Abstract
Phase angle (PhA) is a valuable tool for evaluating the nutritional and inflammatory status, which can accompany acute and severe disorders. PhA is a cellular health biomarker, whose value is particularly substantial due to the negative consequences of these situations in the pediatric population. Relevant literature was collected with the aim of comprehensively analysing the evidence on the association between an altered PhA can serve as a predictive-marker for mortality and poor-outcomes in at-risk-pediatric patients. Understanding this relationship could have significant implications for identifying high-risk individuals and implementing timely interventions. A systematic review with meta-analysis was conducted in the primary electronic databases from inception until January 2023. Overall, four studies with a total of 740 patients were eligible for our analysis. Evidence demonstrates that PhA is associated with nutritional status, reflecting undernutrition and changes in body composition related to illness. This review suggests that PhA can indeed be used as an indicator of nutritional status and a tool for predicting prognosis, including mortality and poor-outcomes, in hospitalized pediatric patients. A low PhA was associated with a significant mortality risk [RR:1.51;95%CI (1.22–1.88),p = 0.0002;I2 = 0%,(p = 0.99)] and an increased complications risk [OR:8.17;95%CI (2.44–27.4),p = 0.0007;I2 = 44%,(p = 0.18)]. These findings highlight the importance of taking a comprehensive approach to clinical nutrition, integrating multiple evaluation aspects to establish an accurate diagnosis and personalized therapeutic plans. While PhA emerges as a valuable tool for assessing the risk of malnutrition and as a prognostic-indicator for poor-outcomes in pediatric patients. Further future studies are needed to focus on investigating this relationship in larger and diverse population to strengthen the evidence base.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background
In clinical practice there is no single tool to assess the nutritional status of a patient. Traditionally, basic anthropometric parameters such as weight, height and body mass index (BMI) have been used for this. These parameters are essential and must continue to be taken into account, but they are not sensitive enough to assess the different components of weight or changes in body composition. In order to evaluate these changes, the need to incorporate new nutritional assessment parameters such as the Phase angle (PhA) is raised, which, although it still have limitations within clinical practice, may be more sensitive, specific and reproducible [1].
Bioelectrical impedance (BIA) is a simple, non-invasive method that, due to its ease of use and low cost, has been used in recent decades to estimate the body composition of healthy and sick subjects. Despite its widespread use in clinical nutrition, this technique does not directly measure body composition, and the lack of standardized methods, quality control procedures, and the fact that its accuracy depends on the formulas used makes this technique subject to possible biases [2].
Bioelectrical vector impedance (BIVA), developed by Piccoli et al. [3], allows to directly measure the body's opposition to alternating current, that is, the impedance (Z), which consists of two components: resistance (R) and reactance (Xc) [4]. Resistance is the opposition offered by the body to the flow of an alternating electrical current and is inversely related to the water and electrolyte content of the tissue. Reactance is related to the capacitance properties of the cell membrane, and variations may occur depending on its integrity, function, and composition [5]. These two parameters provide direct information on hydration status, body cell mass, and cell integrity, without algorithm-dependent errors and without requiring assumptions such as constant tissue hydration [4]. From the vector relationship between these two crude impedance parameters, the PhA can be calculated by means of the following formula: tan−1(Xc/R) × 180/π (Fig. 1A).
A Geometric relationships of impedance components. B RXc graph. The BIVA nomogram uses tolerance ellipses to plot reference values and assess the position of vectors. Values outside the 95th percentile are considered abnormal. The vector's position and length provide information about disease status and cell membrane function. A longer vector indicates high or low R values, indicating dehydration (quadrant 1) or overhydration (quadrant 3) respectively. Sideways migration of the vector due to high or low XC indicates an increase (quadrant 2) or decrease (quadrant 4) in dielectric mass of soft tissues. The interpretation remains the same when using Z scores. Quadrant divisions are indicative rather than strict distinctions. Xc = reactance; R = resistence; Φ = Phase angle; z = impedance; H = height (meters). References [8, 9]
1.1 Fundamentals and methodology for Phase angle measurement
The PhA is physiologically characterized as an index of the integrity and vitality of the cell membrane and expresses the quantity and quality of the soft tissues. PhA is positively correlated with lean body mass and cell mass, but is inversely related to the ratio of extracellular to intracellular fluid in healthy adults and children [6]. It is considered an indicator of cellular health, since high PhA values indicate greater cellularity and integrity of the membrane and better cellular function [4, 7]. However, lower PhA values are indicative of a worse prognosis and higher morbidity and mortality. Due to its direct relationship with the state of cellular health, this angle has been proposed as a prognostic marker in certain clinical conditions [2].
To obtain the PhA value, a distal tetrapolar, monofrequency and vectorial BIA equipment is used [8, 9]. To perform its measurement, 4 electrodes are placed on the right side of the body, two of them on the hand and two on the foot. It is important to comply with the measurement protocol, the standardized conditions prior to it, the position of the patient and the placement of the electrodes so that the electrical determinations are not affected [2].
This equipment uses a 50 kHz signal to directly measure R and Xc standardized for height, which can be plotted on the RXc graph. The vector that is produced (Z) has a length inversely related to total body water and the combination of the vector's length and its direction, defined as the PhA, is an indicator of hydration status [7]. The RXc plot (Fig. 1B) is a probability distribution that classifies a vector according to its distance from the mean healthy vector. The variability of the Z vector is represented in tolerance ellipses (50%, 75% and 95%). Vectors within the 50% tolerance ellipse are within normal hydration, while vector elongation to 75% and above this percentage and 95% indicates moderate and severe dehydration, respectively. On the contrary, the shortening of the vectors within these percentages in the reference ellipses in the lower range indicates an increasing fluid overload. Vectors to the left of the major axis reflect increasing cell mass and vectors to the right indicate decreasing cell mass, respectively.
