Abstract
This paper updates previous reviews of the 1993 stock decline of Pacific herring (Clupea pallasi) in Prince William Sound, Alaska, and focuses on hypotheses about subsequent poor recovery. Recent age structured assessment modeling with covariate analysis indicates that the population dynamics of the sound’s herring are influenced by oceanic factors, nutrition, and, most substantially, hatchery releases of juvenile pink salmon. For the 1993 decline, poor nutrition remains the most probable cause with disease a secondary response. Concerning poor recovery, we examined 16 potential factors and found three to be causal: oceanic factors, poor nutrition, and hatchery releases of juvenile pink salmon. Absences of strong year classes at both Sitka and Prince William Sound after 1993 indicate the action of large-scale ocean processes. Beyond regional-scale environmental factors, two factors specific to the sound influence the population dynamics of herring and are likely impeding recovery. First, pink salmon fry releases have increased to about 600 million annually and may disrupt feeding in young herring, which require adequate nutrition for growth and overwintering survival. Juvenile pink salmon and age-1 herring co-occur in nearshore areas of bays in late spring and summer, and available data on dietary overlap indicates potential competition between the age-1 juvenile herring and juvenile pink salmon. Field studies demonstrate that juvenile herring reduce food intake substantially in the presence of juvenile pink salmon. Second, overwintering humpback whales may consume potentially large amounts of adult herring, but further studies must confirm to what extent whale predation reduces herring biomass.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Because of the commercial and ecological importance of the Pacific herring (Clupea pallasi) population in Prince William Sound (PWS) Alaska, there is concern for the present and future states of PWS herring. In a comprehensive review, Hay et al. (2001) found that during the last century many herring stocks around the world have collapsed. Intense fishing has often been associated with such collapses, but habitat loss and environmental change have also led to collapses of herring and other species (Hilborn 1997). Recovery of most collapsed herring stocks usually took a decade or more (Hay et al. 2001). For example, after 15 years of no fishing, the Georges Bank herring returned to stock levels above those before the 1977 collapse. Some stocks, however, have not returned to their former abundance even after several decades. For example, the Hokkaido-Sakhalin stock has remained extremely depressed since its decline in the late 1950s. Most collapsed stocks appear to recover, but the present status of some stocks is “worrisome.” One stock considered worrisome is that in PWS (Hay et al. 2001).
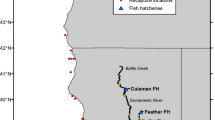
PWS herring biomass increased steadily through the 1980s (Fig. 1). Funk (1993, 1994) used ASA (age structured assessment) modeling to predict that the 1993 PWS herring spawning biomass would be a record high of over 121,000 metric tons. However, the predicted biomass failed to materialize (Brown et al. 1994, 1996; Carls et al. 2002). Brown et al. (1994) report that the observed PWS spawning biomass was about 27,000 metric tons in spring 1993 or about 20% of expectation.
After the decline, the fishery was closed from 1994 through 1996. The PWS herring stock increased to the point where modest harvests of 3,629 to 4,536 metric tons were permitted in 1997 and 1998. Then, in 1999, only half the predicted PWS biomass was found (Marty et al. 2003). In 1999, the PWS herring fishery was closed and remains closed because of insufficient biomass.
Before the early 1990s, the PWS herring population and fishery were sustained by recruitment of strong year classes about every 4 years (Funk and Sandone 1990; Funk 1994; Williams and Quinn 2000a, b). Recovery of PWS herring would be greatly aided by the recruitment of a highly successful year class. However, no strong year class has emerged in PWS since the 1988 year class recruited in 1991 and 1992 (Fig. 2).
The two aims of this paper are (1) to briefly update the review of Pearson et al. (1999) concerning the decline of PWS Pacific herring (Clupea pallasi) stock in 1992–1993 (Table 1) and (2) to assess hypotheses concerning the subsequent poor recovery of the stock. For the first aim, the decline, we focus on information that has become available since the late 1990s, particularly work subsequent to Pearson et al. (1999), which includes studies by Marty et al. (2003, 2004, 2007, 2010) on disease, a comprehensive disease review by Elston and Meyers (2009), and ASA modeling focused on PWS herring (Quinn et al. 2001; Deriso et al. 2007, 2008; Hulson et al. 2008). Deriso et al. (2008) used ASA modeling with covariate analysis to quantitatively assess 19 decline and recovery hypotheses. ASA models have been well described in the literature (Quinn and Deriso 1999; Deriso et al. 2007, 2008) and will not be discussed in detail here. An important aspect of these models is that their statistical nature enables them to be used appropriately to provide estimates of uncertainty and to test hypotheses. Deriso et al. (2008) used 19 different time series of data generally for the period from the early 1970s to 2005 as single and multi-factor covariates in their ASA modeling. Modeling results revealed the need to assess predation and competition in greater detail and led to substantial effort and analysis in those areas in this paper.
Identifying the hypotheses
We have identified hypotheses from literature reviews and have distinguished the hypotheses about the decline from those about poor recovery (Table 2). Herring have supported important fisheries worldwide for centuries (Hay et al. 2001; Stephenson 2001), and high variability in abundance has been known for decades to be a common and inherent feature of herring stocks (Hjort 1914; Smith 1988; Rothschild 1986; Southward et al. 1988; Hay et al. 2001). Prominent among the various causes proposed for herring stock fluctuations have been the influence of ocean climate on recruitment processes and the effects of intense fisheries (Hay et al. 2001; Stephenson 2001). Unlike other declines that involve recruitment failure (Hilborn 1997), the PWS 1992–1993 decline was a one-time event with an acute increase in adult mortality, not a recruitment failure (Pearson et al. 1999; Quinn et al. 2001; Carls et al. 2002; Deriso et al. 2008; Hulson et al. 2008). Hypotheses concerning the decline must provide mechanisms that increase mortality in all adult age classes between the spring of 1992 and the spring of 1993. Mechanisms governing poor recovery cannot be assumed to be the same as those for the decline. Hypotheses for poor recovery must provide mechanisms for (1) no recruitment of strong year classes after the 1988 year class recruited and (2) continued low herring biomass.
Hypotheses for the decline of PWS herring
The hypotheses concerning the decline and acceptance and rejection of certain hypotheses have evolved substantially since 1993. The ASA models of Quinn et al. (2001), Hulson et al. (2008), and Deriso et al. (2008) all require an abrupt increase in mortality in 1992–1993 to achieve reasonable model fits. Any hypothesis for the decline must explain this abrupt increase in adult mortality between 1992 and 1993. We briefly describe the evolution of the hypotheses below and assess the two hypotheses, oil exposure from the 1989 Exxon Valdez oil spill (EVOS) and disease, about which some debate continues.
At the time of the decline, the major hypotheses concerning its cause(s) centered on two factors: oil exposure and disease. After identifying viral hemorrhagic septicemia virus (VHSV) in Pacific herring, Meyers et al. (1994) concluded that the role of VHSV in the 1992–1993 decline could not be established with the 1993 data. Brown et al. (1996) conjectured that the decline was due to an epizootic, and Marty et al. (1998) implicated VHSV disease as the cause of the 1992–1993 decline.
Later, Pearson et al. (1999) reviewed 13 hypotheses concerning the 1993 decline (Table 1). These 13 hypotheses fell into groups related to the EVOS, harvest, and natural processes, especially disease. Pearson et al. rejected hypotheses concerning oil-spill effects based on the high population levels following the spill, the lack of change from the expected age-class structure, and the low level of oil exposure following the spill. They also found no evidence to support hypotheses about harvesting effects and judged hypotheses concerning water temperature, predation, and competition to have insufficient information to accept or reject them. Condition, as weight at specific length, is a measure of nutritional state of fish (Winters and Wheeler 1994; Cardinale et al. 2003). The 1993 mortality was preceded by sharply declining condition (weight at specific length) in PWS herring from about 1986 (Fig. 3) and by lower zooplankton abundance (Fig. 4). Pearson et al. concluded that poor nutrition, either alone or in combination with disease or other natural factors, probably caused the decline.
Herring condition as weight at specific length in spring for Prince William Sound and Sitka by calendar year. Weight at specific length was calculated following Winters and Wheeler (1994). Data from: ADFG (unpublished data, See Appendix), Biggs et al. (1992), Brady et al. (1987, 1988, 1990, 1991a, b), Donaldson et al. (1992), Dressel (2006), Sandone (1988)
Settled volume of spring zooplankton in Prince William Sound. Data from: Cooney et al. (2001)
Since the earlier review (Pearson et al. 1999), new information is available about the decline relative to oil exposure, harvest, disease, poor nutrition, and oceanic factors. Hypotheses relating the decline to oil-spill effects, harvest effects, spawning-habitat loss, or the spawn-on-kelp fishery continue to be unsupported by the available evidence (Table 3). The declining herring condition and low zooplankton abundance (Figs. 3 and 4) described in Pearson et al. (1999) still support the conclusion that poor nutrition was a major factor in the 1993 decline. The results of the ASA modeling of Deriso et al. (2008) offer evidence for both oceanic factors (winter sea-surface temperature) and poor nutrition in the 1993 decline (Table 4). Since the 1999 review, other investigators also report that the 1993 decline was most likely derived from poor nutrition with disease as a secondary factor (Carls et al. 2002; Marty et al. 2003; Hulson et al. 2008; Rice and Carls 2007; Elston and Meyers 2009). Poor nutrition remains the best explanation of the 1992–1993 decline, although some debate remains about potential influence from the oil spill and about the role of disease agents.
Oil exposure
Recent evidence continues to support the earlier conclusions of Pearson et al. (1999) that oil exposure during or after the EVOS did not play a role in the 1992–1993 decline. Boehm et al. (2007) synthesized the available information on the water-column concentrations of total polycyclic aromatic hydrocarbons (TPAH) with emphasis on nearshore waters in the spill path and concluded that “water column concentrations of TPAH resulting for the spill returned to background levels by 1990, ranging from 0.001 to 0.10 ppb TPAH.” An oil-exposure index derived from the TPAH burdens in mussels confirms that oil exposure was not a significant factor affecting adult mortality in the single-factor or multi-factor ASA models of Deriso et al. (2008) (Table 4). Carls et al. (2002), Marty et al. (2003), Rice and Carls (2007) and Hulson et al. (2008) find factors other than the EVOS (e.g., poor nutrition) to be the likely direct cause of the 1992–1993 decline.
Thomas and Thorne (2003) and Thorne and Thomas (2008) suggest that the decline actually began in 1989 and was associated with the EVOS. As a mechanism for exposure to oil and to subsequent mortality, they postulate direct contact with oil when herring break the water surface during “gulping” of air to fill their swim bladders. At dusk, herring schools ascend from deeper waters to surface waters to feed (Blaxter 1985). While at the surface, herring may gulp air to fill their swim bladders and regain buoyancy. The extent of gulping is unknown.
Gulping is a plausible way for herring to contact oil on the water surface, but such contact would be brief and intermittent. Behavioral avoidance of slicks and sheens is also plausible and would prevent or minimize exposure. Herring avoid suspended dredged materials (Johnston and Wildish 1981), but the extent to which herring avoid oil is unknown.
Assuming herring were unable to avoid slicks and sheens, the window for significant direct contact with EVOS oil in slicks and sheens would have been limited primarily to spring 1989 and would have ended when EVOS sheens decreased to background by the fall of 1990. The oil slicks from the Exxon Valdez began to exit PWS about one week after the beginning of the spill (Wolfe et al. 1994). By April 1989, floating oil within PWS had fallen to primarily surface sheens (Wolfe et al. 1994).
At their greatest extent, sheens associated with EVOS covered only a very small area of PWS on a few days. The occurrence, volume, and source of PWS sheens were surveyed from summer 1989 until fall 1990 (Taft et al. 1995). The majority of the sheens (75%) were small (<1 l) with only 19 of the total 827 sheens being greater than 1,000 l in oil volume. A rainbow-colored sheen has a thickness of 0.0003 mm (Taft et al. 1995) so that a rainbow sheen of 1,000 l would cover an area of about 3,000 m2. Of these 19 sheens, 13 large-volume sheens associated with the EVOS occurred on October 24, 1989, after a storm had released oil from beach sediments. These 13 sheens had volumes from 1,000 to 54,000 l and covered areas from 0.003 km2 about 0.34 km2. If all 13 were of the maximum observed extent, these sheens would have covered about 2.2 km2 or about 0.049% of PWS total area of 9,059 km2. After March 1990, no sheens from EVOS were greater than 65 l in oil volume. By September of 1990, PWS sheens from EVOS had decreased to smaller volumes than those associated with normal vessel traffic.
If direct and lethal contact between herring and oil had been widespread in 1989, then large, observable herring kills would be expected, but none was observed. In contrast, mass mortalities of herring have been observed during epizootics (Tibbo and Graham 1963; Patterson 1996; Meyers et al. 1999) and an industrial spill of elemental phosphorus (Winters et al. 1986). However, even with the massive boat traffic throughout PWS during the 1989 and 1990 cleanup—and with scientists specifically looking for fish kills—no herring (or other fish) kills were observed.
