Abstract
Phase angle (PhA) has been identified as a poor prognostic factor in patients with COVID-19. This study aimed to achieve a systematic review, where we discussed the potential role of PhA value as a prognostic marker of adverse clinical outcomes such as mortality and complication in hospitalized with SARS-CoV2 infection and established the strength of recommendations for use. A systematic literature review with meta-analysis was done in the main electronic databases from 2020 to January 2023. The selected articles had to investigate adverse consequences of the COVID-19 population and raw bioimpedance parameters such as PhA and published in peer-reviewed journals. GRADE tools regarded the quality of the methodology. The review protocol was registered in PROSPERO. Only eight studies, 483 studies, were eligible for the analysis. In general, differences in PhA were seen between the comparative study groups. Patients with a low PhA experienced poor outcomes. A low PhA was associated with a significantly increased mortality risk [RR: 2.44; 95% CI (1.20–4.99), p = 0.01; I2 = 79% (p = 0.0008)] and higher complications risk [OR: 3.47, 95% CI (1.16 – 10.37), p = 0.03; I2 = 82% (p = 0.004)] in COVID-19 patients. Our analysis showed four evidence-based recommendations on the prognostic value of PhA with two strong recommendations, one of moderate and another of low-moderate quality, for predicting mortality and complications, respectively. We recommend using PhA as a prognostic marker for mortality and complications in this population. Although the results are promising, future studies must identify the PhA cut-off to guide therapeutic decisions more precisely.
Registration code in PROSPERO: CRD42023391044
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The outbreak of Coronavirus Disease 2019 (COVID-19) due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has caused a global pandemic with a substantial spread of the infection and death [1]. This disease is associated with more than 660 million cases confirmed worldwide and more than 6.7 million deaths from march 2020 until January 2023 [2]. COVID-19 is a respiratory disease with high clinical variability, having identified specific clinical risk factors related to life-threatening illness, including comorbidities, older age, male gender, and host genetic variants (e.g., type I interferon auto-antibodies) [3,4,5,6].
COVID-19 patients may develop acute respiratory, nervous and musculoskeletal symptoms, leading to complications like sepsis, acute respiratory distress syndrome, thromboembolic events, coagulopathies, renal or cardiac failure, and even systemic organ failure. Therefore, it is essential to adapt support therapies such as intensive mechanical ventilation (IMV), precise fluids management, corticosteroids, and anticoagulant vasopressor treatments to care COVID-19 [7,8,9].
In the subacute phase, some patients may develop post-viral sequels or complications such as gastrointestinal symptoms, dysphagia, decreased food and protein intake, malabsorption, inflammation, low vitamin D levels, anabolic resistance, malnutrition, sarcopenia, fatigue syndrome or “long COVID” [10,11,12].
Researchers continue to identify prognostic factors for morbidity and mortality of SARS-CoV-2, focusing on both blood biochemical, drug treatments (angiotensin-converting enzyme inhibitors and/or angiotensin receptor blockers), the basic clinical variables (oxygen (O2) saturation, temperature, heart rate) [3, 5, 6, 13], as well as, tools for assessing body composition (malnutrition, sarcopenia, obesity or excess fat mass overhydration states) and the individual's cellular health status [14,15,16]. Thus, a more comprehensive insight into COVID-19 has enabled early diagnosis, stratification of disease severity, identification of potential sequelae, and an individualized therapeutic approach to patient management based on the severity of the disease [17].
In patients with severe COVID-19, malnutrition is often uncovered because of a direct effect of the virus resulting in systemic organ failure, long hospitalization, or intensive care unit (ICU) stay with prolonged immobilization [18]. Bioelectrical impedance (BI) assesses a patient´s nutritional status and body composition. Mainly, the BI measured using 50 kHz phase-sensitive devices uses whole-body measurements to classify and monitor hydration and cell mass without the use of multiple regression equations, instead focusing on raw bioelectric parameters, such as resistance (R) and reactance (Xc) [19, 20]. Resistance (R) is the opposition of the flow of low-level alternating current due to ionic fluids, and reactance (Xc) is the delay of current entry into cells related to cell membranes and cell interfaces. Phase angle (PhA) describes the lag between voltage and current and characterizes fluid distribution between the extracellular and intracellular compartments (E/I) [19, 20].
Thus, PhA is a cellular health biomarker that discloses the malnutrition and inflammatory status which can accompany these acute and/or serious disorders. PhA is a unique predictor of mortality in diverse clinical conditions [19, 21], including SARS-CoV2 infection and a potentially helpful screening tool for prognosis [22, 23]. Some studies have reported its association with poor outcomes, such as length of stay (LOS), mortality, or the need for intensive support therapies [21, 24,25,26]. However, the routine use of PhA to assess hospitalized COVID-19 patients has not been established due to the lack of a focused evaluation of clinical findings.