1.2 Phase angle and malnutrition in pediatric population. Predictor of mortality and complications
Malnutrition is very prevalent in critically ill children. Disease-related malnutrition is characterized by an early exchange of fluids from the intracellular water space to extracellular water with a concomitant decrease in cell mass. These disease-related alterations in fluid distribution are reflected by decreasing PhA [4].
Several studies in developing countries showed that malnutrition may affect 50% of children and adolescents admitted to the pediatric intensive care unit (PICU) [10], and in turn is associated with increased morbidity and mortality, including increased risk of infection due to transient immune disorders, inadequate wound healing, reduced bowel function, increased dependence on mechanical ventilation and longer hospital stays [1,2,3].
Body composition appears to be able to predict clinical prognosis, which is extremely important as early identification of severity allows anticipation of therapeutic measures that may be decisive in patient’s outcomes. Studies have shown that reduced muscle mass is an indirect risk factor for mortality and is associated with a longer length of hospital stay [11, 12].
There are several techniques to assess body composition, such as mid-upper arm circumference and triceps skinfold thickness[13, 14] that describe fat deposits and lean mass [15], but the most complete technique and the one that gives us more values regarding body composition is the bioelectrical impedance analysis.
PhA is a cellular health marker, that detects malnutrition and inflammation that can accompany acute and/or severe pathologies, it has has been used in recent years as an evaluation technique with a prognostic factor for mortality or complications in different pathologies [16,17,18,19,20,21] like cancer [22,23,24,25,26], liver diseases [27,28,29], kidney diseases renal [30, 31] and critically ill patients [32,33,34]. In addition, there are strong indications of an association between decreased PA values and mortality [35]. One of the most studied pathologies is cancer, where it is observed that the PhA value can detect a higher or lower survival during treatment and in the evolution of the disease depending on the cellular status of the patient [36, 37]. Therefore, PhA can be considered as a reliable prognostic marker and should be considered as a screening tool for the identification of patients at risk of deterioration of nutritional and functional status [21, 38, 39]. BIA is used as a tool to obtain data that helps to better understand the nutritional status of the patient, being a non-invasive, relatively inexpensive and easily transportable technique [38, 40]. BIA works by passing a low intensity electrical current through the body, measuring the primary components, and estimating fat mass (FM), fat free mass (FFM) and total body water. Among the BIA parameters, PhA which indirectly represents FFM, is the most clinically established as it has demonstrated a strong ability to predict outcomes in a wide variety of clinical situations [41].
PhA predicts mortality in various clinical situations [42, 43] and a potentially useful screening tool for mortality prognosis [44]. Some studies show its association with poor disease outcome, such as length of stay (LOS), mortality or need for intensive supportive therapies [43,44,45]. However, the use of PhA to assess pediatric patients has not been established. Further studies are needed to validate and establish recommendations for this tool in routine clinical practice.
1.3 Normal values PhA in healthy children
Numerous studies have estimated normal PhA values in healthy pediatric populations. Several considerations should be made in this regard. De Moraes et al. [46] found gender differences in the PhA values among adolescents, with boys exhibiting significantly higher values compared to girls, even after controlling for age group and sexual maturity status. Additionally, it was found that PhA values tended to increase with advancing age and maturity. Moreover, when examining the relationship between PhA and proximity to predicted age at peak height velocity (PHV), a stronger association was observed in boys than in girls. Incorporating body mass into the multilevel models revealed that changes in overall body mass accounted for a substantial portion of the influence exerted by maturity status and age group on the PhA. These findings indicate that body size plays a significant role in shaping the relationship between PhA and developmental factors. The present study highlights the multifactorial nature of PhA variability, indicating its dependence on inter-individual differences in sex, age, maturity status, and body size. Therefore, when investigating PhA in adolescents, it is recommended to employ multilevel modeling with standardized parameters as the default approach to effectively control for the concurrent influence of sex, age, maturity status, and body size. Ballarin et al. [47] described that PhA increases progressively over the first 2 decades of life and is higher in male than female adolescents, especially after the age of 13 yrs. Less consistent evidence has been reported in younger subjects. Schmidt et al. [48]reported in a representative German sample that Percentile curves for body composition parameters are similar between boys and girls until puberty. Subsequently, girls show a higher FMI than boys, and boys increase their FFM, BCM, and PA time-shifted, in that order. Differences in FMI between the overall and the normal weight sample increase with age, showing an age-dependent prevalence for overweight and obesity.
In the study conducted by Redondo-del-Río et al. [49], tolerance ellipses were utilized to analyze the Spanish child and adolescent population. The results revealed a displacement of the mean impedance vector across various age groups, with a few exceptions. Notably, there were no significant displacements observed in girls aged 12–13 years, girls aged 15–18 years, and boys aged 16–18 years. Remarkably, the study also highlighted sex-related differences in the mean impedance vector across all age ranges, including in prepubertal children. These differences persisted throughout adolescence. The observed patterns of vector displacement were found to align with the expected timing of normal growth and development, indicating that they can be attributed to the maturation process.