If herring had direct and lethal contact with spilled oil, adult herring survival would have been lowered in 1989. In fact, adult herring survival was highest in 1989 and lowest in 1992 (Hulson et al. 2008)―opposite to the expectation if direct contact with slicks was inducing substantial adult herring mortality.
Viral hemorrhagic septicemia virus
Viral hemorrhagic septicemia virus (VHSV) infects Pacific herring and other marine fish species along the west coast of North America (Meyers et al. 1994; Hedrick et al. 2003). Recent studies suggest that VHS is primarily a disease of age-0 herring and that the disease is either rapidly resolved or fatal within 2 weeks following infection (Kocan et al. 2001; Hershberger et al. 2007; Elston and Meyers 2009). Fish surviving VHS acquire immunity. VHSV is an opportunistic pathogen in adult fish that produces disease when fish are stressed by environmental or other factors (Meyers et al. 1994; Elston and Meyers 2009).
Based on an analysis of VHSV-positive herring samples in 1993 from PWS and other areas, Meyers et al. (1994) concluded that the role of VHSV in the 1992–1993 decline could not be established with the 1993 data. A critical and comprehensive review of the role of disease in PWS herring population dynamics from 1989 to 2005 led Elston and Meyers (2009) to conclude that VHS was not a primary cause of the 1992–1993 decline for several reasons: (1) VHSV is principally a pathogen of juvenile herring (Kocan et al. 1999a); (2) the mortality in 1992–1993 was distributed across all age classes including those age classes resistant to the disease (Elston et al. 1997); (3) no mass mortality was observed; (4) the poor condition of the herring itself was sufficient to cause the decline; and (5) most herring simply did not return to spawning grounds in 1993 and could not be examined for cause of death. The designation of the herring population crash of 1992–1993 as an “epidemic,” implying a primary infectious cause (e.g., Marty et al. 2003; Marty 2007) is without basis. Elston and Meyers (2009) evaluated all evidence regarding VHS in PWS herring from 1989 to 2005 and found no evidence to support any assignment of significant effects of VHSV on PWS herring biomass or recruitment.
Additionally, hypothesis tests using the ASA modeling by Deriso et al. (2008) did not find VHSV to be a significant factor when tested in the model as a single factor. The VHSV indices used for younger fish were selected by the hypothesis test procedure as the fourth factor in a four-factor model, however the regression coefficient had the incorrect (i.e., positive) sign.
Viral hemorrhagic septicemia virus and oil exposure
Pearson et al. (1999) found no evidence that either acute or chronic oil exposure from the EVOS contributed to the 1993 decline. Carls et al. (2002) stated that the consequences of high biomass, disease, and poor nutrition are the more likely explanations of the 1993 decline but indicated that indirect links to the EVOS could not be entirely ruled out. Carls et al. (2002) and Marty et al. (2007) find a link from oil exposure to the decline unlikely, in part because of the time delay between oil exposure and the decline.
An indirect link from oil exposure through disease is suggested by one experiment of Carls et al. (1998) but is not supported by subsequent experiments. In reviewing experiments that attempted to induce or exacerbate VHSV in herring by weathered or fresh crude oil exposure (Carls et al. 1998; Kennedy et al. 1999; Kocan et al. 1999b; Sanders 2005), Elston and Meyers (2009) found that none of the experiments with juvenile and adult herring demonstrated a causal link between any oil effect and VHSV at oil-exposure concentrations as high as 178 to 328 ppb TPAH (range of highest treatments used) for between 14 and 28 days. Rice and Carls (2007) now also consider the EVOS not to be a causal or contributing factor in the decline.
Pathogen Ichthyophonus hoferi
The pathogen Ichthyophonus hoferi has long been associated with epizootics leading to mass mortalities and low population levels in Atlantic herring (Tibbo and Graham 1963; Patterson 1996; Kramer-Schadt et al. 2010). An alternative or additional causative agent, I. hoferi, was identified by Meyers et al. (1994) and Marty et al. (1998) but was believed an incidental finding at the time and subsequently (Quinn et al. 2001; Marty et al. 2003).
Recent work by Kramer-Schadt et al. (2010), studying Norwegian spring spawning herring (NSSH), and a reiteration of the ASA model (Marty et al. 2010) suggest that I. hoferi can be an important factor in herring population declines. However, Kramer-Schadt et al. (2010) demonstrate that representative sampling of herring populations to estimate prevalence, severity, and impact of I. hoferi requires extensive effort to account for broad geographic migration patterns, differential distribution of the disease by age class, and clustering of infected fish in schools more vulnerable to sampling.
Sampling of PWS herring for the presence of I. hoferi prior to the decline was limited but two points derived from all available data are contrary to the I. hoferi as the causative agent in the decline. First, mass mortalities with large numbers of moribund and dead fish in shallow areas have been associated with I. hoferi in Atlantic herring (Sindermann 1958), but the geographically limited mortality observed in PWS herring in 1993 (Meyers et al. 1994) showed a very low prevalence of I. hoferi. Removal of moribund fish by predation, as observed by Kramer-Schadt et al. (2010) for Atlantic herring, remains a possibility, but it also must remain an unknown for which there is no evidence in PWS in 1993. Second, the prevalence of I. hoferi infection in PWS (based on very limited sampling, and determined by histology, Table 5) before and during the decline was low, i.e., 5.7% in spring 1992 and 5.1% in spring 1993 but increased to 29% in 1994 after the decline (Table 5). This pattern of low levels before the decline and high levels after the decline is the reverse of the expectation of high prevalence before and lower prevalence after the decline if I. hoferi was causal in the decline. The expectation would be that the prevalence decreases after the epizootic because infected fish succumb to disease. The prevalence data is contrary to the notion that the I. hoferi infection was causative in the PWS decline (Table 5).
Hypotheses concerning poor recovery
Hypotheses for poor recovery must provide mechanisms for (1) no recruitment of strong year classes in PWS after recruitment of the 1988 year class and (2) low herring biomass. Recovery hypotheses examined (Table 3) include oil exposure, harvesting, disease, oceanic factors, predation, and competition.
Oil exposure
Among the post-decline exposure pathways that could limit development of strong year classes, and hence recruitment, the exposure of most concern is that of juvenile (age-0+ and 1+) herring to water-column TPAH concentrations. Nearshore areas of PWS bays and fjords provide nursery areas for juvenile herring (Stokesbury et al. 1999, 2000; Norcross et al. 2001). In such locations, mussels provide composite water sampling of intertidal and shallow subtidal areas.
The use of bivalves for monitoring the marine environment dates back to the 1970s (Farrington and Quinn 1973; Dunn and Stich 1976; Dunn and Young 1976). Mussel tissues have been used extensively in determining levels of total TPAH in the waters of PWS (Short and Harris 2006; Boehm et al. 2004, 2007). For PWS, Neff and Burns (1996) applied an equilibrium-partitioning technique to relate tissue burdens of TPAH in mussels to contaminant levels in the water column. Direct water-column sampling was discontinued several years after the EVOS because TPAH concentrations were at background or below method detection limits. Boehm et al. (2004, 2007) produced an extensive database of the concentrations of TPAH in mussel samples collected from 1989 to 2005 from oiled and unoiled shorelines including sites of past human activity (e.g., mines, canneries) in PWS.
Figure 5 shows the arithmetic means of TPAH water concentrations derived from analyses of mussel tissues from oiled and unoiled shorelines using the conversion of Neff and Burns (1996). The profile of exposure concentrations in Fig. 5 shows the peak of 3.3 ppb in 1989 (the year of the spill), followed by exponential decay to less than 0.1 ppb in 1990 and to low background levels (<0.02 ppb) since 1995.
The TPAH levels were too low to cause toxic effects. Even in 1989, the TPAH levels (3.3 ppb) were below the 28 ppb that Carls et al. (1998) found associated with VHSV in one laboratory experiment. For all years except 1989, water concentrations were also below the value of 0.4 ppb, the value which Carls et al. (1999) postulated as the threshold level for the development of abnormal larvae in herring eggs exposed to oil. While evidence of localized effects on herring eggs were observed by Pearson et al. (1995) in 1989, there was no evidence of effects on eggs in 1990 (Brown et al. 1996; Pearson et al. 1995). In 1995, Johnson et al. (1997) incubated eggs from mature herring collected from oiled and unoiled areas of PWS and found no regional differences in fertilization, hatching times, hatching success, or larval viability or abnormalities. The water TPAH data and experimental studies provide no evidence that exposure to oil in the water column has affected herring or prevented their recovery in PWS.
Subsurface oil residues are not a source of exposure for herring. Juvenile herring forage in the water column and do not dig in sediments. Other factors also limit the potential of subsurface oil residues as an exposure source for herring. First, there was little overlap between herring spawning grounds and oiling at the time of the EVOS (Fig. 6) (Pearson et al. 1995, 1999) and much less between the residual oiling and spawning areas (Rice and Carls 2007). Second, the bioavailability of subsurface oil residues is low. In 2002, Neff et al. (2006) analyzed beach sediment, beach pits, and intertidal biota for 17 sites heavily oiled in 1989 and found low TPAH concentrations in biota (e.g., means of 22.4 ± 59 (SD) ppb in fish from oiled sites, 6.4 ± 4.9 ppb in unoiled sites, and 108 ± 165 ppb in unoiled sites with human activity). In 2002, TPAH concentrations in the tissue of the cockscomb gunnel, which inhabits the intertidal and shallow subtidal zones of PWS, and in the tissues of clams, mussels, and worms, did not differ significantly between previously oiled sites and unoiled reference sites and are predominantly from combustion sources—not from crude oil (Neff et al. 2006).
Herring spawning areas and oiled shorelines in PWS during 1989. Base map from Pearson et al. (1999) reproduced with permission. The boundaries of the Herring Spawning Areas designated by ADFG have changed slightly over the year, and the 1989 boundaries given by Biggs et al. (1992) appear as an overlay on the base map
The ASA modeling results do not support an effect of oil exposure on recruitment. Deriso et al. (2008) used TPAH concentrations in PWS nearshore areas as a covariate in their ASA modeling. The TPAH levels fell during the spill and have been at background levels for over two decades. The oil-exposure index derived from the TPAH burdens in mussels was not a significant factor affecting recruitment in the single-factor or multi-factor ASA models (Deriso et al. 2008).
Harvest
The evidence that harvest effects are not involved in the poor recovery of PWS herring derives from the lack of strong year classes after 1993 in both PWS and Sitka (Fig. 2). Fishing has continued at Sitka but not PWS. This parallelism between PWS and Sitka indicates that the lack of strong year classes in PWS since 1993 is due to some regional-scale environmental change rather than a harvest effect. Also, Deriso et al. (2008) found that the commercial harvest of PWS herring was commensurate with harvests being actively managed to be below 20% of the spawning biomass.
Spawning habitat loss
Loss of spawning habitat is one of the three main general causes of fishery declines listed by Hilborn (1997) and is an impediment to stock success (Stephenson 1997). Spawning habitat, however, is not limited and has not been lost in PWS. Spawning sites in PWS are not subject to shoreline urbanization or other widespread human disturbances that degrade or fragment herring spawning habitat in other regions.
Shifts in PWS herring spawning sites since the 1970s (Fig. 7) are typical of those in other herring stocks. Spawning sites change over the years, to be abandoned and then reoccupied (McQuinn 1997; Hay et al. 2001). Both Atlantic and Pacific herring are repeat spawners at particular sites but with varying degrees of site fidelity (McQuinn 1997; Hay et al. 2001). This repeat spawning is not natal homing because herring do not necessarily return to the site where the fish were hatched. Herring spawning habitat in PWS appears to be in excess of what herring require, and, in any event, little if any spawning habitat was impacted or lost as a consequence of the 1989 spill. The shifts in spawning locations (Fig. 7) are not related to oiled or unoiled condition following EVOS. For these reasons, we reject the hypothesis that the poor recovery of herring can be attributed to habitat loss from the 1989 oil spill.
Viral hemorrhagic septicemia virus
VHSV primarily affects juvenile herring so that the potential role of VHSV in reducing recruitment and limiting recovery needs to be examined. Quinn et al. (2001) incorporated disease indices in their ASA model of PWS herring and found that negative correlations between VHSV prevalence and recruitment or the logarithm of recruitment at lags of 0 year and 1 year were not significant. Lagging the VHSV indices by 1 year improved the negative correlation with recruitment, but the correlation was still not significant. Quinn et al. (2001) suggest that disease may still be a factor in recruitment, the effect of which was masked by other factors in their study. Based on a later version of the ASA model, Marty et al. (2003) conclude that both VHSV and Ichthyophonus hoferi may have affected the PWS herring population since the 1993 decline. Elston and Meyers (2009) reviewed all VHSV data from PWS herring from 1989 through 2005, including correlation analysis with recruitment lags of 0, 1, 2, and 3 years and concluded that there was no evidence that VHSV affected recruitment processes after the decline. These authors also provided corrected prevalence data showing that the prevalence of VHSV in PWS herring never exceeded 7.0% from 1994 to 2002 in contrast to Marty et al. (2003). The ASA modeling of Deriso et al. (2008) does not find VHSV adversely influencing ages-3 and -4 herring recruitment. The ASA modeling of Hulson et al. (2008) finds only small influence of VHSV prevalence on herring mortality in PWS and supports Elston and Meyers (2009). As discussed in more detail in Elston and Meyers (2009), the evidence is not sufficient to assign VHSV as a major factor limiting recovery.
