mar Sci 67:1948–1956. https://doi.org/10.1093/icesjms/fsq084
Watling L, Norse EA (1998) Disturbance of the seabed by mobile fishing gear: a comparison to forest clearcutting. Conserv Biol 12:1180–1197. https://doi.org/10.1046/j.1523-1739.1998.0120061180.x
Wheeler JP, Winters GH (2011) Homing of atlantic herring (Clupea harengus) in Newfoundland waters as indicated by tagging data. Can J Fish Aquat Sci 41:108–117. https://doi.org/10.1139/F84-010
Wood H (1960) The herring of the Clyde estuary. Mar Res 1:1–24
Wood RJ (1971) Autumn-spawning herring grounds to the west of Scotland. Rapp Proces-Verbaux Des Reun 160:16–40
Wood H, Parrish BB, McPherson G (1954) Review of Scottish herring tagging experiments 1948–1953. ICES C 1954:1–29
Wood H (1930) Scottish herring shoals: pre-spawning and spawning movements. Fishery Board for Scotland Scientific Investigations No. 1. Edinburgh
Woods HA, Podolsky RD (2007) Photosynthesis drives oxygen levels in macrophyte-associated gastropod egg masses. Biol Bull 213:88–94. https://doi.org/10.2307/25066621
Zhou S, Smith ADM, Punt AE et al (2010) Ecosystem-based fisheries management requires a change to the selective fishing philosophy. Proc Natl Acad Sci USA 107:9485–9489
Óskarsson GJ, Taggart CT (2009) Spawning time variation in Icelandic summer-spawning herring (Clupea harengus). Can J Fish Aquat Sci 66:1666–1681. https://doi.org/10.1139/F09-095
Šaškov A, Šiaulys A, Bǔcas M, Daunys D (2014) Baltic herring (Clupea harengus membras) spawning grounds on the Lithuanian coast: current status and shaping factors. Oceanologia 56:789–804. https://doi.org/10.5697/oc.56-4.789
Acknowledgements
We would like to thank the anonymous reviewers for their constructive suggestions and comments. We also wish to thank the William Grant Foundation for funding and the Skye and Wester Ross Fisheries Trust (SWRFT)/Skye and Lochalsh Rivers Trust (SRLT) for support and collaboration. We acknowledge the following persons for provision of information: Peter Cunningham (Wester Ross Fisheries Trust) for sharing his knowledge on herring and fisheries in Wester Ross; Sue Pomeroy (Little Loch Broom Marine Life), who kindly provided material on historic fisher accounts and the Wester Ross region; Dr Ed Farell (EDF Scientific Limited); Helen McGregor (Marine Scotland Science), Linda Fitzpatrick (Scottish Fisheries Museum), Dr Karen Buchanan (Gairloch Heritage Museum), Rachel Culver (SAMS institute), and Dr Clive Fox (SAMS institute) for providing or pointing the authors in the right direction of a variety of literature. We also thank Jackie Daly from SubSeaTV, on behalf of Andy Jackson’s estate, for allowing us to use his herring spawning bed image.
Funding
This study was funded by the William Grant Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Consent to participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Frost, M., Diele, K. Essential spawning grounds of Scottish herring: current knowledge and future challenges. Rev Fish Biol Fisheries 32, 721–744 (2022). https://doi.org/10.1007/s11160-022-09703-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11160-022-09703-0