Mattiello et al. [50] conducted a systematic review and meta-analysis involving 46 studies and 249,844 subjects to explore age-related variations in PhA and gender differences. The results showed that for males, the mean PhA increased from 3.6 (95% CI: 3.0–4.1) in infants (0–2 years) to 7.3 (95% CI: 7.0–7.5) in teenagers (16–18 years), stabilized in adults (18–38 years), and decreased to 5.3 (95% CI: 4.5–6.0) in elderly individuals (> 80 years). Females had similar patterns, with PhA starting at 3.7 (95% CI: 3.2–4.3) in infants, reaching 6.4 (95% CI: 6.1–6.8) in teenagers, stabilizing in adults (18–48 years), and decreasing to 5.4 (95% CI: 5.3–5.6) in elderly individuals (> 80 years). Males generally had higher PhA estimates than females, except for infants and subjects older than 80 years old.
Considering the importance of body composition analysis as a complementary nutritional assessment and as a possible predictor of morbidity and mortality in many clinical situations, studies are needed to demonstrate this ability, especially in critically ill children, because data are still scarce.
The GRADE method aims at grading the quality of evidence and grading the strength of recommendations. It has been approved to reduce the risk of bias, inconsistency of results between studies, indirect evidence, imprecision and publication bias [16, 51].
This systematic review of the literature aims to determine whether the PhA value is a good prognostic marker of morbidity and mortality and to establish the reliability of recommendations intended for use in routine clinical practice guidelines.
The PICO question asked was: In hospitalized or ambulatory PEDIATRIC patients (with disease-related malnutrition or at risk of malnutrition) does the existence of an altered PHASE ANGLE predict higher mortality and/or morbidity (short and long term)?
2 Methods
2.1 Study design
A systematic review was performed comparing studies based on the PICO (Patient, Intervention, Comparison, Outcome) framework. A MESH search was performed by applying the appropriate filters in PubMed.
The evidence was evaluated using the parameters or recommendations of the GRADE (Grading of Recommendations Assessment, Development and Evaluation) method [16], which defines the quality of evidence as the degree of confidence we have that the estimate of an effect is adequate to make a recommendation.
2.2 Literature search
To obtain published studies related to the topic of interest, the following websites were consulted: MEDLINE or PubMed, Scopus, Embase and Web of Science (from base inception to January 2023). The following terminology was used in the title, abstract or keyword fields: ("malnutrition"[MeSH Terms] OR "malnutrition"[All Fields]) AND "phase angle"[All Fields] AND (("mortality"[Subheading] OR "mortality"[All Fields] OR "mortality"[MeSH Terms]) OR "Lenght of stay"[All Fields] OR "Quality of life"[All Fields] OR "complications"[All Fields]) AND "humans"[MeSH Terms] AND (English[lang] OR Spanish[lang]), to identify the main bioimpedance parameters along with the population of interest. In addition, the following filters were used to select the pediatric population: Filters applied: Child: birth-18 years, Infant: birth-23 months, Infant: 1–23 months, Newborn: birth-1 month, Preschool Child: 2–5 years, Child: 6–12 years, Adolescent: 13–18 years. Articles published in English or Spanish were selected for critical synthesis, and only significant associations are reported.
The clinical questions that guided the literature search were elaborated by the scientific committee with a focus on the population of interest. To determine the eligibility criteria, the PICOS strategy was adopted [52]: where "P" (patients), corresponded to pediatric patients, all genders and ethnicities; "I" (intervention), was designated as PhA bioimpedance assessment, "C" (comparison), was defined as altered versus normal PhA results, "O" (outcomes), were mortality, LOS or PICU admissions, and "S" (study design), were related to observational or clinical trials.
Exclusion criteria were as follows (i) articles did not include a full text description of the study; (ii) were not in English or Spanish; (iii) PhA differences were not assessed in relation to outcomes (e.g., mortality, length of stay, complications, or sequelae); (iv) studies published in non-peer-reviewed journals; (v) meta-analyses, reviews, protocols, case series or reports, editorials, and letters to the editor; (vi): studies on animal models.
2.3 Risk of bias assessment
GRADE method is an approach that enables an explicit evaluation of evidence and provides a framework to develop recommendations [16] GRADE was used to evaluate the evidence regarding the prognostic value of morphofunctional assessment tools in terms of mortality and complications. For each of the seven topics, an expert reviewed the literature, selecting outcomes from the studies, rating their importance, and evaluating outcomes across studies; then the evidence profile tables for each outcome was created, including a rating of the quality of the evidence, using GRADEpro GDT software (https://gradepro.org). The tables included outcomes, number of studies, study design, risk of bias, effect, quality of evidence, and importance. Another author from the scientific committee reviewed the evidence tables and conclusions drawn from the literature. The overall quality of evidence was graded across outcomes based on the lowest quality of critical outcomes. The scientific committee then made recommendations for each topic based on the literature findings and balancing consequences (e.g., benefits/harms, values and preferences, feasibility).
2.4 Statistical analysis
We used Review Manager 5.3 statistical software for the meta-analysis. Risk Ratio (RR) or Odds Ratio (OR) and 95% confidence interval (CI) were used in this study for continuous binary variables, respectively. it indicates that the index is statistically different between studies, and Random Effects Model (Random) is used to combine. If the heterogeneity test p > 0.05 and I2 < 50%, it indicates that there is no statistical difference in this indicator between studies, and the Fixed Effects Model (Fixed) is used to integration.
3 Results
3.1 Results in tables: evidence map and GRADE table
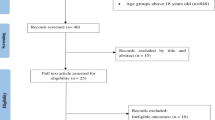
In our review we obtained a total of 701 studies, as can be seen in the Flow Chart (Fig. 2). Of these articles identified from the databases, 503 were removed before the screening process through duplication. The analysis of titles, keywords and abstracts, based on our inclusion criteria (PICOS) and exclusion criteria, identified 26 potentially eligible studies. After reading the full text, 4 relevant studies were finally included in our systematic review about PhA and pediatric patients [53,54,55,56]. 22 reports were excluded due to a lack of evaluation of PhA and poor outcomes for incomplete data in the pediatric population, without essential data or OR and HR analysis.