Viral hemorrhagic septicemia virus and oil exposure
As with the decline, the experimental evidence does not support any causal relationship between oil exposure and VHSV in the poor recovery of PWS herring. With the oil levels in PWS at background levels throughout the period of poor recovery and lack of evidence for a significant effect of VHSV on herring with or without oil present (Elston and Meyers 2009), a linkage between oil exposure, VHSV, and poor recovery is not warranted.
Pathogen Ichthyophonus hoferi
The marked and progressive increase in observed infection rate of herring by of I. hoferi after the decline (Table 5) suggests some involvement of disease in the recovery process. Ichthyophonus hoferi is a pathogen producing chronic disease that progressively affects older age classes of herring more severely (Marty et al. 1998; Hershberger et al. 2002). I. hoferi is associated with adult Atlantic herring mortality, in some cases on a massive scale (Tibbo and Graham 1963; Patterson 1996). Marty et al. (2003) concluded from ASA modeling that the PWS prevalence of I. hoferi did not affect adult herring survival from 1994 to 2000. The available PWS disease data suggest that the I. hoferi infection rate is more influential in mortality of adult PWS herring than VHSV, especially since 2000 (Marty et al. 2010).
This hypothesis may ultimately be proven correct, but the findings from Kramer-Schadt et al. (2010) indicate the I. hoferi prevalence data for PWS suffer in two aspects that preclude drawing strong conclusions from the prevalence data. First, the field observational technique used to identify infected fish needs to be corrected for sensitivity to achieve an estimated match with histology diagnosis (a high significant correlation was shown between field observations and histology [Marty et al. 2010]). Measurements with the field technique underestimate infection rate (Table 5; Kocan et al. 2011; Kramer-Schadt et al. 2010) as determined by the more accurate tissue explant method. The time series produced by correction from field observation to histology appears reasonably consistent, but total infection rate likely remains underestimated (Kocan et al. 1999a, b, 2011). There is also a lack of correlation between prevalence by histology or visual examination and prevalence as determined by tissue explant culture, so that the extent to which prevalence was underestimated by the first two techniques likely varied substantially between samples. Kocan et al. (2011) visualized tissue sections from infected hosts three-dimensionally, and found non-random distribution of the parasite, establishing a mechanism for the variability in sensitivity encountered when using histology for the detection of I. hoferi in infected hosts. Second, sampling error for the PWS data is likely large because Holst (1996) and Kramer-Schadt et al. (2010) found infection rates were up to 3 times higher in small compared to large catches. Swimming impairment in infected fish appears to separate them from the larger schools and can also be expected to make infected fish more vulnerable to sampling. Finally, the consequences of infection severities and the time course of the disease under various infection scenarios remain poorly understood. The observed spatial and seasonal patchiness of infected fish suggests that sampling herring for prevalence without taking such variation into account could yield sampling bias and erroneous conclusions.
Multiple disease factors
Several authors have introduced the hypothesis that filamentous bacteria played a role in the decline and lack of recovery of PWS herring populations (Hulson et al. 2008; Marty 2007; Marty et al. 2007). In the only reported, confirmed infection of this type in Pacific herring, Carls et al. (1998) reported a low mortality rate (3%), scale loss, and raised white cutaneous foci associated with filamentous bacterial rods in captive herring from near Juneau, Alaska. However, culture of kidneys from PWS herring sampled from 1994 to 2002 failed to produce any filamentous bacterial isolates. Filamentous marine bacteria (such as Tenacibaculum maritimum, formerly Flexibacter maritimus) are a part of the normal external flora of marine fish and often, if not always, require an injury to the tissues as a portal of entry (Avendaño-Herrera et al. 2006). However, once established, T. maritimum can cause an aggressive infection and contribute to mortality. Herring are susceptible to stress, which can cause blood vessel dilation, hemorrhaging, and external injuries to skin and fins, opening the portals for secondary pathogens. There is no hard evidence to show that T. maritimum was responsible for the skin ulcers observed in PWS herring between 1993 and 2005 (Elston and Meyers 2009), but it could have played a role in exacerbating lesions in stressed fish. Given the low prevalence of observed ulcers in PWS herring and the complete absence of any data confirming infection by filamentous bacteria, the assertion of a primary role of filamentous bacteria in controlling PWS herring populations is not supported.
Predation
The synthesis of Spies (2007) provides substantial detail on trophic interactions in PWS and GOA (Gulf of Alaska) and a background for assessing effects of predation on herring. Forage fish in PWS include juvenile Pacific herring, Pacific sand lance, capelin, eulachon, juvenile salmon, juvenile pollock, and some small mesopelagic fishes. Predators utilizing this complex of forage fish include marine mammals, marine birds, and larger fish. The high fat content of sand lance, capelin, and juvenile herring make these fish attractive prey to predators; for example, Pacific herring, sand lance, and capelin comprise 58% of the diet by weight of 14 seabirds in PWS (Spies 2007).
The ASA modeling of Deriso et al. (2008) finds PWS hatchery releases of juvenile pink salmon to consistently be the single factor affecting recruitment (Table 4) but could not discern whether the mechanism was predation or competition; the paper states “The salmon factors always were highly significant and reduced the spawning biomass to 20% of its size as calculated by removal of the hatchery effect in the ASA [model].” In contrast, the ASA modeling did not find adult coho predation applied to age-1 and age-2 herring to be a significant factor influencing recruitment. Similarly, the modeling did not find adult sea lion predation to be a significant factor influencing adult herring mortality.
Whale predation on herring can be significant. Minke whale predation on Norwegian spring-spawning herring varies annually but can account for 45% of the mortality (Tjelmeland and Lindstrom 2005). For Atlantic herring on Georges Bank, both fin and humpback whale predation removes substantial portions of the herring biomass while seabird predation removed about 100 times less biomass (Overholtz and Link 2007). This section examines recovery hypotheses concerning predation on herring by pink salmon and humpback whales.
Pink salmon predation
Unlike all other herring fishery areas, PWS has pink salmon hatcheries that contribute substantial numbers of fry above and beyond wild fry entering the Sound. Juvenile pink salmon releases from PWS hatcheries began in the mid-1970s. The annual releases first reached 600 million in 1989 and have been about 600 million since the mid-1990s (Fig. 8). Here we examine the potential for predation by juvenile pink salmon on juvenile herring. We do not consider predation by returning adult pink salmon because previous studies (Okey and Pauly 1999) report that returning pink salmon “feed little while in [PWS], if at all.”
In contrast to adult pink salmon, which feed primarily on zooplankton and micronekton (Ruggerone and Nielsen 2004), juvenile pink salmon in PWS consume substantial amounts of fish (Sturdevant 1999; Willette et al. 1999a). Stomach analyses of PWS juvenile pink salmon by Sturdevant (1999) reveal that fish are 28% of the diet by weight over all months but are as high as 80% in July. Juvenile pink salmon and age-0 herring overlap in the nearshore nursery areas from July through at least August.
Fish in general select prey that are 20–30% of their body size, but they can select prey that are up to 50% of their size when they commence piscivory (Willette et al. 1999b). Juvenile herring are smaller than pink salmon when the herring enter PWS. Age-0 herring enter the Sound in May at less than 0.2 g average body weight (Okey and Pauly 1999) and grow to 6.9 g the first summer (Sturdevant et al. 2001). Pink salmon, by contrast, enter the Sound in mid-May at 0.26 and 0.53-g average body weights for wild and hatchery fish, respectively, and grow to 99 g by October (Cross et al. 2005). In May, age-1+ herring weigh 12 g and are much larger than any of the other 0+ forage fish. Juvenile pink salmon grow more quickly than the age-1+ herring but do not outweigh them until August. Predation by juvenile pink salmon most likely affects age-0 rather than age-1 herring.
Humpback whale predation
The humpback whale (Megaptera novaeangliae) is a predator that targets herring aggregations (Hjort and Ruud 1929). Such targeted predation leads Brown (2004) to suggest that PWS herring are now in “a predator pit,” where recruitment cannot exceed losses from predation.
Humpback whales visiting PWS are part of the Central North Pacific Stock of humpback whales, which has increased in recent years, primarily from increases in Southeast Alaska (Angliss and Outlaw 2005). A recent estimate for the Central North Pacific Stock has 2,036 whales with 147 comprising the PWS population. Whales observed in PWS are transient, being observed elsewhere in the GOA during the same summer (Von Ziegesar et al. 1994; Angliss and Outlaw 2005) and in other parts of the range in other seasons (Calambokidis and Steiger 2001).
Whale abundance in PWS peaks in July and falls to low levels by mid-September. The number of humpbacks identified in PWS from 1988 to 1993 averaged 49 with a range from 26 to 66 (Von Ziegesar et al. 1994; Calambokidis and Steiger 2001). Von Ziegesar (pers. comm.) estimates that PWS has 50 to 70 whales during the summer and 10 to 20 whales during the winter. Von Ziegesar et al. (1994) estimated summer residence time for individual whales in PWS to be 49 days for summer resident whales and 27 days for other more transient whales. During summer feeding in PWS, humpback distribution in 1988 to 1990 centered on the southern end of Knight Island and Montague Strait (Von Ziegesar et al. 1994).
Preliminary results from ongoing studies of humpback whales in PWS indicate that the overwintering population is higher in recent years (2008, 2009) than that reported by Von Ziegesar et al. (1994) for the 1990’s. PWS-wide surveys estimated the numbers of whales overwintering in PWS to be 67 in September 2008 and with a peak of 112 in October 2008 (Rice 2009). Slightly over 50 whales were estimated to be overwintering in November and December 2008, and only 8 whales, in January through March 2009. The total PWS whale population also appears to have increased to 160 (Rice 2009).
The diet of humpback whales in their North Pacific feeding grounds consists mainly of zooplankton and small schooling fish (Angliss and Outlaw 2005). Reconstruction of current humpback diets near Kodiak Island estimated that the bulk of the diet was comprised of walleye pollock (37%), capelin (29%), and euphausiids (22%) with herring being only 0.03% of the diet (Witteveen et al. 2006). Stomach analyses of humpback whales conducted in the 1920s near Kodiak Island indicated that surf smelt and euphausiids were the major dietary items (Witteveen et al. 2006). Based on sonar surveys and scat analysis, the majority of the potential prey in PWS during the summer were euphausiids, but herring and sand lance also were present (Matkin and Hobbs 1999). Based on observations of the winter distribution of herring and humpbacks, Matkin and Hobbs (1999) and Brown (2004) have inferred that the overwintering whales are feeding on herring. However, studies have shown that high euphausiid abundance leads to aggregations of humpback and other whales (Hjort and Ruud 1929 cited in Sverdrup et al. 1942; Everson 2000). Also plausible is the inference that the observed winter co-occurrence results from pursuit by both herring and humpbacks of euphausiids, probably a major, if not the dominant, zooplankton in winter. Preliminary results of ongoing visual and acoustic observations along with fatty acid analyses indicate that overwintering whales in PWS frequently feed on herring (Rice 2009).
Tables 6 and 7 present estimates of the consumption of herring by humpbacks in PWS during the summer feeding season and the overwintering period. Median value for the standing stock of humpbacks of 60 whales in the summer is taken from the range given by Von Ziegesar et al. (2006 pers. comm.). The monthly values for overwintering whales are taken from Rice (2009). A range of values for the daily consumption rates was used to estimate consumption. The information on the diet composition for summering whales leads us to use a worst-case high estimate of consumption by assuming that 50% of the diet is fish and that 95% of the fish are herring. Similarly, we estimate predation by overwintering whales, assuming that the winter diet is 80% herring.
In the summer, the whales would feed primarily on age-2 herring because the younger age classes are in nearshore nursery areas inaccessible to whales and adult age classes are outside PWS or in eastern parts of PWS where whales do not aggregate in summer (Stokesbury et al. 1999; Brown et al. 2002). Assuming an estimated weight of 74 g for individual age-2 herring, estimated consumption by 60 summering whales would range from 0.011 to 44.9 million sub-adult herring with an average of about 19.6 million sub-adult herring (Table 6). Assuming an average weight of 158.4 g for individual adult herring, estimated consumption by overwintering whales ranged from 17 to 102 million herring with an average of about 51.3 million adult herring (Table 7).