This systematic review of the literature with meta-analysis aims to establish the clinical value of PhA as a prognostic marker of adverse clinical outcomes such as mortality and complication/sequelae in hospitalized with SARS-CoV2 and to establish the strength of recommendations intended for use by health systems in clinical practice guidelines.
2 Methods
2.1 Methodology and protocol registration
This study was prepared using the recommendations of the preferred reporting items for systematic reviews (PRISMA) guide [27]. The review protocol was registered on PROSPERO with the number: CRD42023391044. Additionally, the quality of the evidence of the present systematic literature review was evaluated with the GRADE methodology (Grading of Recommendations Assessment, Development and Evaluation) [28] to develop evidence-based recommendations. The authors developed the clinical questions that guided the literature search and the requests using the PICO (Patient, Intervention, Comparison, Outcome) framework [29].
2.2 Literature search
We conducted our systematic literature identifying potential studies with a comprehensive search in MEDLINE or PubMed, Scopus, Embase, and Web of Science databases (from database inception to January 2023) to identify studies addressing the PhA evaluation and COVID-19. A combination of the following medical subject headings and keywords was used in the title, abstract or keywords fields: “SARS-CoV2,” “COVID,” “COVID-19” AND “bioelectrical impedance”, “BIA”, “bio-impedance”, “phase angle”, “PhA”, to identify the main bioimpedance parameters together with the population of interest. The additional search terms for primary outcomes are mortality, length of hospitalization, and complications, such as sarcopenia, malnutrition, dysphagia and invasive mechanical ventilation. Also, the hand-searching of databases was completed by two authors. Articles published in English or Spanish were selected for critical synthesis, and only significant associations are reported.
2.3 Literature inclusion and exclusion criteria
The clinical questions that guided the literature search and the recommendations were developed by the scientific committee composed of four authors with experience in body composition study and COVID-19 (I.C.-P.; J.M.G.-A.; D.B.-G. and F.J.T.) To determine the eligibility criteria, the PICO strategy [29] was adopted: in which "P" (patients), corresponding to COVID-19 patients of all genders and ethnicities; “I” (intervention), was designated as bioimpedance assessment with phase angle, “C” (comparison), was defined as altered results vs normal phase angle results, “O” (outcomes), was the mortality, LOS, severity disease or short- and long-term complications or sequels.
Exclusion criteria were as follows: (i) articles did not include a full-text description of the study; (ii) not in Spanish or English language; (iii) differences in phase angle are not evaluated regarding outcomes (e.g., mortality, length of stay, severe disease, complications or sequels); (iv) studies not published in peer‐reviewed journals; (v) meta‐analyses, reviews, protocols, case series or reports, editorials, and letter to the editor; (vi): pregnant or lactating women studies; and (vii) studies using animal models.
2.4 Study selection
The selection process was conducted by four independent authors (I.C.-P., J.M.G.-A., D.B.-G. and F.J.T.). The reference lists of all included studies were hand-searched for missing publications. Three authors (I.C.-P., J.M.G.-A. and D.B.-G.) independently screened the selected articles for eligibility after testing the abstract and full text. Differences of opinion while selecting the articles were resolved by consensus between authors. One author (F.J.T.) reviewed each opinion difference and decided for inclusion or exclusion in this study.
2.5 Risk of bias assessment
The methodological quality assessment of the studies was achieved using the GRADE methodology [28]. The GRADE method to provide is a standardized tool for rating the quality of evidence and grading the strength of recommendations. Many organizations have endorsed GRADE method to decrease the risk of bias, inconsistency of results across studies, indirectness of evidence, imprecision and publication bias [30]. It proposes specific criteria that should be considered, particularly in observational studies [31].
GRADE’s four categories of quality of evidence (high, moderate, low, very low) imply a gradient of confidence in estimates of the effect of a diagnostic test strategy on patient-important outcomes. This GRADE approach examines methodological quality by analysing the studies potential limitations, focusing on aspects such as study design, risk of bias, directness, indirectness outcomes, patient populations, diagnostic tests, comparison tests, indirect comparisons, inconsistency in study results and imprecise evidence. The GRADE approach to grading the quality of evidence and strength of recommendations for diagnostic tests provides a comprehensive and transparent approach [28].
The authors reviewed the literature, selecting outcomes from the studies, rating their importance, and evaluating outcomes across studies. Then the evidence profile tables for outcomes were created, including a rating of the quality of the evidence, using GRADEpro GDT software (https://gradepro.org). The tables include outcomes, number of studies, study design, risk of bias, effect, quality of evidence, and importance. The overall quality of evidence was rated across outcomes based on the lowest quality of critical outcomes. The authors then made recommendations for each topic based on the literature findings and balancing consequences (e.g., benefits/harms, values, preferences, feasibility).