3.2 Characteristics, methodology and outcomes of the included studies
Marino et al. [53] analysed the relationship between nutritional status, PhA and post-operative outcomes in children with congenital heart disease in a sample of 122 patients, aged from birth to 8 years (Table 1). They observed that lower PhA was related to worse nutritional status and longer hospital stay in PICU. ROC curves at different time points revealed that 40th percentile at day 0 PhA = 2.6° (p = 0.03), 30th percentile at day 2 PhA = 2.7° (p = 0.03) and 50th percentile PhA = 3.4° (p = 0.01), with predictive accuracy of adverse clinical outcomes at hospital discharge. Children with a PICU stay of > 4 days had significantly lower PhA value on day 0, 2 and at discharge compared to children whose PICU stay was < 4 days. These authors showed that a lower PhA was associated with worse outcome and longer hospital stay. A Pha < 2,7º on day 2 of admission leads to an increase in hospital days > 4 days, OR = 7.8 (2.7–22.45) p < 0.001 (Table 2). These are cardiac patients, many of them with elective surgery, as nowadays the age of intervention of patients with congenital heart disease has decreased considerably due to the refinement of surgical techniques and the skill of cardiac surgeons. In many cases they are operated on very early. The timing of surgery should be carefully selected and optimized according to the PhA in order to avoid the complications described above (Table 3).
In an observational study by Zamberlan et al. [54] it was observed that in a sample of 247 patients (with different pathologies) aged between 2 and 18 years (Table 1), the cut-off point of PhA was 2.8° correlated with the risk of mortality, since this increased significantly in PICU hospitalized patients, showing a ROC curve analyzed under the AUC = 0.65 with a confidence interval between 0.58–0.71, sensitivity of 37% and specificity of 86% (Table 2). Thirty-six deaths occurred during hospitalization in the tertiary PICU, corresponding to 14.6% of cases. This article concluded that the lower the PhA value, the higher the risk of morbidity and mortality and the worse the nutritional status of the patient. In addition, most deaths occurred while the patient was hospitalized in PICU and a mean hospital stay of 4 days was observed, with a mortality rate 2.7 times higher, so it could also be said that the lower the PhA, the longer the hospital stay and the higher the risk of admission to PICU. This analysis was adjusted for sex and age with Cox regression and revealed that children with PhA ≤ 2.8° were more likely to stay in PICU compared to those with PA > 2.8° [HR: 1.64 (1.09–2.47); p = 0.003], (Table 3).
In a prospective analysis, Almeida de Azevedo et al. [55] conducted bioelectrical impedance measurements on 145 children aged between one month and six years who were initially not in septic shock upon admission to the PICU (Table 1). The researchers analyzed serial measurements of PhA to determine its sensitivity and specificity in accurately identifying children who later developed septic shock. The results of the study revealed that lower PhA values were associated with an increased occurrence of septic shock and longer stays in the PICU. PhA < 3.27° had an OR 9.58 (1.29–71.47) for developing septic shock the next day, with a sensitivity of 95.8%, specificity of 29.4%, and an AUC of 0.62 (0.58–0.67). Moreover, the presence of a PhA < 2.64° showed an OR of 14.2 (4.47–45.1), with an AUC of 0.77 (0.70–0.84), for developing septic shock on the same day (Tables 2 and 3).
In an observational study conducted by Xiong et al. [56], 231 pediatric patients admitted to the PICU were included, with 31.6% of them being malnourished (Table 1). The study aimed to assess the relationship between the PhA and 90-day survival. The results showed that children with a higher PhA had a longer survival time compared to those with a lower PhA (cut-off PhA = 3.0°, sensitivity 83%, specificity 53%). The area under the curve (AUC) was 0.69 (95% CI: 0.53–0.85, p < 0.05). The OR for survival with a higher PhA was 1.51 (1.10–2.07, p = 0.01) (Tables 2 and 3).
Furthermore, the study compared the duration of admission to the PICU among different degrees of malnutrition (non-malnourished, moderately malnourished, severely malnourished) and found no significant difference (p = 0.86). However, a lower PhA was associated with a longer duration of mechanical ventilation in the PICU (r = -0.42). These findings suggest that low PhA values can serve as prognostic markers for mortality risk in pediatric patients.
3.3 Quality of studies
From the initial literature review which yielded 701 studies, only 4 articles covered all four topics related to PICOs issues. The quality of the evidence was evaluated following the GRADE method (Table 4), to make evidence-based recommendations on the prognostic and clinical value of PhA measurement (Table 5). There was insufficient evidence to make recommendations for the systematic use of PhA as an indicator of the length hospital stay. However, we could make recommendations for the usefulness of the PhA as a prognostic marker for poor outcomes such as mortality or complications in hospitalized pediatric patients. Thus, PhA, which has a weak strength of recommendation and low-very low quality of evidence, could suggest mortality and complications in hospitalized pediatric patients.