The abundance of the PWS adult herring population was about 900 million per year from 1980 through 1993 (estimated from Fig. 1) and about 200 million per year from 1994 to 2005 (Fig. 2). The overwintering whales appear to be consuming about 26% of the recent adult population. The abundance of age-3 herring in PWS was about 81 million fish per year from 1994 to 2005. We have no estimates of the age-2 population. Assuming the age-2 population to be generally greater than the age-3 population, the summering whales would then be consuming less than 24% of the age-2 population.
Poor nutrition and oceanic factors
Modeling by Rose et al. (2007) illustrates how temperature and other oceanic factors can influence weight at age and recruitment in herring populations. Oceanic factors have been found to influence herring recruitment in Alaska (Collie 1991; Williams and Quinn 2000a, b; Zebdi and Collie 1995). The analysis of 14 herring stocks by Williams and Quinn (2000a, b) show that the GOA stocks, which include the PWS and Sitka stocks, demonstrated similar interannual patterns in recruitment and weight at age that set them apart from herring stocks in the Bering Sea or British Columbia. The similarities among the GOA stocks indicate that they respond in a similar way to regional-scale environmental factors occurring over the GOA (Williams and Quinn 2000b).
Evidence for operation of oceanic factors in PWS and GOA comes from a comparison of recruitment (the numbers of herring at age-3 and age-4) that reveals that emergence of strong years occurred at the same time at PWS and Sitka from 1980 to 1993 (Fig. 2, Table 8). More importantly, strong year classes emerged at the same time in both PWS and Sitka until 1993; after 1993, strong year classes have not emerged in either PWS or Sitka (Fig. 2). Autocorrelation analysis supports such synchrony. The 4-year lag provides the highest positive autocorrelation for the numbers of both age-3 and age-4 herring at PWS and Sitka from 1980 through 1993 (Table 9). After 1993, the high positive autocorrelations at 4-year lag are no longer evident at either PWS or Sitka. The parallel trends are strong evidence that population dynamics of both PWS and Sitka herring are being influenced at the same time by GOA-wide ocean processes.
Further, the nutritional state of the PWS herring after the decline remains much poorer than that at Sitka (Table 10). With the data available, we may not be able to separate the relative roles of poor nutrition and other oceanic factors, but the field observations of Foy and Norcross (2001) and the analysis by Norcross and Brown (2001) suggest how these factors play a role in determining year-class strength in PWS herring. Elsewhere, changes in the ocean environment have apparently changed the zooplankton prey field and reduced overwintering larval survival in Atlantic herring, in turn leading to six sequential years of poor recruitment (Payne et al. 2009).
Poor nutrition associated with oceanic factors is a plausible hypothesis for poor recovery in PWS herring and has empirical evidence for its occurrence. However, some additional process beyond environmentally-derived poor nutrition must be occurring in PWS but not at Sitka. The ASA modeling outcomes suggest that the effects of the hatchery releases of pink salmon fry operate as well and are of great influence on recruitment and recovery.
Competition with juvenile pink salmon
Results from the ASA modeling with covariates by Deriso et al. (2008) appear in Table 4. These results led us to evaluate mechanisms of competition and predation by which juvenile pink salmon could influence population dynamics in PWS herring. We assessed the competition hypotheses on the basis of both the ASA modeling results and the available information from field studies.
Juvenile pink salmon releases into PWS now average about 600 million annually (Fig. 8). ASA modeling by Deriso et al. (2008) found releases of hatchery pink salmon fry to consistently be the most influential single factor on PWS herring recruitment and the common factor in all their multi-factor models (Table 4). The impact analysis of Deriso et al. (2008) reveals that the pink salmon fry releases have the most substantial impact, reducing the spawning herring biomass to an estimated 20% of the level without the releases. However, Deriso et al. (2008) could did not address whether the mechanism behind these results was predation, competition, or a combination of the two.
Here we examine potential competition mechanisms by which the pink salmon fry releases influence PWS herring recruitment. To assess competition hypotheses, we examine several questions: (1) Do the competing species overlap in space and time? (spatio-temporal overlap), (2) Do the diets of the competitors include shared prey? (dietary overlap), (3) Does consumptive demand exceed food availability (consumptive demand), (4) Is the feeding of one competitor substantially reduced in the presence of the other? (feeding disruption), and (5) Does lowered prey availability or feeding disruption adversely affect growth and survival (growth and survival). To affirm a competition hypothesis, we must find evidence for all of the following: spatio-temporal overlap, dietary overlap, consumptive demand or feeding disruption, and effects of food limitation on herring growth and survival.
Spatio-temporal overlap
There is evidence for spatial and temporal overlap between juvenile pink salmon and juvenile herring. The degree and timing of overlap between pink salmon and age-0 herring differs from that between pink salmon and age-1 herring. Some previous discussions of the overlap (e.g., Cooney et al. 2001) have only considered the limited spatial overlap between age-0 herring and pink salmon in May and June.
Wild pink salmon fry enter the Sound in late April or May (Boldt and Haldorson 2003), and release of hatchery pink salmon fry is timed to optimally correspond with the spring zooplankton bloom, usually mid-May (Cross et al. 2005). Once salmon fry have entered the Sound, they initially aggregate in shallow, nearshore bays (Boldt and Haldorson 2003). Young-of-the-year salmon stay within the 20-m isobath (Okey and Pauly 1999). Furthermore, most of the hatchery-released pink salmon inhabit the corridor running from Port Valdez to Esther Island and to Knight Island Passage, roughly one-half of the Sound (Okey and Pauly 1999; Willette et al. 2001). Juvenile pink salmon can inhabit PWS through October but begin to migrate out of PWS into the coastal waters of the GOA in July (Armstrong et al. 2005). The vast majority of juvenile pink salmon have emigrated from the Sound by September.
The geographic distributions of age-0 and age-1 herring overlap substantially with the distribution of hatchery juvenile pink salmon within PWS. In Fig. 9, the one-dimensional migration route for hatchery pink salmon fry taken from Willette et al. (2001) is superimposed on the distribution of age-0 and age-1 herring in July of 1995 and 1996 taken from Norcross et al. (2001). Age-0 herring are in the larval drift in May and June and, after metamorphosis, enter PWS bays and fjords in June and July (Norcross et al. 2001; Stokesberry et al. 2000). From distribution studies over several years, Stokesbury et al. (1999, 2000) and Norcross et al. (2001) conclude that the age-0 and age-1 herring use the nearshore areas of PWS bays and fjords as nursery areas throughout the year. In western PWS, juvenile pink salmon overlap with age-0 herring from July onwards and with age-1 herring from May onwards. July is probably the time of maximum overlap when there are substantial numbers of juvenile pink salmon still within the PWS nearshore and both age-0 and age-1 herring are in PWS bays.
Several lines of evidence indicate that the extent of spatial and temporal overlap is biologically meaningful. First, Wertheimer and Celewycz (1996) state that their beach seining captured “large numbers” of Pacific herring and other fish “coincident with juvenile [pink] salmon” during April to June of 1989 and 1990 in PWS. Second, Willette et al. (1997) indicate that in August and September of 1994, at a low stock level for PWS herring, co-occurrence of juvenile pink salmon and juvenile herring in the same net hauls was 27%. Willette et al. (1997) used an anchovy purse seine that was 20-m deep; therefore, these are not beach samples. The mean length of the herring indicates that they were age-1 and with perhaps some age-2. Third, of the 17 stations described by Sturdevant (1999) where either allopatric or sympatric herring and pink salmon occurred, about 25% met a rigorous definition of sympatry, i.e., both species being captured in the same net haul (Fig. 9). Sturdevant’s (1999) study was not intended to be a survey of all the allopatric and sympatric conditions for juvenile herring and other species. In her discussion, Sturdevant suggests that sympatry among the forage fishes may be greater than her collection data suggest. Fourth, Moulton (1997) finds substantial numbers of juvenile pink salmon and juvenile herring occurring in the same tow net hauls in northern Cook Inlet. The tow nets were conducted away from the beaches and sampled to a depth of 3.1 m. Analysis of Moulton’s raw data shows that the sympatry between juvenile pink salmon and age-1 herring in tow net sets in the surface waters of northern Cook Inlet during the months of June and July is 81 and 58% (Table 11). Age-0 herring, but not juvenile pink salmon, are caught in September. The available field evidence indicates that the rate of co-occurrence of juvenile pink salmon and juvenile herring ranges from 25% to about 80% depending on timing.
Dietary overlap
Both herring and pink salmon juveniles are highly omnivorous, with both species consuming small and large copepods, larvaceans, and euphausiids (Sturdevant 1999; Boldt and Haldorson 2003). Both species prefer large copepods in May (Foy and Norcross 1999; Willette 2001). In general, pink salmon avoid small copepods (Cross et al. 2005), although small copepods may be underrepresented in diet studies because of their small size (Boldt and Haldorson 2003). The degree of dietary overlap varies, depending on the time of year, the date of sampling, and the study, but overlap in food resources appears greatest at the beginning of the summer, when both species are consuming the seasonally abundant calanoid copepods, and both are occupying nearshore habitats. Later in the summer, herring primarily consume smaller prey than that preferred by pink salmon but eat larger prey items such as larvaceans as a fraction of their diet. Larger prey items are not numerically abundant in the diets of juvenile herring, but these prey items contribute disproportionately to the overall amount of calories ingested and hence increase the energy content of juvenile herring (Sturdevant 1999).
Consumptive demand and prey availability
Cross et al. (2005) suggest that the consumption demands of pink salmon may exceed food availability in PWS. They used a bioenergetics model (Hansen et al. 1997) to predict the consumption demand of juvenile pink salmon, and compared the demand for certain prey with the availability of these taxa within the prey fields. Estimated consumptive demand of high-energy-content prey, such as large copepods, larvaceans, and hyperiid amphipods, exceeded supply in PWS. One advantage of the Cross study is that they were able to take zooplankton tows at the same sites where they collected fish and thereby were able to compare demand and regional availability. Sturdevant (1999) also found locally decreased zooplankton populations in areas with high densities of sympatric juvenile salmon and herring.
Disruption of feeding during sympatry
Sturdevant’s (1999) examination of dietary overlap of 14 species of forage fish collected from PWS includes field observations of feeding disruption that provide strong evidence of food competition between juvenile pink salmon and herring. When Pacific herring occur sympatrically with pink salmon, Sturdevant observed significant and substantial decreases for herring in four feeding indices: median stomach fullness, prey weight in stomach as a percentage of body weight, median prey number, and median prey biomass (Table 12). These observations indicate that when herring are food-limited in the presence of salmon, they do not shift diet composition, but instead decrease feeding. The observations of decreased feeding indices under sympatry led Sturdevant (1999) to conclude that if these fishes regularly co-occur, the carrying capacity of PWS may be exceeded. The spatio-temporal overlap observations discussed above indicate that co-occurrence is common.
Effects of food limitation on growth and survival of juvenile herring
Field observations in PWS indicate that food limitation influences growth and survival of juvenile herring; the ability of herring juveniles to gain sufficient fat reserves to live through the winter determines their overall recruitment (Cooney et al. 2001; Norcross et al. 2001).
Paul and Paul (1998) experimentally starved young-of-the-year herring to determine the critical energy content of herring juveniles to survive winter. They found that fish that died during laboratory starvation had a whole-body energy content (WBEC) of 3.2 kJ g−1, whereas the WBEC of young-of-the-year herring in PWS was 3.4 kJ g−1, suggesting that juvenile herring use most of their reserves during the winter (Paul and Paul 1998). Fish with low WBEC are inferred to be at greater risk of predation, because they probably leave predator refuges in search of food (Paul and Paul 1998).
Field, laboratory, and modeling studies have led Norcross et al. (2001) to consider adequate nutrition (stored energy) to be essential for overwintering survival of age-0 herring, which in turn is considered important to year-class strength. Foy and Norcross (2001) reported that warmer water temperatures in nursery bays in 1997 were associated with lower zooplankton densities and taxonomic diversity, and that the frequency of empty stomachs among juvenile herring (a mixture of age-0 and age-1 in unknown proportion) was three times higher in October of the year with warmer water temperatures. In the past, strong year classes have typically emerged in PWS and other GOA herring populations every 4 years (Funk and Sandone 1990; Williams and Quinn 2000a; Hay et al. 2001). The 1996 year class would have been expected to be a strong one in the four-year cycle. However, the 1996 year class (age-1 at the time of the warmer water temperatures) had one of the lowest recruitments (less than one million age-3 herring) in the PWS recruitment time series (Fig. 2). Norcross and Brown (2001) and Norcross et al. (2001) suggest that no one early life stage is the “key” to year class strength in PWS herring, and the above observations suggest that, at least in the case at hand, nutritional state, low zooplankton densities, and warmer water temperatures at age-1 were associated with an extremely weak year class (Brown 2004; Brown and Norcross 2001).