2.6 Data analysis, processing and data synthesis
The authors manually included the selected articles in a Microsoft Excel table. This Excel document contains the characteristics of selected articles, such as first author, study country, study design, comparative groups, number and type of participants [general or ICU hospitalized patients] participants characterization [sex and age], measurement time, follow-up time, BI device [and frequency (kHz)], PhA value and reference values, raw bioelectrical parameters (R and Xc) nutrition status [fat-free mass (FFM), skeletal muscle mass index (SMI), soft lean mass (SLM), skeletal muscle mass (SMM), appendicular muscle mass index (AMMI), hydration status [extracellular water/total body water (ECW/TBW) ratio, TBW/FFM] and reference value, outcomes [mortality, LOS, complications or sequels], results [(number of events/total population) ratio and effect rate [95% confidence interval (CI)], and conclusions.
Tables summarize data, which were grouped by similar categories to allow comparisons among studies. Likewise, an analysis was carried out following the GRADE methodology to evaluate the quality of the studies and develop recommendations for clinical application of PhA.
2.7 Statistical analysis
The meta-analysis used Review Manager 5.3 statistical software. Risk Ratio (RR) or Odds Ratio (OR) and 95% CI were used for continuous binary variables, respectively. If the heterogeneity test revealed p ≤ 0.05 or I2 > 50%, we concluded that the index is statistically different between the studies, and the Random Effects Model (Random) is used. If the heterogeneity test p > 0.05 and I2 < 50%, it indicates that there is no statistical difference in this indicator between studies, and the Fixed Effects Model (Fixed) is used for merging.
3 Clinical characteristics of bioelectrical phase angle
Bioimpedance analysis (BIA) is an indirect method to measure body composition based on the human body’s ability to conduct electricity. The current is transmitted through liquids and electrolytes, while fat and bone are not conductors [32]. Through raw impedance parameters, such as R and Xc, the PhA can be obtained: PhA (°) = [arctan (Xc/R) × (180°/π)]. By definition, PhA is positively associated with tissue Xc, as related to cell mass, integrity, and function, and negatively associated with R, which depends mainly on the degree of tissue hydration [33] (Fig. 1).
PhA is measured directly with BIA, which is fast, portable, non-invasive, reproducible, and sensitive. In addition, it has been considered a valuable tool in various clinical situations. In healthy subjects, the PhA oscillates between 5° and 7°. A low PhA (< 4°) indicates unbalanced proportion between cells and fluids. Normality curves have been established for different populations. Age, body mass index (BMI), sex, and race influence PhA values among healthy individuals. In this way, although the absolute value of the measure is a parameter that must be assessed, the standardized PhA is obtained by adjusting the PhA obtained by age and sex variables and represents an additional crude measure. That is, this value is important because it allows comparison with respect to healthy population references with the same characteristics (age and sex).
PhA is considered a reliable indicator of cell integrity and has been proposed as a nutritional status marker for adults and children after findings in numerous pathologies. It has also been proposed as a useful prognostic marker for several clinical conditions. In clinical practice, the determination of PhA allows the characterization of a patient relative to a healthy group and facilitates the follow up in the clinical care of patients at nutritional risk, such as screening and to evaluate prognosis and mortality in very diverse pathologies (HIV, cancer, anorexia, liver cirrhosis, haemodialysis, short bowel, cardiac pathology, lung disease, surgery, neurological pathology, surgical pathologies, geriatric diseases, hospitalized patients, critical patients, infectious pathologies such as SARS-CoV2, etc.) [21]. A recent meta-analysis reported normality curves and population percentiles derived in a sample of more than 250,000 patients; in general, lower levels of PhA suggest a worse prognosis and greater morbidity and mortality [21].
Other aspects of the PhA assessment have raised interest. The reported relationship between low PhA values and age-related muscle depletion and its correlation with reduced muscle function (strength and endurance) assessed with dynamometry opens a new path in the value of determining PhA in the assessment of malnutrition and sarcopenia [19, 34, 35]. Emerging interest in monitoring PhA values as an indicator of inflammatory states and oxidative stress in obesity and metabolic diseases reinforces its use in patients with SARS-CoV2 infection as a factor in the evaluation of the prognosis of the patient [36].
Beyond the PhA as a primary measurement, we can analyse the raw bioelectrical data. In this sense, the height (H)-adjusted R and Xc results are standardized when compared to the population reference pattern. The standardization of the PhA adjusted by age and sex concerning population references allows us to compare different clinical populations [37]. In the same way, the analysis of the raw bioelectrical components R/H transformed into hydration status, and Xc/H transformed into nutrition status with their mean values and population standard deviation allow us to analyse segregated results of the hydration and nutrition components contained in the PhA [38, 39].