3.4 Impact of the PhA as a prognostic factor of poor outcomes in pediatric patients
Finally, we examined the usefulness of the PhA as a prognostic factor for poor outcomes. We operated for meta-analysis a randomized-effect or fixed-effect model if the tests were characterized as heterogeneous or homogeneous, respectively. Meta-analysis of data from 408 patients indicated a significantly increased mortality risk in pediatric patients with lower PhA [RR: 1.51; 95% CI (1.22 – 1.88), p = 0.0002]. Homogeneity between studies: I2 = 0%, (p = 0.99). A significantly increased complications risk was found in 262 pediatric patients with lower PhA [OR: 8.17; 95% CI (2.44 – 27.4), p = 0.0007]. Heterogeneity between studies: I2 = 44%, (p = 0.18). However, PhA was not a significant predictor of longer hospital stay [RR: 3.30, 95% CI (0.72 – 15.10), p = 0.12] (Fig. 3).
Analyses of PhA as a prognostic marker for poor outcomes in hospitalized pediatric patients. The data of OR or HR and 95% CI from 4 studies were combined in this meta-analysis and the result of the meta-analysis was described as a forest plot. Four studies were grouped, in the main poor outcomes studied: mortality (A), complications (B) and length of hospital stay (C). OR: Odds ratio; HR: Hazard ratio; CI: confidence interval
4 Discussion
PhA is indeed a reliable indicator of cellular health and is typically not less than 4° in a healthy pediatric population [46, 47, 49, 50]. Its measurement is simple and can provide valuable insights into the prognosis and evolution of patients. The study conducted by Marino et al. [53] further highlights the significance of PhA in assessing the prognosis of children with congenital heart disease. The results demonstrate that children who experienced longer stays in the PICU, exceeding four days, had significantly lower PhA values on day 0, day 2, and at discharge compared to those with shorter stays. This finding suggests that monitoring PhA can potentially serve as a useful tool in identifying patients at higher risk of complications and prolonged hospitalization. It is important to note that the study population of Marino et al. [53] primarily consisted of cardiac patients, many of whom underwent elective surgeries. Over time, the age at which patients with congenital heart disease undergo surgical interventions has significantly decreased due to advancements in surgical techniques and the expertise of cardiac surgeons. Consequently, surgical interventions are often performed at a very early age. Considering this, the timing of surgeries should be carefully selected and optimized based on the PhA to minimize the risk of complications associated with prolonged hospital stays and unfavorable outcomes.
The results have shown that measurements should be performed on a regular basis and can serve as a guide to predict the evolution of patients. It would be useful to have data correlating PhA values with other parameters and scores predictive of mortality in pediatric patients admitted to the PICU. This is the case for the Pediatric Index of Mortality (PIM), the Pediatric Risk of Mortality (PRISM) [57], the vasoactive-inotropic score [58], albumin or lactic acid levels whose values have not yet been correlated with the PhA [59].
The data resulting from this GRADE review with meta-analysis on the evidence of PhA as a predictor of morbidity and mortality show that a lower PhA value relates to a higher risk of complications such as sepsis and lower survival. In our systematic review was a trend for a longer hospital stay with a low PhA, but it found no statistically significant association between PhA and long length hospital stay.
It is worth remembering that in surgical pediatric patients, the intervention is not always urgent and may be postponed sometimes to find the ideal moment. In these cases, it may be advisable to optimize nutritional support and overhydration or inflammation status according to the underlying pathology by PhA monitoring.
Looking to the future, the integration of PhA measurements into routine clinical practice holds great promise. As healthcare technologies continue to advance, it is conceivable that PhA monitoring could become more accessible, allowing for frequent and non-invasive assessments of cellular health. This would enable healthcare providers to obtain real-time data on patients' inflammatory degree, as well as, nutritional-hydration status and overall well-being, facilitating timely interventions and personalized treatment plans.
Moreover, future studies could explore the potential of combining PhA measurements with other clinical parameters and predictive models to enhance risk stratification and prognostic accuracy. By incorporating PhA into existing scoring systems, such as the PIM or PRISM, healthcare professionals may gain a more comprehensive understanding of a patient's condition and make more informed decisions regarding their care [60].
As research progresses, it would be valuable to investigate the underlying mechanisms linking PhA to outcomes in pediatric patients. Consequently, understanding the physiological basis of the association between PhA and morbidity/mortality could provide insights into the complex interplay between cellular health, immune function, and disease progression in this population. This knowledge may open avenues for personalized and targeted interventions and therapeutic strategies aimed at improving outcomes and reducing complications [61].
Lastly, interdisciplinary collaborations among clinicians, researchers, and technologists will be vital in advancing the field of PhA monitoring. By combining expertise from various domains, innovative approaches can be developed to refine PhA measurement techniques, establish standardized protocols, and harness the potential of machine learning and artificial intelligence to extract meaningful patterns and predictive algorithms from PhA data.
In summary, the future of PhA as a predictor of poor outcomes and mortality in pediatric patients is promising. With continued research and technological advancements, PhA monitoring has the potential to revolutionize pediatric healthcare by enabling early detection of clinical deterioration, optimizing and personalizing treatment strategies, and ultimately improving patient outcomes. This new tool offers important opportunities to enhance the quality of care and promote better health outcomes for the children population in the years to come.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- BI:
-
Bioelectrical impedance
- CI:
-
Confidence interval
- ECW:
-
Extracellular water
- FFM:
-
Fat-free mass
- GRADE:
-
Grading of Recommendations Assessment, Development and Evaluation
- HR:
-
Hazard ratio
- PICU:
-
Pediatric intensive care unit
- IMV:
-
Intensive Mechanical ventilation
- LOS :
-
Length of stay
- OR :
-
Odds ratio
- HR:
-
Hazard ratio
- PhA :
-
Phase angle
- PICOS:
-
Patient, Intervention, Comparison, Outcome, Studies
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews
- R:
-
Resistance
- Xc:
-
Reactance
- RR:
-
Risk Ratio
- AUC:
-
Under the curve
- PICOS:
-
Patient, Intervention, Comparison, Outcome
References
García J, García C, Bellido V, Diego B. Nuevo enfoque de la nutrición. Valoración del estado nutricional del paciente: función y composición corporal. Nutr Hosp. 2018;35(3):1–14.