Discussion
Based on Platt’s (1964) guidance for making inferences, we organize the summary information in Table 3 under these four questions: (1) Is there correlation or constant association between the hypothesized factor and herring mortality, biomass, or recruitment? (constant association test), (2) Is there a biologically plausible mechanism for the hypothesized factor to produce the observed effect? (mechanism test), (3) Is there evidence for the occurrence of such a mechanism? (test of supporting evidence for mechanism), and (4) Is there contrary evidence? (test for contrary evidence).
Decline
We find no evidence that oil exposure, harvest effects, spawning habitat loss, or the spawn-on-kelp fishery caused or contributed to the 1993 decline (Table 3). The evidence supports the contention that at least a portion of the PWS herring population was stressed by poor nutrition. Further evidence supports the argument that infectious disease effects during the decline were a secondary response to poor nutrition.
Most investigators now agree with the conclusion of Pearson et al. (1999) that the decline was not related to the oil spill. The link between the decline and the oil spill postulated by Thomas and Thorne (2003) and Thorne and Thomas (2008) has a plausible mechanism for exposure to oil during the initial year of the spill but lacks empirical evidence for its occurrence in PWS. The limited distribution of oil slicks over the large area of PWS and intermittent nature of gulping at the water surface indicates a limited exposure via gulping during the spill year and essentially negligible exposure after the initial year. Further, the results of ASA modeling (Deriso et al. 2008; Hulson et al. 2008) and other available evidence is contrary to the notion of a mortality event in adult herring during the spill year or year other than 1992 or 1993. Consequently, we reject the hypothesis that the decline started in 1989 and was associated with the 1989 EVOS.
The sum of the observations is not sufficient to assign VHSV as a cause of the 1992–1993 decline. Recent detailed analysis (Elston and Meyers 2009) demonstrates that the preponderance of evidence does not support any primary role of infectious disease in the decline. VHSV appears to have been a secondary response in an undetermined portion of the PWS herring population when the herring had already been stressed by poor nutrition.
The prevalence patterns of I. hoferi are contrary to the notion that this pathogen caused or contributed to the decline, although there is evidence that I. hoferi may play a role in PWS herring population dynamics some years after the decline. Because of the potential for sampling bias and changing measurement techniques, the data on the prevalence of I. hoferi requires cautious use. Despite these limitations, the prevalence patterns during the decline do not match the expected pattern if I. hoferi was a causative agent in the decline.
Poor nutrition preceding severe I. hoferi disease is a plausible mechanism for the recent emergence of the disease in PWS. Kramer-Schadt et al. (2010) hypothesize that the interactions of several factors drive the progression from I. hoferi infection to disease and point out that a dominant causal factor did not emerge from their study of herring epizootics. They suggest that high fish density facilitates disease transmission and that poor condition precedes disease expression. Laboratory experiments by Kocan et al. (1999a, b) showed that I. hoferi prevalence can increase with fish age without an associated increase in mortality. This finding suggested to Kramer-Schadt et al. (2010) that Pacific herring in good condition appear able to endure high infection intensities, although the fish may be at high risk if external conditions become unfavorable. Similar to VHSV, disease expression and morbidity caused by I. hoferi infection may result from, and perhaps exacerbate, the effects of poor nutrition and decreased condition, but there is insufficient knowledge to fully understand the apparent interactive effects of these factors. Certainly, more focused research may reveal the possible synergistic mechanisms by which these factors affect herring health and condition, but the available evidence continues to indicate that poor nutrition preceded disease expression for both VHSV and I. hoferi during the decline.
Our re-examination of available information and recent modeling outcomes supports earlier conclusions (Pearson et al. 1999) that poor nutrition is the probable cause of the 1993 decline. Nutritional status of PWS herring clearly began to decline in the mid-1980s and reached a low in 1993. Zooplankton abundance in PWS was low before, during, and following the decline. In recent years, other investigators have come to conclude that the 1993 decline most likely derived from poor nutrition with disease as a secondary factor (Carls et al. 2002; Marty et al. 2003; Hulson et al. 2008; Rice and Carls 2007; Elston and Meyers 2009).
Recovery
We find no evidence that oil spill effects or oil exposure, harvest effects, spawning habitat loss, or VHSV caused or contributed to poor recovery of PWS herring after 1993 (Table 3). Oceanic factors, poor nutrition, and interactions with hatchery releases of juvenile pink salmon are supported by the evidence. Available information about disease from I. hoferi and predation by overwintering whales suggest roles for these factors in limiting recovery but more information on these potential factors is needed to confirm them as causal.
We attribute the lack of strong year classes in both PWS and Sitka principally to oceanic factors operating at the scale of the GOA, and the nutritional state of the adult PWS herring remains poorer than that at Sitka and probably influences biomass levels of adult herring in PWS. The condition of adult herring in PWS would have been expected to improve under the low biomass levels after the decline. Some factor specifically related to PWS appears to be acting beyond the regional-scale oceanic factors.
ASA modeling clearly and strongly indicates 600-million-per-year juvenile pink salmon releases as a substantial factor affecting PWS herring recruitment. Evidence presented above supports the position that juvenile pink salmon releases affect juvenile herring through predation on age-0 herring and food competition with the age-1 herring.
Predation by juvenile pink salmon on age-0+ herring may be intense in mid-summer. Because age-0 herring are the most abundant forage fish in PWS waters (Okey and Pauly 1999), a substantial fraction of juvenile pink salmon predation likely occurs on herring. Also, the extent to which avoidance of such predation limits feeding by age-0 herring is not known. More specific identification of the fish found in the PWS juvenile pink salmon stomachs by Sturdevant (1999) would be useful. The hypothesis that juvenile pink salmon predation substantially influences age-0 herring survival has supporting evidence and no contrary evidence.
Our findings that juvenile pink salmon and age-1 herring probably compete for limiting food resources is in contrast with some other published reports on the interactions between these species. Cooney (1993) studied the influence of the release of hatchery-reared pink salmon fry on the carrying capacity of PWS and concluded that the release of hatchery-reared fry are unlikely to have much impact on zooplankton populations. Hence, Cooney (1993) did not expect that the fry releases would cause competition with other forage-fish species. However, his estimates were based on the total production (as grams of carbon) of broad trophic classes of organisms, such as zooplankton or planktivores. This simple approach assumes that all organisms within a given trophic class are potential prey for the next trophic level, regardless of size, energy content, or the ease of capture. This approach also does not include the potential for differences in prey quality or the selection of high-energy-content prey, which studies indicate have a strong bearing on growth and survival of PWS juvenile herring (Paul and Paul 1998; Cooney et al. 2001; Norcross et al. 2001).
If PWS adult herring condition has been declining since the mid-1980s, how does the strong PWS 1988 year class reconcile with oceanic factors and juvenile pink salmon releases as impediments to recovery? Pink salmon interactions are a combination of predation by pink salmon juveniles on age-0 herring and competition of pink salmon juveniles with age-1 herring. The 1984 and 1988 year classes were 1 year old in 1985 and 1989, respectively, which were two years of the highest zooplankton abundance (Fig. 4). The pink salmon fry releases first reached 600 million in 1989. There are no years of high zooplankton abundance in the years following 1989 (Fig. 4).
There is evidence for food competition between juvenile herring and juvenile pink salmon in PWS. Age-0 herring and pink salmon fry overlap in the nearshore areas of PWS from July through the fall. Age-1 herring and juvenile pink salmon overlap in PWS nearshore areas from May through the fall. There is sufficient overlap in the preferred food items among the juvenile forage fishes in PWS to indicate food competition, especially for prey of high-energy content. The field studies of Sturdevant (1999) found statistically significant and substantial decreases in four feeding indices of juvenile herring when they co-occur with juvenile pink salmon. Poor nutrition in juvenile herring, which could result from competition with pink salmon, is related to growth, survival, and recruitment (Foy and Norcross 2001; Norcross et al. 2001; Brown and Norcross 2001; Norcross and Brown 2001). Because juvenile herring need sufficient energetic reserves to survive the winter, decreased nutrition from competition in the spring and summer, followed by a poor prey field in the fall, likely would seriously compromise overwintering ability and, in turn, PWS herring year-class strength.
Marty et al. (2010) posit that I. hoferi affects both recruitment and biomass in PWS herring, but a mechanism for the effect on recruitment was not offered and is not clear. The potential for a biologically plausible mechanism for I. hoferi to produce low biomass appears to be greater. Poor condition in Atlantic herring was associated with severe I. hoferi infections (Kramer-Schadt et al. 2010). Also, I. hoferi affects older fish and a loss of older herring could decrease the biomass. But as discussed previously, poor nutrition appears to precede disease expression.
Marty et al. (2010) used ASA modeling with two disease covariates to assess the relative roles of VHSV and I. hoferi in the decline and poor recovery and concluded that both VHSV and I. hoferi had a substantial influence on the population dynamics of PWS herring―reducing the stock biomass to levels about three times below those that otherwise might have been. Deriso et al. (2008) also used ASA modeling but with 19 covariates and found some support for both VHSV and I. hoferi using the chi-square test but not when using the more accurate randomization test. Interactions with hatchery-release juvenile pink salmon proved to be the most consistent and strongest influence on PWS herring population dynamics (Deriso et al. 2008). Marty et al. (2010) does not include the hatchery releases in their ASA model so that the assessment of the higher relative influence of juvenile pink salmon from hatchery releases versus disease remains that of Deriso et al. (2008).
The impact of I. hoferi infection on herring populations is well documented regarding Pacific herring (Hershberger et al. 2006), and the ASA modeling of Deriso et al. (2008) (Table 3) and Hulson et al. (2008) indicate that I. hoferi could be influencing the population dynamics of PWS herring. The observed prevalence patterns of I. hoferi in PWS herring through 2006 (Table 5) suggest that the pathogen could be limiting survival of herring, particularly in the older year classes (Hershberger et al. 2002). Given the poorly understood relationship between infection intensity and prevalence on population mortality risk, we conclude that these factors need to be more fully understood before accurate conclusions and predictions relating I. hoferi infection to population effect can be made.
The influence of I. hoferi on PWS herring recruitment is not supported, but some secondary role of I. hoferi in producing low biomass in PWS herring cannot be dismissed. The increase in prevalence of I. hoferi after 2000 offers some evidence of a role and the outcomes of the more recent ASA models posit this hypothesis. The loss of older fish to disease from I. hoferi offers a plausible mechanism for lower biomass. Experimental examination of the disease process to determine whether poor condition is the precursor to disease related morbidity and mortality is needed before we can more definitively assess whether there is a causal role for I. hoferi in the low post-decline biomass levels in PWS herring.
Whale predation appears to be reducing adult biomass rather than the sub-adult biomass. Summer feeding by humpback whales appears to be primarily on euphausiids. Whale predation during the summer is on the 2-year-old herring because age 0 and age 1 herring are in the nursery areas and the adults are in the GOA. Our estimates indicate that summering whales may be taking less than 24% of the PWS recent sub-adult herring population. Because there are no estimates of the age-2 biomass or numbers, we used the age-3 abundance in our estimate. Consequently, a more realistic estimate from the available information would be that summering whales remove substantially less than 24% of the sub-adult herring population.
Our estimates are sensitive to the daily consumption rate, the proportion of herring in the diet and the number of whales. Our estimates for the overwintering period are especially sensitive to the high number of whales (112) observed in October and by the preliminary diet assumption that 80% of the overwintering whale diet is herring. This predation falls on adult herring overwintering in PWS. Because of the wide range of potential influence that whale predation may have on adult herring mortality, we recommend assessments of overwintering whale abundance and diet over several years and some studies to confirm the summer diet.
From ongoing studies, overwintering whales now appear to be in greater abundance than previously supposed and appear to feed on herring in the fall and winter. Our estimates indicate that overwintering whales may be taking about 26% of the recent levels for the adult population. Preliminary estimates from ongoing bioenergetics modeling suggest that overwintering whales take 18% to 23% of the pre-winter PWS herring biomass (Rice 2008). Whereas these estimated levels of whale predation are approximately equivalent to a 20% commercial harvest and worthy of concern, the wide range of estimates, the variety of assumptions, and the preliminary nature of recent observations make it premature to declare that a classic “predator pit” from whale predation is the cause of poor recovery in PWS herring.
Furthermore, whale predation cannot be the sole factor impairing recovery in PWS herring. Condition and weight at age has not rebounded in PWS. Enhanced growth under lower population levels would have occurred if whale predation was removing large portions of the sub-adult and adult population and other factors remained the same. Perhaps, overwintering whales take not only herring but also consume large amounts of the euphausiid prey also needed for growth and survival of overwintering adult herring. Whereas whale predation appears to be a credible hypothesis for contributing to low biomass, how whale predation influences recruitment and lack of strong year classes remains less clear. We need to examine other factors in PWS that would affect recruitment.