PhA and SPhA are BIA measurements that are novel options for practical assessment and clinical evaluation of impaired nutritional status and prognosis among hospitalized COVID-19 patients and could potentially contribute to enhanced patient care and clinical outcomes. The literature review reported that a lower PhA increased the odds of COVID-19 complications and mortality during variable period-time [14, 22, 23, 40,41,42,43,44].
In SARS-CoV2 patients, the interpretation of PhA requires careful consideration of the raw bioelectrical data to identify the R and Xc changes, due to both overhydration status associated with an increased inflammatory process and malnutrition status contributing to a decline in the PhA value [16, 22] (Fig. 2).
Interpretation of the bioelectrical component value of Phase Angle in COVID-19. PhA is a crude measurement. The analysis of its raw bioelectrical components R/H transformed into hydration status and Xc/H transformed into nutrition status allow us to analyse segregated results of the hydration/inflammation and nutrition components contained in the PhA. R: resistance; H: height; Xc: reactance; PhA: phase angle
4 Results
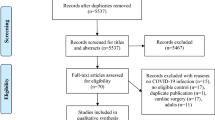
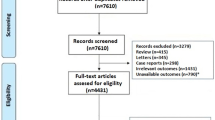
Our search procedure produced a total of 483 studies, as exposed in the flow-chart (Fig. 3). Of these articles identified from the databases, 272 were removed before the screening process through duplication. Based on our inclusion criteria (PICO) and exclusion criteria, the analysis of titles, keywords, and abstracts identified 22 potentially eligible studies. After reading the complete text, eight relevant studies were finally included in our systematic review of PhA and SARS-CoV2 infection [14, 22, 23, 40,41,42,43,44]. Fourteen reports were excluded due to lack of evaluation of PhA for clinical outcomes or relevance for SARS-CoV2 infection.
4.1 Characteristics of the included studies
Eight studies were included, six were prospective observational cohort studies from one centre [22, 23, 40,41,42, 44], while one of them was multicentre with two referral centres [41], one was an observational cross-sectional cohort study [14] and other was a retrospective observational study [43]. Most of the studies were conducted in European countries [14, 22, 23, 43, 44] and Mexico [40,41,42] (Table 1).
A total of 854 admission patients with SARS-CoV2 infection participated in our systematic review. In all the studies, the male sex was predominant (> 60%). The mean age of the European studies was higher, prevailing at a mean age of > 65 years, while the mean age in two of the Mexican studies [41, 42] was lower with a mean of 55 years, corresponding to studies focused on ICU patients (Table 1).
The BIA device used for the measurement were InBody S10®, a multifrequency model [14, 23, 41, 42], BIA 101 BIVA (Akern), a 50 kHz phase-sensitive model [22, 43], SECA® model mBCA 525, a multifrequency model [44], and BIA Quantum V RJL Systems, a phase-sensitive model [40] (Table 2). In general, the PhA measurements were carried out in the first 24-72 h of admission and follow-up time for adverse outcomes ranged from 20 to 90 d. The measurement technique was performed with patients in a supine position [22, 40,41,42,43,44] and using a frequency of 50 kHz [14, 22, 23, 40, 41] in most of the studies (Table 2).
4.2 Findings
The average PhA ranged from 4.4 to 5.6º. The lowest PhA values were recorded in the studies that included patients with the most severe SARS-CoV2 infection (ICU patients) [14, 22, 41, 42]. The studies focused on COVID-19 patients admitted to general ward had higher mean PhA values [40, 43, 44], than those involving patients admitted to the ICU [14, 22, 41, 42]. In all studies, significantly lower PhA values were found in patients with poor-outcome compared to the comparison groups, except Moone et al. [23] and Del Giorno et al. [43] studies that reported no significant differences between general ward and ICU patients and patients with and without nutritional risk, respectively (Table 2).
A decrease in R and Xc both contribute to the overhydration state for an increase in the inflammatory process and the malnutrition state that contributes to a decrease in PhA value. The publications analysed raw bioelectrical parameters such as R, Xc, and SPhA, as well as parameters related to hydration-inflammation status and those related to cell mass and nutritional status (Table 2). These raw bioelectrical measurements were only analysed in one of the studies [22], while the SPhA value was described in two of the studies [22, 42]. The principal finding was significant differences between the comparative groups (survivors vs. non-survivors) for all studies. Importantly, the mean SPhA was -2.5 (i.e., 2.5 SD less than the healthy population of his age and sex) in critically ill patients while in general ward patients, it was -0.8 (i.e., 2.5 SD less than the healthy population of his age and sex). Thus, patients with more severe disease (critically ill patients) had SPhA further away from the reference population compared to patients with more stable disease (general hospital ward) (Table 2).