Porca Fernández C, Tejera Pérez C, Bellido Castañeda V, Manuel J, Almeida G, Guerrero DB. Nuevo enfoque en la valoración de la ingesta dietética. Nutr Clin Med. 2016;X(2):95–107.
Piccoli A, Rossi B, Pillon L, Bucciante G. A new method for monitoring body fluid variation by bioimpedance analysis: The RXc graph. Kidney Int. 1994;46(2):534–9.
Norman K, Stobäus N, Pirlich M, Bosy-Westphal A. Bioelectrical phase angle and impedance vector analysis - Clinical relevance and applicability of impedance parameters. Clin Nutr. 2012;31(6):854–61.
Barbosa-Silva MCG, Barros AJD, Wang J, Heymsfield SB, Pierson RN. Bioelectrical impedance analysis: Population reference values for phase angle by age and sex. Am J Clin Nutr. 2005;82(1):49–52.
Gonzalez MC, Barbosa-Silva TG, Bielemann RM, Gallagher D, Heymsfield SB. Phase angle and its determinants in healthy subjects: Influence of body composition. Am J Clin Nutr. 2016;103(3):712–6.
Lukaski HC, Kyle UG, Kondrup J. Assessment of adult malnutrition and prognosis with bioelectrical impedance analysis: Phase angle and impedance ratio. Curr Opin Clin Nutr Metab Care. 2017;20(5):330–9.
Molina Vega M, García Almeida JM, Vegas Aguilar I, Muñoz Garach A, Gómez Pérez AM, Cornejo Pareja I, et al. Revisión sobre los fundamentos teórico-prácticos del ángulo de fase y su valor pronóstico en la práctica clínica. Nutr Clin Med. 2017;XI(3):129–48.
Brantlov S, Jødal L, Andersen RF, Lange A, Rittig S, Ward LC. An evaluation of phase angle, bioelectrical impedance vector analysis and impedance ratio for the assessment of disease status in children with nephrotic syndrome. BMC Nephrol. 2019;20(1):331.
Delgado AF, Okay TS, Leone C, Nichols B, Del Negro GM, Vaz FAC. Hospital malnutrition and inflammatory response in critically ill children and adolescents admitted to a tertiary intensive care unit. Clin Sao Paulo Braz. 2008;63(3):357–62.
Weijs PJM, Looijaard WGPM, Dekker IM, Stapel SN, Girbes AR, Oudemans-van Straaten HM, et al. Low skeletal muscle area is a risk factor for mortality in mechanically ventilated critically ill patients. Crit Care Lond Engl. 2014;18(2):R12.
Pichard C, Kyle UG, Morabia A, Perrier A, Vermeulen B, Unger P. Nutritional assessment: Lean body mass depletion at hospital admission is associated with an increased length of stay. Am J Clin Nutr. 2004;79(4):613–8.
Reilly JJ. Mid-upper arm circumference (MUAC): new applications for an old measure. Arch Dis Child. 2017;102(1):1–2.
Craig E, Bland R, Ndirangu J, Reilly JJ. Use of mid-upper arm circumference for determining overweight and overfatness in children and adolescents. Arch Dis Child. 2014;99(8):763–6.
Feferbaum R, Delgado AF, Zamberlan P, Leone C. Challenges of nutritional assessment in pediatric ICU. Curr Opin Clin Nutr Metab Care. 2009;12(3):245–50.
Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336(7653):1106–10.
Cichoż-Lach H, Michalak A. A Comprehensive Review of Bioelectrical Impedance Analysis and Other Methods in the Assessment of Nutritional Status in Patients with Liver Cirrhosis. Gastroenterol Res Pract. 2017;2017:6765856.
Jun MH, Kim S, Ku B, Cho J, Kim K, Yoo HR, et al. Glucose-independent segmental phase angles from multi-frequency bioimpedance analysis to discriminate diabetes mellitus. Sci Rep. 2018;12;8(1):648.
Dittmar M, Reber H, Kahaly GJ. Bioimpedance phase angle indicates catabolism in Type 2 diabetes. Diabet Med J Br Diabet Assoc. 2015;32(9):1177–85.
Barrea L, Macchia PE, Di Somma C, Napolitano M, Balato A, Falco A, et al. Bioelectrical phase angle and psoriasis: a novel association with psoriasis severity, quality of life and metabolic syndrome. J Transl Med. 2016;10;14(1):130.
Markaki AG, Charonitaki A, Psylinakis E, Dimitropoulakis P, Spyridaki A. Nutritional status in hemodialysis patients is inversely related to depression and introversion. Psychol Health Med. 2019;24(10):1213–9.
Norman K, Stobäus N, Zocher D, Bosy-Westphal A, Szramek A, Scheufele R, et al. Cutoff percentiles of bioelectrical phase angle predict functionality, quality of life, and mortality in patients with cancer. Am J Clin Nutr. 2010;92(3):612–9.
Lee SY, Lee YJ, Yang JH, Kim CM, Choi WS. The association between phase angle of bioelectrical impedance analysis and survival time in advanced cancer patients: Preliminary study. Korean J Fam Med. 2014;35(5):251–6.