The recovery hypotheses remaining are not mutually exclusive and could be acting on different aspects of herring population dynamics at the same time. A multifactor hypothesis would include the following elements. First, regional-scale oceanic factors set the limits for the prey field and, in turn, the growth and survival of PWS herring. Second, interactions with pink salmon juveniles released from the hatcheries reduce age 0 herring survival through predation and reduce age 1 growth and survival through food competition and feeding disruption. Third, disease as a secondary response following poor nutrition could reduce biomass levels. Fourth, overwintering whales could remove large portions of adult herring biomass through predation.
Implications for management and restoration
To be most effective, any management or restoration efforts should address the mechanism behind the decline and lack of recovery. If the mechanisms are still operational, then restoration efforts that enhance the population before any bottleneck will be wasted effort. For example, because spawning habitat is not limiting in PWS, enhancing spawning habitat is unlikely to improve herring recovery.
When oceanic factors are influencing recovery, then little can be done other than to manage the commercial herring harvest. It is important to remember that several important herring stocks took decades to recover even under severe fisheries management efforts. Recovery of PWS herring could well depend on a future change in the conditions of the marine environment and, especially, the prey field.
Similarly, how to influence recovery if disease is either a primary or secondary casual factor is elusive. Perhaps all the fisheries manager can do is to incorporate the disease variables, along with all other available and relevant variables, into an ASA model to predict the spawning biomass more accurately. If the disease variables are a proxy for a population showing the effects of an environmental stressor rather than a causal factor, their incorporation in the ASA models may still be of practical use in fisheries management. Marty et al. (2010) cites recent instances of forecasts that prevented unnecessary mobilization of the herring fishing fleet. The available information indicates that closer attention to sampling design and the use of the improved techniques to measure I. hoferi need to be incorporated into any field efforts to monitor infectious diseases in PWS herring.
Whale predation may prove to be removing large amounts of PWS herring, but protected status precludes manipulating whale populations or individuals. The only viable remedy to any effects of whale predation may still be to manage the commercial herring harvest with improved forecasts of the influence of whale predation. Tjelmeland and Lindstrom (2005) found that including minke whale predation in a stock assessment model of Barents Sea herring was both feasible and beneficial in improving stock assessments to support management decisions. Adding whale predation to PWS assessment models will require ongoing studies of whale abundance and predation to be completed over a sufficient number of years to provide reliable long-term information.
Improving nutrition among overwintering juvenile herring clearly would be beneficial to PWS herring recruitment, but how to do so on a scale that would be effective is not immediately obvious. One approach may be to switch the timing of hatchery pink salmon fry release from the peak of plankton production to smaller releases over a longer time period. Reduction in the total amount of releases may also be effective in preventing juvenile pink salmon from depleting any zooplankton stocks that also benefit juvenile herring. If ocean survival of juvenile and adult salmon is the main factor governing the magnitude of returns, then an evenly timed release or even a reduced total release may not have a great influence on the overall returns of adult pink salmon to PWS.
In their review of the lessons learned from the collapse and recovery of North Sea herring, Dickey-Collas et al. (2010) call for not only more research on the mechanisms for collapse and recovery but also for a move away from single-species management approaches to multispecies management and, in the long term, to ecosystem management. When commitments to the management of several species are in conflict (which appears to be the case with PWS), reconciling the conflicting commitments will be best done at the ecosystem level (Dickey-Collas et al. 2010).
Conclusions
We have assessed the evidence for and against the principal decline and poor recovery hypotheses and find no evidence that oil exposure from the Exxon Valdez oil spill, harvest effects, spawning habitat loss, the spawn-on-kelp fishery, or disease have led to either the decline or poor recovery of PWS herring.
Our re-examination of available information and recent modeling outcomes supports earlier conclusions (Pearson et al. 1999) that poor nutrition is the probable cause of the 1993 decline. Nutritional status of PWS herring clearly began to decline in the mid-1980s and reached a low in 1993 and was associated with low zooplankton abundance. Recent information, especially the disease analysis of Elston and Meyers (2009), decreases the role of disease in the decline. The evidence supports the contention that disease during the decline was a secondary response after a portion of the PWS herring population was stressed by poor nutrition.
Poor recovery probably results from several factors. Since 1993, no strong year classes have emerged in the GOA herring populations, including that at PWS. This lack of strong year classes appears to derive from regional-scale ocean environmental factors. Beyond the regional-scale factors, two other factors specific to PWS appear to be reducing herring biomass and recruitment. First, predation by an increasing number of overwintering humpback whales may prove to be removing a substantial proportion of the adult herring in PWS. Second, interactions with juvenile pink salmon released from PWS hatcheries may be influencing nutrition in juvenile herring and their subsequent growth, survival, and recruitment. Continued research is recommended on PWS humpback whale predation and on herring recruitment processes. Also recommended is a move away from single-species management to multi-species management, if not to the management of PWS as a whole ecosystem.
References
Angliss RP, Outlaw RB (2005) Killer whale (Orcinus orca): eastern north Pacific northern resident stock. In: Alaska marine mammal stock assessments, National Oceanic and Atmospheric Administration Technical Memorandum NMFS-AFSC-161, Seattle, pp 95–99
Armstrong JL, Boldt JL, Cross AD et al (2005) Distribution, size, and interannual, seasonal and diel food habits of northern Gulf of Alaska juvenile pink salmon, Oncorhynchus gorbuscha. Deep-Sea Res Pt II 52:247–265
Ashe D, Gray D, Lewis B et al. (2005) Prince William Sound management area 2004 annual finfish management report. Fishery Management Report 05–65, Alaska Department of Fish and Game, Anchorage, http://www.sf.adfg.state.ak.us/FedAidPDFs/fmr05-65.pdf
Avendaño-Herrera R, Toranzo AE, Magariños B (2006) Tenacibaculosis infection in marine fish caused by Tenacibaculum maritinum: a review. Dis Aquat Organ 71:255–266
Biggs ED, Haley BE, Gilman JM (1992) Historic database for Pacific herring in Prince William Sound, Alaska, 1973–1991. Regional Information Report 2C91-11, Alaska Department of Fish and Game, Anchorage
Blaxter JHS (1985) The herring: a successful species? Can J Fish Aquat Sci 42(Suppl 1):21–30
Boehm PD, Neff JM, Page DS (2007) Assessment of hydrocarbon exposure in the waters of Prince William Sound after the Exxon Valdez oil spill: 1989–2005. Mar Pollut Bull 54:339–367
Boehm PD, Page DS, Brown JS et al (2004) Polycyclic aromatic hydrocarbon levels in mussels from Prince William Sound, Alaska, USA, document the return to baseline conditions. Environ Toxicol Chem 23(12):2916–2929
Boldt JL, Haldorson LJ (2003) Seasonal and geographic variation in juvenile pink salmon diets in the northern Gulf of Alaska and Prince William Sound. T Am Fish Soc 132:1035–1052
Brady J, Morstad S, Simpson E et al (1991a) Prince William Sound management area annual finfish management report 1989. Regional Information Report 2C90-07, Alaska Department of Fish and Game, Cordova
Brady J, Morstad S, Simpson E et al (1991b) Prince William Sound management area 1990 annual finfish management report. Alaska Department of Fish and Game, regional information report No.2C91-14, Anchorage, Alaska
Brady JA, Schultz K, Simpson E et al (1990) Prince William Sound area annual finfish management report 1988. Regional Information Report 2C90-02, Alaska Department of Fish and Game, Cordova
Brady J, Sharr S, Roberson K et al (1987) Prince William Sound area annual finfish management report 1986. Alaska Department of Fish and Game, Cordova
Brady JA, Sharr S, Roberson K et al (1988) Prince William Sound area annual finfish management report 1987. Alaska Department of Fish and Game, Cordova
Brown ED (2004) Stock structure and environmental effects on year class formation and population trends of Pacific herring, Clupea pallasi, in Prince William Sound, Alaska. Dissertation, University of Alaska, Fairbanks
Brown ED, Baker TT, Funk F et al (1994) The Exxon Valdez oil spill and Pacific herring in Prince William Sound, Alaska: a summary of injury from 1989–1994. In: Brown ED, Baker TT (eds) Injury to Prince William Sound following the Exxon Valdez oil. Final Report Fish/Shellfish Study Number 11. Alaska Department of Fish and Game, Anchorage
Brown ED, Norcross BL (2001) Effect of herring egg distribution and environmental factors on year-class strength and adult distribution: preliminary results from Prince William Sound, Alaska. In: Funk F, Blackburn J, Hay D et al (eds) Proceedings of the 18th Lowell Wakefield fisheries symposium, Herring 2000: expectations for a new millennium, Anchorage, AK, February 23–26, 2000, AK-SG-01–04. University of Alaska Sea Grant College Program, Fairbanks, pp 335–345
Brown ED, Norcross BL, Short JW (1996) An introduction to studies on the effects of the Exxon Valdez oil spill on early life history stages of Pacific herring, Clupea pallasi, in Prince William Sound, Alaska. Can J Fish Aquat Sci 53:2337–2342
Brown ED, Seitz J, Norcross BL et al (2002) Ecology of herring and other forage fish as recorded by resource users of Prince William Sound and the outer Kenai Peninsula, Alaska. Alsk Fish Res Bull 9(2):75–101
Bruce G (2006) Herring data from LCI, PWS, and Sitka. Alaska Department of Fish and Game, Division of Commercial Fisheries, Juneau, Alaska
Calambokidis J, Steiger GH (2001) Movements and population structure of humpback whales in the north Pacific. Mar Mamm Sci 17(4):769–794
Cardinale M, Casini M, Arrhenius F et al (2003) Diel and spatial distribution and feeding activity of herring (Clupea harengus) and sprat (Sprattus sprattus) in the Baltic Sea. Aquat Living Resour 16:283–292
Carls MG, Marty GD, Hose JE (2002) Synthesis of the toxicological impacts of the Exxon Valdez oil spill on Pacific herring (Clupea pallasi) in Prince William Sound, Alaska, USA. Can J Fish Aquat Sci 59(1):153–172
Carls MG, Marty GD, Meyers TR et al (1998) Expression of viral hemorrhagic septicemia virus in prespawning Pacific herring (Clupea pallasi) exposed to weathered crude oil. Can J Fish Aquat Sci 55(10):2300–2309
Carls MG, Rice SD, Hose JE (1999) Sensitivity of fish embryos to weathered crude oil: part I: low-level exposure during incubation causes malformations, genetic damage, and mortality in larval Pacific herring (Clupea pallasi). Environ Toxicol Chem 18(3):491–493
Collie JS (1991) Herring population dynamics and management in Sitka Sound, Alaska. In: Wespestad V, Collie J, Collie E (eds) Lowell Wakefield fisheries symposium: proceedings of the international herring symposium, Anchorage, AK, October 23–25, 1990, AK-SG-91–01. University of Alaska Sea Grant College Program, Fairbanks, pp 463–476
Cooney RT (1993) A theoretical evaluation of the carrying capacity of Prince William Sound, Alaska, for juvenile Pacific salmon. Fish Res 18:77–87
Cooney RT, Allen JR, Bishop MA et al (2001) Ecosystem controls of juvenile pink salmon (Oncorhynchus gorbuscha) and Pacific herring (Clupea pallasi) populations in Prince William Sound, Alaska, fisheries. Fish Oceanogr 10(Suppl 1):1–13
Cross AD, Beauchamp DA, Armstrong JL et al (2005) Consumption demand of juvenile pink salmon in Prince William Sound and the coastal Gulf of Alaska in relation to prey biomass. Deep-Sea Res Pt II 52:347–370