Seven studies investigated the hydration status of the patients with COVID-19. Five studies [14, 23, 41, 42, 44] reported hydration status as the ECW/TBW by BIA, and two reports provided [22, 43] the TBW/FFM ratio by BIA. The mean hydration status ranged from 0.39 to 0.45 for EWC/TBW and 73.8% for TBW/FFM. Significant differences were detected in the hydration status of the comparative groups analysed [14, 22, 23, 41, 42, 44]. However, Del Giorno et al. [43], found no significant differences in hydration status between COVID-19 patients with and without nutritional risk (Table 2).
Various assessments of lean soft tissue mass compared in the studies [14, 22, 23, 40, 43, 44] (Table 2). Moonen et al. [14, 23] identified depletion in indices of muscle and FFM between ICU and general ward patients. Significant reductions in BCM [22, 40], AMMI [40] and FFMI and SMMI [44] were found between comparison control groups (survivors vs. non-survivors, dysphagic vs non-dysphagic patients and malnourished vs. non-malnourished patients). However, Del Giorno et al. [43] found no significant differences between the study groups (Table 2).
4.3 Poor outcomes researched in admission patients with COVID-19
The principal outcomes under analysis were mortality [14, 22, 40, 42,43,44] and disease severity, defined as the need for mechanical ventilation or a composite score, including the need for ICU admission [14, 23, 43, 44]. The presence of complications, such as thrombo-embolic event, renal failure, delirium, pulmonary fibrosis, dysphagia post-extubating [14, 23, 41] and LOS [23, 43] constituted additional poor outcomes (Table 1).
-
(I)
Mortality
The studies that explored the association of PhA as an independent prognostic factor for mortality used the odds ratio (OR) or hazard ratio (HR) analysis. The highest HR observed for 90-d mortality was 3.912 [95% CI (1.322–11.572), p = 0.014] in an adjusted model for sex, age, BMI, comorbidities, and hydration status in 127 COVID-19 patients (ICU and general ward admission) [22]. The PhA value cut-off point for predicting mortality was 3.95º with a sensitivity of 93.8% and specificity of 66.7% [AUC = 0.839, p = 0.001] [22].
In an adjusted model by sex, age, comorbidities, prognostic scales (CURB-65 and SOFA), and AMMI in 104 hospitalized patients followed up for 20-d, a low PhA < 3.66º had an HR = 2.571[95% CI (1.217–5.430), p = 0.013] [40]; while an adjusted model including nutrition risk (NUTRICscore) and age in 67 critical ill COVID-19 patients demonstrated 60-d mortality HR = 3.08 [95% CI (1.12–8.41), p = 0.02] [42]. Further, a PhA < 5.25° [AUC = 0.74, 95% CI (0.60–0.88), p = 0.003) for males and < 3.85º [AUC = 0.83, 95% CI (0.60–0.99), p = 0.03) in females were predictive mortality markers with a sensitivity (72% and 66.7%, respectively) and specificity (72% and 90%, respectively) [42]. In 54 hospitalized COVID-19 patients (ICU and general ward), a higher PhA value was associated with a significantly lower risk of 28-d mortality [OR: 0.208, p = 0.025] [14].
-
(II)
Length of stay (LOS)
A greater PhA was also related to a lower hospital LOS [OR = 0.875, 95% CI (0.765–1.001), p = 0.037] [23] in a population of 150 patients hospitalized in the ICU and general ward. Osuna-Padilla et al. also found that lower PhA was associated with a longer LOS [r = −0.33, p = 0.03] without deepening multivariate analysis with adjusted models [42]. However, in the study of 90 hospitalized COVID-19 patients investigators did not find a similar association [OR = 1.04, 95%CI (0.12–8.63), p = 0.974] [43].
-
(III)
Severity of disease
Phase angle adjusted for age, sex and BMI was significantly associated with the need to IMV [HR = 1.007, 95% CI (0.714–1.422), p = 0.007] in 150 hospitalized ward patients [44]. Similarly, Osuna-Padilla et al. found a significant negative correlation between PhA and IMV duration [r = −0.42, p = 0.005] without exploring multivariate analyses with adjusted models [42].
Other studies of patients on the ICU and general ward support the prognostic value of PhA. Among 150 patients, a higher PhA was associated with a lower rate of admission to the ICU [OR = 0.531, 95% CI (0.285–0.989), p = 0.021] [23]. PhA adjusted for age, sex and BMI was significantly associated with IMV [HR = 1.007, 95% CI (0.714–1.422), p = 0.007] in hospitalized ward patients [44]. In the composite score studies referred to in the mortality section, PhA was significantly related to the severity of disease in Moonen et al. studies [14, 23], while Del Giorno et al. [43] found no significant differences.