Gupta D, Lammersfeld CA, Vashi PG, King J, Dahlk SL, Grutsch JF. Bioelectrical impedance phase angle as a prognostic indicator in breast cancer. BMC Cancer. 2008;8:1–7.
Gupta D, Lammersfeld CA, Vashi PG, King J, Dahlk SL, Grutsch JF, et al. Bioelectrical impedance phase angle in clinical practice: Implications for prognosis in stage IIIB and IV non-small cell lung cancer. BMC Cancer. 2009;9:1–6.
Sánchez-Lara K, Turcott JG, Juárez E, Guevara P, Nez-Valencia C, Oate-Ocaa LF, et al. Association of nutrition parameters including bioelectrical impedance and systemic inflammatory response with quality of life and prognosis in Patients with Advanced Non-Small-Cell Lung Cancer: A prospective study. Nutr Cancer. 2012;64(4):526–34.
Belarmino G, Gonzalez MC, Torrinhas RS, Sala P, Andraus W, D’Albuquerque LAC, et al. Phase angle obtained by bioelectrical impedance analysis independently predicts mortality in patients with cirrhosis. World J Hepatol. 2017;9(7):401–8.
Pirlich M, Schütz T, Spachos T, Ertl S, Weiß ML, Lochs H, et al. Bioelectrical impedance analysis is a useful bedside technique to assess malnutrition in cirrhotic patients with and without ascites. Hepatology. 2000;32(6):1208–15.
Peres WAF, Lento DF, Baluz K, Ramalho A. Ángulo De Fase Como Una Herramienta Para Evaluar El Estado Nutricional En Todas Las Etapas De La Enfermedad Hepática Crónica. Nutr Hosp. 2012;27(6):2072–8.
Lee JE, Jo IY, Lee SM, Kim WJ, Choi HY, Ha SK, et al. Comparison of hydration and nutritional status between young and elderly hemodialysis patients through bioimpedance analysis. Clin Interv Aging. 2015;10:1327–34.
Mushnick R, Fein PA, Mittman N, Goel N, Chattopadhyay J, Avram MM. Relationship of bioelectrical impedance parameters to nutrition and survival in peritoneal dialysis patients. Kidney Int Suppl. 2003;64(87).
Berbigier MC, Pasinato VF, De Almeida RB, Moraes RB, Perry IDS. Bioelectrical impedance phase angle in septic patients admitted to intensive care units. Rev Bras Ter Intensiva. 2013;25(1):25–31.
Jian-hui C, Iskandar EA, Cai SI, Chen CQ, Wu H, Xu JB, et al. Significance of Onodera’s prognostic nutritional index in patients with colorectal cancer: a large cohort study in a single Chinese institution. Tumor Biol. 2016;37(3):3277–83.
De Lima E Silva RR, Pinho CPS, Rodrigues IG, De Moura Monteiro Júnior JG. Ángulo De Fase Como Indicador Del Estado Nutricional Y Pronóstico En Pacientes Críticos. Nutr Hosp. 2015;31(3):1278–85.
Garlini LM, Alves FD, Ceretta LB, Perry IS, Souza GC, Clausell NO. Phase angle and mortality: a systematic review. Eur J Clin Nutr. 2019;73(4):495–508.
Kovarik M, Hronek M, Zadak Z. Clinically relevant determinants of body composition, function and nutritional status as mortality predictors in lung cancer patients. Lung Cancer Amst Neth. 2014;84(1):1–6.
Lee SY, Lee YJ, Yang JH, Kim CM, Choi WS. The Association between Phase Angle of Bioelectrical Impedance Analysis and Survival Time in Advanced Cancer Patients: Preliminary Study. Korean J Fam Med. 2014;35(5):251–6.
Norman K, Stobäus N, Pirlich M, Bosy-Westphal A. Bioelectrical phase angle and impedance vector analysis–clinical relevance and applicability of impedance parameters. Clin Nutr Edinb Scotl. 2012;31(6):854–61.
Caccialanza R, Cereda E, Klersy C, Bonardi C, Cappello S, Quarleri L, et al. Phase angle and handgrip strength are sensitive early markers of energy intake in hypophagic, non-surgical patients at nutritional risk, with contraindications to enteral nutrition. Nutrients. 2015;7(3):1828–40.
Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, et al. Bioelectrical impedance analysis–part I: Review of principles and methods. Clin Nutr Edinb Scotl. 2004;23(5):1226–43.
Grundmann O, Yoon SL, Williams JJ. The value of bioelectrical impedance analysis and phase angle in the evaluation of malnutrition and quality of life in cancer patients–a comprehensive review. Eur J Clin Nutr. 2015;69(12):1290–7.
Lukaski HC, Kyle UG, Kondrup J. Assessment of adult malnutrition and prognosis with bioelectrical impedance analysis: Phase angle and impedance ratio. Curr Opin Clin Nutr Metab Care. 2017;20(5):330–9.
Garlini LM, Alves FD, Ceretta LB, Perry IS, Souza GC, Clausell NO. Phase angle and mortality: A systematic review. Eur J Clin Nutr. 2019;73(4):495–508.
Paiva SI, Borges LR, Halpern-Silveira D, Assunção MCF, Barros AJD, Gonzalez MC. Standardized phase angle from bioelectrical impedance analysis as prognostic factor for survival in patients with cancer. Support Care Cancer. 2011;19(2):187–92.