Deriso RB, Maunder MN, Pearson WH (2008) Incorporating covariates into fisheries stock assessment models: Salmon hatcheries inhibit herring recovery. Ecol Applications 18(5):1270–1286
Deriso RB, Maunder MN, Skalski JR (2007) Variance estimation in integrated assessment models and its importance for hypothesis testing. Can J Fish Aquat Sci 64(2):187–197
Dickey-Collas M, Nash RDM, Brunel T et al (2010) Lessons learned from stock collapse and recovery of North Sea herring: a review. ICES J Mar Sci 67:1875–1886
Dillingham P, Ryding K, Skalski JR (2004) Assessment of fine-scaled interactions between Steller sea lions and trends of local fisheries. Final report to National Oceanographic and Atmospheric Administration by School of Fisheries, University of Washington, Seattle
Donaldson W, Morstad S, Sharp D et al (1995) Prince William Sound management area 1993 annual finfish management report. Regional Information Report 2A95-20, Alaska Department of Fish and Game, Anchorage
Donaldson W, Morstad S, Simpson E et al (1992) Prince William Sound management area 1991 annual finfish management report. Regional Information Report 2A92-09, Alaska Department of Fish and Game, Anchorage
Donaldson W, Morstad S, Simpson E et al (1993) Prince William Sound management area 1992 annual finfish management report. Regional Information Report 2A93-12, Alaska Department of Fish and Game, Anchorage
Dressel S (2006) ADF&G age-structured assessment Excel spreadsheet program for Sitka Sound herring. Alaska Department of Fish and Game, Division of Commercial Fisheries, Douglas
Dunn BP, Stich HE (1976) Monitoring procedures for chemical carcinogens in coastal waters. J Fish Res Bd Canada 33(9):2040–2046
Dunn BP, Young DR (1976) Baseline levels of benzo(a)pyrene in southern California mussels. Mar Pollut Bull 7(12):231–234
Elston RA, Drum AS, Pearson WH et al (1997) Health and condition of Pacific herring Clupea pallasi from Prince William Sound, Alaska, 1994. Dis Aquat Organ 31:109–126
Elston RA, Meyers TR (2009) Effect of viral hemorrhagic septicemia virus on Pacific herring in Prince William Sound, Alaska, from 1989 to 2005. Dis Aquat Organ 83:223–246
Everson I (2000) Krill: biology, ecology and fisheries. Blackwell Science, Oxford, p 372
Farrington C (2004) Alaska salmon enhancement program 2003 annual report. Regional Information Report 5J04-02, Alaska Department of Fish and Game, Juneau
Farrington JW, Quinn JG (1973) Petroleum hydrocarbons in Narragansett Bay. I. Survey of hydrocarbons in sediments and clams (Mercenaria mercenaria). Estuar Coast Mar S 1:71–79
Foy RJ, Norcross BL (1999) Spatial and temporal variability in the diet of juvenile Pacific herring (Clupea pallasi) in Prince William Sound, Alaska. Can J Zool 77:697–706
Foy RJ, Norcross BL (2001) Temperature effects on zooplankton assemblages and juvenile herring feeding in Prince William Sound, Alaska. In: Funk F, Blackburn J, Hay D et al (eds) Proceedings of the 18th Lowell Wakefield fisheries symposium, herring 2000: expectations for a new millennium, Anchorage, Alaska, February 23–26, 2000, AK-SG-01–04. University of Alaska Sea Grant College Program, Fairbanks, pp 21–35
Funk F (1993) Preliminary forecasts of catch and abundance for the 1993 Alaska herring fisheries. Regional Info Rep No 5J93-06, Alaska Department of Fish and Game, Juneau
Funk F (1994) Forecast of the Pacific herring biomass in Prince William Sound, Alaska. Regional Information Report 5J94-04, Alaska Department of Fish and Game, Juneau
Funk F, Sandone G (1990) Catch-age analysis of Prince William Sound, Alaska, herring, 1973–1988. Fish Res Bull 90-01, Alaska Department of Fish and Game, Juneau
Hansen PC, Johnson TB, Schindler DE et al (1997) Fish bioenergetics 3.0 for windows. Center for Limnology, University of Wisconsin, Madison
Hay DE, Toresen R, Stephenson R et al (2001) Taking stock: an inventory and review of world herring stocks in 2000. In: Funk F, Blackburn J, Hay D et al (eds) Proceedings of the 18th Lowell Wakefield fisheries symposium, herring 2000: expectations for a new millennium, Anchorage, Alaska, February 23–26, 2000, AK-SG-01–04. University of Alaska Sea Grant College Program, Fairbanks, pp 381–454
Hedrick RP, Batts WN, Yun S et al (2003) Host and geographic range extensions of the North American strain of viral hemorrhagic septicemia virus. Dis Aquat Organ 55:211–220
Hershberger PK, Gregg J, Pacheco C et al (2007) Larval Pacific herring, Clupea pallasi (Valenciennes), are highly susceptible to viral haemorrhagic septicaemia and survivors are partially protected after their metamorphosis to juveniles. J Fish Dis 30:445–458
Hershberger P, Hart A, Gregg J et al (2006) Dynamics of viral hemorrhagic septicemia, viral erythrocytic necrosis, and ichthyophoniasis in confined juvenile Pacific herring Clupea pallasi. Dis Aquat Org 70:201–208
Hershberger PK, Stick K, Bui B et al (2002) Incidence of Ichthyophonus hoferi in Puget Sound fishes and its increase with age of Pacific herring. J Aquat Anim Health 14:50–56
Hilborn R (1997) The frequency and severity of fish stock declines and increases. In: Hancock DA, Smith DA, Grant DC, Beumer A et al (eds) Developing and sustaining world fisheries resources, the state of the science and management, proceedings of the 2nd world fisheries congress, Brisbane, Australia, July 28–August 2, 1996. CSIRO Publishing, Collingwood, pp 36–38
Hjort J (1914) Fluctuations in the great fisheries of the Northern Europe viewed in the light of the biological research. Cons Perm Int Explor Mer Rapp et Proc-Verb Reun 20:1–228
Hjort J, Ruud JT (1929) Whaling and fishing in the North Atlantic. Cons Perm Int Explor Mer Rapp et Proc Verb Reun 56(Paper 1):1–123
Holst JC (1996) Estimating the prevalence of Ichthyophonus hoferi (Plehn and Mulsow) in a herring stock (Clupea harengus L.): observed effects of sampling gear, target school density and migration. Fish Res 28:85–97
Hulson P-JF, Miller SE, Quinn TJ II et al (2008) Data conflicts in fishery models: incorporating hydroacoustic data into the Prince William Sound Pacific herring assessment model. ICES J Mar Sci 65:25–43
Johnson SW, Carls MG, Stone RP et al (1997) Reproductive success of Pacific herring, Clupea pallasi, in Prince William Sound, Alaska, six years after the Exxon Valdez oil spill. Fish Bull 95(4):748–761
Johnston DW, Wildish DJ (1981) Avoidance of dredge spoil by herring (Clupea harengus harengus). Bull Environ Contam Toxicol 26:307–314
Kennedy CJ, Farrell AP, Sanders S (1999) Survival, performance and reproduction in the pacific herring, Clupea harengus pallasi: effects of environmental contamination, viral hemorrhagic septicemia virus and Ichthyophonus hoferi. In: Kocan RM, Marty GD, Kennedy CJ (eds) Investigations of disease factors affecting declines of Pacific herring populations in Prince William Sound, Final Report, Restoration Project 97162, Exxon Valdez Oil Spill Trustee Council, Anchorage, pp III-1–III-40, http://www.evostc.state.ak.us/Store/FinalReports/1998-98162-Final.pdf
Kocan RM, Hershberger PK, Elder N et al (2001) Epidemiology of viral hemorrhagic septicemia among juvenile Pacific herring and Pacific sand lances in Puget, Sound, Washington. J Aquat Anim Health 13:77–85
Kocan RM, Hershberger PK, Mehl P et al (1999a) Pathogenicity of Ichthyophonus hoferi for laboratory-reared Pacific herring Clupea pallasi and its early appearance in wild Puget Sound herring. Dis Aquat Organ 35:23–29
Kocan RM, Hershberger PK, Winton JR et al (1999b) Controlled field and laboratory studies on VHS and Ichthyophonus in Pacific herring. In: Kocan RM, Marty GD, Kennedy CJ (eds) Investigations of disease factors affecting declines of Pacific herring populations in Prince William Sound, Final Report, Restoration Project 97162, Exxon Valdez Oil Spill Trustee Council, Anchorage, pp II-i–II-73, http://www.evostc.state.ak.us/Store/FinalReports/1998-98162-Final.pdf
Kocan RM, Dolan H, Hershberger P (2011) Diagnostic methodology is critical for accurately determining the prevalence of Ichthyophonus infections in wild fish populations. J Parasit (in press)
Kramer-Schadt S, Holst JC, Skaen D (2010) Analysis of variables associated with the Ichthyophonus hoferi epizootics in Norwegian spring-spawning herring, 1992–2008. Can J Fish Aquat Sci 67:1862–1973
Marty GD, Freiberg EF, Meyers TR et al (1998) Viral hemorrhagic septicemia virus, Ichthyophonus hoferi, and other causes of morbidity in Pacific herring, Clupea pallasi, spawning in Prince William Sound, Alaska, USA. Dis Aquat Organ 32:15–40
Marty GD, Quinn TJ II, Carpenter G et al (2003) Role of disease in abundance of a Pacific herring (Clupea pallasi) population. Can J Fish Aquat Sci 60:1258–1268
Marty GD, Quinn TJ, Miller SE et al (2004) Effect of disease on recovery of Pacific herring in Prince William Sound, Alaska. Restoration Project 030462, Final Report, Exxon Valdez Oil Spill Trustee Council, Anchorage, http://www.evostc.state.ak.us/Store/FinalReports/2003-030462-Final.pdf
Marty GD (2007) Evidence that the Exxon Valdez oil spill did not cause the 1993 disease epidemic in the Pacific herring population of Prince William Sound Alaska. Chapter 2. In: Rice SD, Carls MG Prince William Sound Herring: An updated synthesis of population declines and lack of recovery. Exxon Valdez Trustee Council Restoration Project 050794, Final Report
Marty GD, Miller SE, Hulson P-JF, Quinn TJII, Moffitt SD, Merion RA, Meyers TM. (2007) Role of Ichthyophonus hoferi, viral hemorrhagic septicemia virus, and cutaneous ulcers in preventing recover of a Pacific herring (Clupea pallasi) population. Chapter 5, in: Rice SD and Carls MG Prince William Sound Herring: An updated synthesis of population declines and lack of recovery. Exxon Valdez Trustee Council Restoration Project 050794, Final Report
Marty GD, Hulson P, Miller SE et al (2010) Failure of population recovery in relation to disease in Pacific herring. Dis Aquat Org 90:1–40
Matkin C, Hobbs R (1999) Mammals: baleen whales. In: Okey TA, Pauly D (eds) Trophic mass-balance model of Alaska’s Prince William Sound ecosystem, for the post-spill period 1994–1996. Final Report, Restoration Project 99330-1, Exxon Valdez Oil Spill Trustee Council, Anchorage, p 56, http://www.evostc.state.ak.us/Store/FinalReports/1999-99330BAA-Final.pdf
McQuinn IH (1997) Metapopulations and the Atlantic herring. Rev Fish Biol Fish 7:297–329
Meyers TR, Short S, Lipson K et al (1994) Association of viral hemorrhagic septicemia virus with epizootic hemorrhages in the skin of Pacific herring Clupea harengus pallasi from Prince William Sound and Kodiak, Alaska, USA. Dis Aquat Organ 19:27–37
Meyers TR, Short S, Lipson K (1999) Isolation of the North American strain of viral hemorrhagic septicemia virus (VHSV) associated with epizootic mortality in two new host species of Alaska marine fish. Dis Aquat Organ 38:81–86
Moffitt S (2006) ADF&G age-structured assessment Excel spreadsheet program and data for Prince William Sound herring. Alaska Department of Fish and Game, Division of Commercial Fisheries, Cordova
Moulton LL (1997) Early marine residence, growth, and feeding by juvenile salmon in northern Cook Inlet, Alaska. Alaska Fish Res Bull 4(2):154–177
Neff JM, Bence AE, Parker KR et al (2006) Bioavailability of polycyclic aromatic hydrocarbons from buried shoreline oil residues thirteen years after the Exxon Valdez oil spill: a multispecies assessment. Environ Toxicol Chem 25(4):947–961
Neff JM, Burns WA (1996) Estimation of polycyclic aromatic hydrocarbon concentrations in the water column based on tissue residues in mussels and salmon: an equilibrium partitioning approach. Environ Toxicol Chem 15(12):2240–2253
Norcross BL, Brown ED (2001) Estimation of first-year survival of Pacific herring from a review of recent stage-specific studies. In: Funk F, Blackburn J, Hay D et al (eds) Proceedings of the 18th Lowell Wakefield fisheries symposium, herring 2000: expectations for a new millennium, Anchorage, Alaska, February 23–26, 2000, AK-SG-01-04. University of Alaska Sea Grant College Program, Fairbanks, pp 535–558
Norcross BL, Brown ED, Foy RJ et al (2001) A synthesis of the life history and ecology of juvenile Pacific herring in Prince William Sound, Alaska. Fish Oceanogr 10(Suppl 1):42–57
Okey TA, Pauly D (1999) Trophic mass-balance model of Alaska’s Prince William Sound ecosystem, for the post-spill period 1994–1996. Final Report, Restoration Project 99330-1, Exxon Valdez Oil Spill Trustee Council, Anchorage, http://www.evostc.state.ak.us/Store/FinalReports/1999-99330BAA-Final.pdf