PhA also was as an independent prognostic factor with a composite outcome such as ICU admission and complications including mortality [OR 0.299, p = 0.046] [14] and [OR = 0.502, 95% CI (0.281–0.898), p = 0.015), respectively [23]. While Del Giorno et al. found no association between PhA and the composite outcome that includes ICU admission and in-hospital mortality [OR = 0.59, 95%CI (0.21–1.71), p = 0.332] [43]. Likewise, the PhA showed not associated with mortality in 150 ward patients into an adjusted model by age, sex and BMI [HR = 1.084, 95% CI (0.803–1.463), p = 0.081] [44].
-
(IV)
Complications
Among 150 patients on an ICU and ward hospitalized patients, a lower PhA was a significant predictor of complications such as thrombo-embolic event, renal failure, delirium, and pulmonary fibrosis [OR = 0.579, 95% CI, (0.344–0.973), p = 0.031] [23]. In contrast, PhA was not a significant prognostic factor for these complication in 54 patients [OR = 0.413, p = 0.061], but PhA was a significantly predictive of severe disease or mortality, as previously shown [14].
Post-extubating dysphagia is emerging as a complication of SARS-CoV2 infection. Among 112 critically ill patients, a PhA < 4.8º was identified as an independent predictor of post‐extubating dysphagia in a model adjusted for age and sex [OR = 12.2, 95% CI(4.3–34.1), p < 0.05] [41]. This initial finding suggests a mechanism by which low PhA can contribute to malnutrition in patients with SARS-CoV2 infection.
Studies using regression analysis to determine the significance of the PhA as a predictive marker of poor outcomes included various independent variables for model development. At the same time, age and sex were employed homogeneously in all studies [14, 22, 23, 40,41,42,43,44], most used an indirect indicator of body composition such as BMI [14, 22, 23, 43, 44] or AMMI [40]. Other studies introduced nutritional risk scales such as NUTRIC score [42], hydration status [22], or cell mass [40]. Only three studies included comorbidities [22, 40, 43] as possible confounding factors, and four studies used the risk or prognostic scales [14, 23, 40, 43], making these analyses heterogeneous. This leads to the fact that there may be some differences in the results among some of the studies evaluated, as described previously in this section.
4.4 Quality of studies
The initial literature review yielded 483 publications, only 8 publications covered all four topics related to PICO issues. The quality of the evidence was evaluated following the GRADE method (Table 3), which allowed the scientific committee to make 4 evidence-based recommendations on the prognostic and clinical value of PhA measurements (Table 4).
PhA can be used, with a strong recommendation strength and moderate evidence quality, to predict mortality in hospitalized patients with SARS-CoV2 infection. Similarly, PhA, which has a weak strength of recommendation and a very low-low quality of evidence, can predict a longer LOS hospital stay and advise increased risk of severe disease in hospitalized patients with SARS-CoV2 infection. Also, PhA, with a strong strength of recommendation and a low to moderate quality of evidence, can be used to predict complications in hospitalized patients with SARS-CoV2 infection.
4.5 Usefulness of the PhA as a prognostic factor of poor outcomes: meta-analysis
A randomized-effect or fixed-effect model used when the tests were characterized as heterogeneous or homogeneous, respectively, was employed for meta-analysis. Meta-analysis of data from 502 patients indicated a significantly increased mortality risk in COVID-19 patients with lower PhA [RR: 2.44; 95% CI (1.20–4.99), p = 0.01]. Heterogeneity between studies: I2 = 79% (p = 0.0008)]. A significantly increased complications risk was found in 316 COVID-19 patients with lower PhA [OR: 3.47, 95% CI (1.16–10.37), p = 0.03; Heterogeneity between studies: I2 = 82% (p = 0.004)]. Nevertheless, PhA was not a significant predictor of severe disease with 444 patients included [RR: 1.59, 95% CI (0.94–2.69), p = 0.08] (Fig. 4).
The subgroups analyses of PhA as a prognostic marker for poor outcomes in hospitalized COVID-19 patients. The severity data of OR or HR and 95% CI from 8 studies were pooled in this meta-analysis and the result of the meta-analysis was described as a forest plot. 8 studies were grouped, in the main poor outcomes studied: mortality (A), severity disease (B) and complications (C). OR: Odds ratio; HR: Hazard ratio; CI: confidence interval; intensive care unit
5 Discussion
The present systematic review with meta-analysis evaluated the predictive capability of PhA on the clinical prognosis of COVID-19 patients. The overall result showed that a low PhA in hospitalized COVID-19 patients could predict a higher risk of death or complications. This systematic review was the first report of the combined use of the GRADE method and incorporating meta-analysis for the evaluation of the value of PhA as a prognostic marker for poor outcomes in hospitalized COVID-19 patients [45]; allowing statistical evaluation of the included studies and to analyse their quality to generate recommendations.