Del Giorno R, Quarenghi M, Stefanelli K, Rigamonti A, Stanglini C, De Vecchi V, et al. Phase angle is associated with length of hospital stay, readmissions, mortality, and falls in patients hospitalized in internal-medicine wards: A retrospective cohort study. Nutrition. 2021;1(85):111068.
de Moraes AM, Quinaud RT, Ferreira GOC, Lima AB, Carvalho HM, Guerra-Júnior G. Age-, sex-, and maturity-associated variation in the phase angle after adjusting for size in adolescents. Front Nutr. 2022;9:939714.
Ballarin G, Valerio G, Alicante P, Di Vincenzo O, Scalfi L. Bioelectrical Impedance Analysis (BIA)- Derived Phase Angle in Children and Adolescents: A Systematic Review. J Pediatr Gastroenterol Nutr. 2022;75(2):120–30.
Schmidt SCe, Bosy-Westphal A, Niessner C, Woll A. Representative body composition percentiles from bioelectrical impedance analyses among children and adolescents. The MoMo study. Clin Nutr. 2019 Dec;38(6):2712–20.
Redondo-del-Río MP, Camina-Martín MA, Marugán-de-Miguelsanz JM, de-Mateo-Silleras B. Bioelectrical impedance vector reference values for assessing body composition in a Spanish child and adolescent population: REDONDO-DEL-RÍO et al. Am J Hum Biol. 2017:e22978.
Mattiello R, Amaral MA, Mundstock E, Ziegelmann PK. Reference values for the phase angle of the electrical bioimpedance: Systematic review and meta-analysis involving more than 250,000 subjects. Clin Nutr. 2020;39(5):1411–7.
Kavanagh BP. The GRADE system for rating clinical guidelines. PLoS Med. 2009;6(9):e1000094.
The use of ‘PICO for synthesis’ and methods for synthesis without meta-analysis: protocol for a survey of current practice in systematic reviews of health interventions - PubMed [Internet]. [cited 2023 Jan 23]. Available from: https://pubmed.ncbi.nlm.nih.gov/33728041/
Marino LV, Meyer R, Johnson M, Newell C, Johnstone C, Magee A, et al. Bioimpedance spectroscopy measurements of phase angle and height for age are predictive of outcome in children following surgery for congenital heart disease. Clin Nutr Edinb Scotl. 2018;37(4):1430–6.
Zamberlan P, Feferbaum R, Doria Filho U, Brunow de Carvalho W, Figueiredo Delgado A. Bioelectrical Impedance Phase Angle and Morbidity and Mortality in Critically Ill Children. Nutr Clin Pract. 2019;34(1):163–71.
Almeida de Azevedo ZMA, Santos Junior BD, Ramos EG, Salú MDS, Mancino da Luz Caixeta D, Lima-Setta F, et al. The importance of bioelectrical impedance in the critical pediatric patient. Clin Nutr Edinb Scotl. 2020;39(4):1188–94.
Xiong ZH, Zheng XM, Zhang GY, Wu MJ, Qu Y. The Use of Bioelectrical Impedance Analysis Measures for Predicting Clinical Outcomes in Critically Ill Children. Front Nutr. 2022;9: 847480.
Rahmatinejad Z, Rahmatinejad F, Sezavar M, Tohidinezhad F, Abu-Hanna A, Eslami S. Internal validation and evaluation of the predictive performance of models based on the PRISM-3 (Pediatric Risk of Mortality) and PIM-3 (Pediatric Index of Mortality) scoring systems for predicting mortality in Pediatric Intensive Care Units (PICUs). BMC Pediatr. 2022;22(1):199.
Sandrio S, Krebs J, Leonardy E, Thiel M, Schoettler JJ. Vasoactive Inotropic Score as a Prognostic Factor during (Cardio-) Respiratory ECMO. J Clin Med. 2022;11(9):2390.
Yuniar I, Hafifah CN, Adilla SF, Shadrina AN, Darmawan AC, Nasution K, et al. Prognostic factors and models to predict pediatric sepsis mortality: A scoping review. Front Pediatr. 2023;23(10):1022110.
Karagiozoglou-Lampoudi T, Daskalou E, Lampoudis D, Apostolou A, Agakidis C. Computer-based malnutrition risk calculation may enhance the ability to identify pediatric patients at malnutrition-related risk for unfavorable outcome. JPEN J Parenter Enteral Nutr. 2015;39(4):418–25.
Nagano M, Suita S, Yamanouchi T. The validity of bioelectrical impedance phase angle for nutritional assessment in children. J Pediatr Surg. 2000;35(7):1035–9.
Funding
Funding for open access publishing: Universidad Málaga/CBUA. I.C.-P. was the recipient of a postdoctoral grant (Río Hortega CM17/00169) and is now the recipient of a postdoctoral grant (Juan Rodes JR 19/00054) from the Instituto de Salud Carlos III and co-funded by Fondo Europeo de Desarrollo Regional-FEDER.
Author information
Authors and Affiliations
Contributions
The authors R.F.-J., R.M.-M, I.C.-P., I.M.V.-A., M.H.-L, F.T.-M., V.M.N-L., D.B.-G., and J.M.G.-A. contributed to the design, search, sorting and data 701 extraction and analysis procedure R.F.-J, R.M.-M and I.C.-P. drafted the manuscript. R.F.-J, R.M.-M and I.C.-P., V.M.N-L., and J.M.G.-A critically revised 198 the manuscript for important intellectual content. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fernández-Jiménez, R., Martín-Masot, R., Cornejo-Pareja, I. et al. Phase angle as a marker of outcome in hospitalized pediatric patients. A systematic review of the evidence (GRADE) with meta-analysis. Rev Endocr Metab Disord 24, 751–765 (2023). https://doi.org/10.1007/s11154-023-09817-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-023-09817-1