Overholtz WJ, Link JS (2007) Consumption impacts by marine mammals, fish, and seabirds on the Gulf of Maine—Georges Bank herring (Clupea harengus) complex during the years 1977–2002. ICES J Mar Sci 64:83–96
Patterson KR (1996) Modeling the impact of disease-induced mortality in an exploited population: the outbreak of the fungal parasite Ichthyophonus hoferi in the North Sea herring (Clupea harengus). Can J Fish Aquat Sci 53:2870–2887
Paul AJ, Paul JM (1998) Comparisons of whole body energy content of captive fasting age zero Alaskan Pacific herring (Clupea pallasi Valenciennes) and cohorts over-wintering in nature. J Exp Mar Biol Ecol 226(1):75–86
Payne MR, Hatfield EMC, Dickey-Collas M et al (2009) Recruitment in a changing environment: the 2000s North Sea herring recruitment failure. ICEAS J Mar Sci 66:272–277
Pearson WH, Elston RA, Bienert RW et al (1999) Why did the Prince William Sound, Alaska Pacific herring (Clupea pallasi) fisheries collapse in 1993 and 1994? Review of hypotheses. Can J Fish Aquat Sci 56:711–737
Pearson WH, Moksness E, Skalski JR (1995) A field and laboratory assessment of oil spill effects on survival and reproduction of Pacific herring following the Exxon Valdez spill. In: Wells PG, Butler JN, Hughes JS (eds) Exxon Valdez oil spill: fate and effects in Alaskan waters. STP 1219, Publication 04-012190-16, American Society for Testing and Materials, Philadelphia, pp 626–661
Platt JR (1964) Strong inference. Science 146:347–353
Quinn TJ II, Marty GD, Wilcock J et al (2001) Disease and population assessment of Pacific herring in Prince William Sound, Alaska. In: Funk F, Blackburn J, Hay D et al (eds) Proceedings of the 18th Lowell Wakefield fisheries symposium, Herring 2000: expectations for a new millennium, Anchorage, Alaska, February 23–26, 2000, AK-SG-01-04. University of Alaska Sea Grant College Program, Fairbanks, pp 363–379
Quinn TJ II, Deriso RB (1999) Quantitative fish dynamics. Oxford University Press, New York
Rice S, Carls MG (2007) Executive summary. In: Rice SD, Carls MG (eds) Prince William Sound herring: an updated synthesis of population declines and lack of recovery. Exxon Valdez Trustee Council Restoration Project 050794, Final Report
Rice S (2008) Significance of whale predation on natural mortality rate of Pacific herring in Prince William Sound. Exxon Valdez Trustee Council Restoration Project 080804, Progress Report
Rice S (2009) Significance of whale predation on natural mortality rate of Pacific herring in Prince William Sound. Exxon Valdez Trustee Council Restoration Project PJ090804, Progress Report
Rose KA, Werner F, Megrey BA et al (2007) Simulated herring growth responses in the northeastern Pacific to historic temperature and zooplankton conditions generated by the 3-dimensional NEMURO nutrient-phytoplankton-zooplankton model. Ecol Model 202:184–195
Rothschild BJ (1986) Dynamics of marine fish populations. Harvard University Press, Cambridge
Ruggerone GT, Nielsen JL (2004) Evidence for competitive dominance of pink salmon (Oncorhynchus gorbuscha) over other salmonids in North Pacific Ocean. Rev Fish Biol Fish 14:371–390
Sanders SM (2005) Immunotoxicology of Pacific herring: determination of reference ranges and their application to assessing exposure to the water-soluble fraction of crude oil. Dissertation, Simon Fraser University, Burnaby, British Columbia
Sandone GJ (1988) Age, sex, and size composition of Pacific herring sampled from the Prince William Sound Management Area, 1984–1987. Regional Information Report 2A88-08, Alaska Department of Fish and Game, Anchorage
Short JW, Harris PM (1996) Petroleum hydrocarbons in caged mussels deployed in Prince William Sound after the Exxon Valdez oil spill. In: Rice SD, Spiess, Rb, Wolfe DA et al (eds) Proceedings of the Exxon Valdez oil spill symposium. American Fisheries Society Symposium 18
Short JW, Maselko JM, Lindberg MR et al (2006) Vertical distribution and probability of encountering intertidal Exxon Valdez oil on shorelines of three embayments within Prince William Sound, Alaska. Environ Sci Technol 40(12):3723–3729
Sindermann CJ (1958) An epizootic in Gulf of Saint Lawrence fishes. In: Trefethen JB (ed) Transactions of the twenty-third north American wildlife conference March 3–5, 1958. St. Louis, Missouri. Wildlife Management Institute, Washington, DC, pp 349–360
Smith TD (1988) Stock assessment methods: the first fifty years. In: Gulland JA (ed) Fish population dynamics, the implications for management, 2nd edn, John Wiley and Sons, New York, ch 1
Southward AJ, Boalch GT, Maddock L (1988) Fluctuations in the herring and pilchard fisheries of Devon and Cornwall linked to change in climate since the 16th century. J Mar Biol Assoc UK 68:423–445
Spies RB (2007) Long-term ecological changes in the Northern Gulf of Alaska. Elsevier, Amsterdam, p 589
Stephenson RL (1997) Successes and failures in the management of Atlantic herring fisheries: do we know why some have collapsed and others survived. In: Hancock DA, Smith DA, Grant DC, Beumer A et al (eds) Developing and sustaining world fisheries resources, the state of the science and management, proceedings of the 2nd world fisheries congress, Brisbane, Australia, July 28–August 2, 1996. CSIRO Publishing, Collingwood, pp 49–54
Stephenson RL (2001) The role of herring investigations in shaping fisheries science. In: Funk F, Blackburn J, Hay D et al (eds) Proceedings of the 18th Lowell Wakefield fisheries symposium, herring 2000: expectations for a new millennium, Anchorage, Alaska, February 23–26, 2000, AK-SG-01-04. University of Alaska Sea Grant College Program, Fairbanks, pp 1–20
Stokesbury KDE, Foy RJ, Norcross BL (1999) Spatial and temporal variability in juvenile Pacific herring, Clupea pallasi, growth in Prince William Sound, Alaska. Environ Biol Fish 56:409–418
Stokesbury KDE, Kirsh J, Brown ED et al (2000) Spatial distributions of Pacific herring, Clupea pallasi, and walleye pollock, Theragra chalcogramma, in Prince William Sound, Alaska. Fish Bull 98:400–409
Sturdevant MV (1999) Forage fish diet overlap, 1994–1996. Final Report, Restoration Project 97163C, Exxon Valdez Oil Spill Trustee Council, Anchorage, http://www.evostc.state.ak.us/Store/FinalReports/1997-97163C-Final.pdf
Sturdevant MV, Brase ALJ, Hulbert LB (2001) Feeding habits, prey fields, and potential competition of young-of-the-year walleye pollock (Theragra chalcogramma) and Pacific herring (Clupea pallasi) in Prince William Sound, Alaska, 1994–1995. Fish Bull 99:482–501
Sverdrup HU, Johnson MW, Fleming RH (1942) The oceans: their physics, chemistry, and general biology. Prentice-Hall, Englewood Cliffs
Taft DG, Egging DE, Kuhn HA (1995) Sheen surveillance: an environmental monitoring program subsequent to the 1989 Exxon Valdez shoreline cleanup. In: Wells PG, Butler JN, Hughes JS (eds) Exxon Valdez oil spill: fate and effects in Alaskan waters. STP 1219, Publication 04-012190-16, American Society for Testing and Materials, Philadelphia, pp 626–661
Thomas GL, Thorne RE (2003) Acoustical-optical assessment of Pacific herring and the predator assemblage in Prince William Sound, Alaska. Aquat Living Resour 16:247–253
Thorne RE, Thomas GL (2008) Herring and the “Exxon Valdez” oil spill: an investigation into historical data conflicts. ICES J Mar Sci 65:44–50
Tibbo SN, Graham TR (1963) Biological changes in herring stocks following an epizootic. J Fish Res Bd Canada 20(2):435–449
Tjelmeland S, Lindstrom U (2005) An ecosystem element added to the assessment of Norwegian spring-spawning herring: implementing predation by minke whales. ICES J Mar Sci 62:284–294
Tynan CT (2004) Cetacean populations on the SE Bering Sea shelf during the late 1990s: implications for decadal changes in ecosystem structure and carbon flow. Mar Ecol Prog Ser 272:281–300
Von Ziegesar O, Miller E, Dahlheim ME (1994) Impact on humpback whales in Prince William Sound. In: Loughlin TR (ed) Marine mammals and the Exxon Valdez. Academic Press, San Diego, pp 173–191
Wertheimer AC, Celewycz AG (1996) Abundance and growth of juvenile pink salmon in oiled and non-oiled locations of western Prince William Sound after the Exxon Valdez oil spill. In: Rice SD, Spies RB, Wolfe DA, et al (eds) Proceedings of the Exxon Valdez oil spill symposium, Anchorage, Alaska, February 2–5, 1993, American Fisheries Society Symposium 18, Bethesda, pp 518–532
White B (2005) Alaska salmon enhancement program 2004 annual report. Fishery Management Report 05-09. Alaska Department of Fish and Game, Anchorage, http://www.sf.adfg.state.ak.us/FedAidPDFs/fmr05-29.pdf
Willette TM (2001) Foraging behaviour of juvenile pink salmon (Oncorhynchus gorbuscha) and size-dependent predation risk. Fish Oceanogr 10(Suppl 1):110–131
Willette, TM, Cooney RT, Hyer K (1999a) Some processes affecting mortality of juvenile fishes during the spring bloom in Prince William Sound, Alaska. In: Ecosystem approaches to fisheries management: proceedings of the symposium on ecosystem considerations in fisheries management, September 30–October 3, 1998, Anchorage, Alaska. Alaska Sea Grant Program AK-SG-99-01, University of Alaska Sea Grant Program, Fairbanks
Willette TM, Cooney RT, Hyer K (1999b) Predator foraging mode shifts affecting mortality of juvenile fishes during the subarctic spring bloom. Can J Fish Aquat Sci 56(3):364–376
Willette TM, Cooney RT, Patrick V et al (2001) Ecological processes influencing mortality of juvenile pink salmon (Oncorhynchus gorbuscha) in Prince William Sound, Alaska. Fish Oceanogr 10(suppl 1):14–41
Willette TM, Sturdevant MV, Jewett S (1997) Prey resource partitioning among several species of forage fishes in Prince William Sound, Alaska. In: Rice SD, Spies RB, Wolfe DA et al (eds) International fisheries symposium, role of forage fishes in marine ecosystems, Anchorage, Alaska, 1996, AK-SG-97-01. University of Alaska Sea Grant College Program, Fairbanks, pp 11–29
Williams EH, Quinn TJ II (2000a) Pacific herring, Clupea pallasi, recruitment in the Bering Sea and north-east Pacific Ocean. I: relationships among different populations. Fish Oceanogr 9(4):285–299
Williams EH, Quinn TJ II (2000b) Pacific herring, Clupea pallasi, recruitment in the Bering Sea and north-east Pacific Ocean. II: relationships to environmental variables and implications for forecasting. Fish Oceanogr 9(4):300–315
Winters GH, Wheeler JP (1994) Length-specific weight as a measure of growth success of adult Atlantic herring (Clupea harengus). Can J Fish Aquat Sci 52:1169–1179
Winters GH, Wheeler JP, Dalley EL (1986) Survival of a herring stock subjected to a catastrophic event and fluctuating environmental conditions. J Cons Int Explor Mer 43:26–42
Witteveen BH, Foy RJ, Wynne KM (2006) The effect of predation (current and historical) by humpback whales (Megaptera noveangliae) on fish abundance near Kodiak Island, Alaska. Fish Bull 104:10–20
Wolfe DA, Hammedi MJ, Galt JA et al (1994) The fate of the oil spilled from the Exxon Valdez. Environ Sci Technol 28(13):561–568
Zebdi A, Collie JS (1995) Effect of climate on herring (Clupea pallasi) population dynamics in the Northeast Pacific Ocean. In: Beamish RJ (ed) Climate change and northern fish populations conference, Victoria, British Columbia, October 19–24, 1992, Can Spec Publ Fish Aquat Sci 121:277–290
Acknowledgments
We thank Michael Anderson, Michael Cobb, Lee Miller, Ann Skillman, Kathryn Sobocinski, and John Southard of Battelle Marine Sciences Laboratory in Sequim, Washington, and Karen Humphrey of Aquatechnics, for their dedicated efforts in compiling and presenting the information in this review. We thank Mark N. Maunder of Quantitative Resource Assessment LLC for his modeling aid in this effort. We thank Lawrence L. Moulton of MJM Research for sharing his raw data on the co-occurrence of pink salmon and herring in his tow net samples. We thank Kenneth A. Rose of Louisiana State University and John R. Skalski of the University of Washington for valuable discussions and for reviewing an earlier version of this paper. We also thank Douglas E. Hay of Nearshore Consulting for reviewing earlier manuscripts. Exxon Mobil Corporation supported this effort.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jack W. Anderson—Deceased.
Appendix: Sources for Unpublished Data from Alaska Department of Fish and Game
Appendix: Sources for Unpublished Data from Alaska Department of Fish and Game
See Table 13.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Pearson, W.H., Deriso, R.B., Elston, R.A. et al. Hypotheses concerning the decline and poor recovery of Pacific herring in Prince William Sound, Alaska. Rev Fish Biol Fisheries 22, 95–135 (2012). https://doi.org/10.1007/s11160-011-9225-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11160-011-9225-7