The reported PhA and other bioelectrical parameter values differed significantly in hospitalized COVID-19 patients between comparison groups. A low PhA was associated with the patient group, which had a poor clinical evolution or a severe disease with complications (critically ill, presence of complications or mortality). The decreased mean PhA values of the patients with COVID-19 are consistent with the findings of patients with other pathologies, such as inflammatory or infectious pathologies [46, 47], pulmonary disease [48], or patients admitted to the ICU [49].
When assessing the association between PhA and mortality risk of hospitalized patients with SARS-CoV2 infection, this analysis included five studies and 502 patients and determined a significant association. This finding was also seen in patients who were critically ill or had cancer, chronic kidney disease, or heart failure [21]. Also, the present review determined an association between low PhA and complications in hospitalized patients with SARS-CoV2 infection using data from three studies and 316 patients. A similar observation was reported in patients undergoing lung cancer surgery [50]. The severity of the disease was also related to low PhA values in liver disease [51]. Other studies discovered that a reduced PhA was predictive of more extended hospital stay in patients hospitalized in internal medicine [25] or medical and surgical patients in general ward [35]. Our systematic review found no significant association for PhA as a predictor of disease severity (four studies and 444 patients).
The inconsistency between the findings of our meta-analysis review and some individual reports may be due to the few studies that evaluated the association of PhA and poor outcomes in COVID-19 patients and specific technical characteristics and differences between studies. Studies were homogeneous for age (overall > 65 y), sex (male gender dominant > 60%) and clinical profile (e.g., hospitalized COVID-19 patients: ICU or general ward). However, they were heterogeneous relative to experimental design (e.g., prospective cohort study (most), cross-sectional study or retrospective study), follow-time (20–90 d), PhA measurement time (24-72 h after admission), BIA device (e.g., BIA InBody S10®; BIA 101 BIVA AKERN®; BIA SECA® model mBCA 525; BIA Quantum V RJL Systems®), and reference PhA values determined as usual. The PhA values were determined with substantially different BIA devices. Measurements were performed using different types of electrodes (gel wet electrodes vs dry contact), different BI technologies (single 50 kHz frequency phase-sensitive devices optimized calibration vs bioelectrical impedance spectroscopy (BIS) with phase sensitivity using phase detection mediated between 4 to 1000 kHz), and most of the studies did not specify the body position during measurement (recumbent vs standing). Use of different BIA devices to determine PhA (50 kHz compared to multifrequency) can influence the reported values [21]. PhA values are maximal near 50 kHz when measured directly and can vary if determined indirectly (e.g., modelled). Although PhA was measured at 50 kHz in most studies, not all studies specified the frequency at which they had measured PhA.
Thus, another significant matter is related to the PhA cut-off or the reference values employed in the analyses of the studies. Currently, there is no known specific and valid PhA value to identify mortality, severe disease or complications in COVID-19 patients. However, the lower PhA percentiles found in the studied groups were used as a cut-off. This problem of lack of reference values in the disease can be partially solved by analysing the SPhA. The age- and sex-adjusted SPhA may be useful to obtain a prognostic value. Thus, in two of the studies analysed in this systematic review, data on SPhA in relation to mortality are provided. SPhA is also associated with poor outcomes in other diseases [24].
The human body may be considered as a network of resistors (R), represented by body fluids and their electrolytes, and capacitors (Xc) consisting of cell membranes and tissue interfaces. Thus, BIA and PhA measurements may indicate fluid distributions (E/I) and cell mass (BCM). The preponderance of the studies of patients with COVID-19 identify simultaneously identify poor outcomes with low PhA values, and a common finding also overhydration status associated with a high degree of inflammation and a lower cell mass related to malnutrition and sarcopenia (Table 2).
Observational reports identify excess fluid accumulation and acral and pulmonary oedema in SARS-CoV-2 infection, especially those patients that progress to acute respiratory distress syndrome [52]. In SARS-CoV-2 infection, overhydration refers to an imbalance in fluid distribution between extracellular and intracellular water volumes with the expansion of ECW associated with systemic inflammation and aggressive fluid administration [53]. The pathogenesis of overhydration is due to the inflammatory component of the disease and the primary fluid retention due to cardiac or renal hemodynamic failure. This mechanism may be similar to the PhA changes that occur in heart failure [54] or kidney failure [55]. Therefore, patients with overhydration status are related with poor outcomes such as, a higher incidence of sepsis or complications with multiple organ failure constitutes inflammatory settings favourable to more fluid retention.
Similarly, patients with acute SARS-CoV2 infection tend to lose weight due to cachexia with catabolic and metabolic alterations that directly impact nutritional status [56]. Obesity is a risk factor for adverse outcomes of COVID-19[57]. It is associated with impaired measures of the quantity and quality of muscle mass and fat mass that exacerbate outcomes in severe SARS-CoV2 infection and can indirectly promote malnutrition [34, 58], which is highly related to mortality [35].
6 Limitations and strength
Heterogeneity in studies is a main limitation of this systematic review. Body composition, sex, or age influence PhA values. Whereas control of these contributing factors is important in studies of potential biomarkers of prognosis, clinical and medical circumstances may not fully allow the avoidance of them. Also, confounding factors such as comorbidities or risk scales [59, 60] may be impractical to control. Statistical analytical methods allow for adjustment in age and gender and body composition, generally BMI, in the assessment of risk.
Similarly, adjustment for indices of nutritional status assessment and prognostic scales is infrequently performed in risk assessments.
The strengths of the systematic review were consistent with the PRISMA statement. This was achieved by using a rigorous research protocol to evaluate relevant publications, allowing adequate eligibility criteria and uniform search strategies to be used. The search utilized different databases and was reviewed by various authors; there were no restrictions made for year of publication or also not the place of execution. Also, this review used the GRADE method, which is a validated tool for the analysis of the quality of the evidence. GRADE proposes specific criteria that should be considered, particularly in observational studies.It provides guidance to describe clinical recommendations about the usefulness of PhA as a predictor of poor outcome markers. The meta-analysis enables the derivation of global results to determine risks of reduced PhA associated with mortality, severity, and complications of SARS-CoV2 infection.
7 Conclusion
This systematic review determined that PhA, by BIA, is a valid prognostic indicator of mortality and complications in hospitalized COVID-19 patients.
Although the results are promising, there is still a deficiency of knowledge about the use of thresholds of the PhA in this population. Future studies are needed to identify PhA cut-off to guide therapeutic decisions more precisely. The reduction in values of PhA can indicate poor outcomes and allow a more adjusted supportive treatment of these patients.
Abbreviations
- AMMI:
-
Appendicular muscle mass index
- BI:
-
Bioelectrical impedance
- BIA:
-
Bioimpedance analysis
- BIS:
-
Bioelectrical impedance spectroscopy
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- COVID-19:
-
Coronavirus Disease 2019
- ECW:
-
Extracellular water
- FFM:
-
Fat-free mass
- GRADE:
-
Grading of Recommendations Assessment, Development and Evaluation
- HR:
-
Hazard ratio
- ICU:
-
Intensive Care Unit
- IMV:
-
Intensive Mechanical ventilation
- LOS:
-
Length of stay
- OR:
-
Odds ratio
- PhA:
-
Phase angle
- PICO:
-
Patient, Intervention, Comparison, Outcome
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews
- R:
-
Resistance
- RR:
-
Risk Ratio
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- SMI:
-
Skeletal muscle mass index
- SML:
-
Soft lean mass
- SMM:
-
Skeletal muscle mass
- TBW:
-
Total body water
- Xc:
-
Reactance
References
Demeco A, Marotta N, Barletta M, Pino I, Marinaro C, Petraroli A, et al. Rehabilitation of patients post-COVID-19 infection: a literature review. J Int Med Res. 2020;48:300060520948382.
WHO Coronavirus (COVID-19) Dashboard. World Health Organization. [Internet]. 2023 [cited 2023 Jan 14]. Available from: https://covid19.who.int.
Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ. 2020;369:m1985.
Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann H-H, Zhang Y, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370:eabd4585.
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62.
Bellou V, Tzoulaki I, van Smeden M, Moons KGM, Evangelou E, Belbasis L. Prognostic factors for adverse outcomes in patients with COVID-19: a field-wide systematic review and meta-analysis. Eur Respir J. 2022;59:2002964.
Qian Z, Lu S, Luo X, Chen Y, Liu L. Mortality and clinical interventions in critically ill patient with coronavirus disease 2019: a systematic review and meta-analysis. Front Med (Lausanne). 2021;8:635560.
Therapeutics and COVID-19: Living guideline. World Health Organization. [Internet]. 2023 [cited 2023 Jan 14]. Available from: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-therapeutics-2022.4.
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–13.
Lopez M, Bell K, Annaswamy T, Juengst S, Ifejika N. COVID-19 guide for the rehabilitation clinician: a review of nonpulmonary manifestations and complications. Am J Phys Med Rehabil. 2020;99:669–73.
Narici M, Vito GD, Franchi M, Paoli A, Moro T, Marcolin G, et al. Impact of sedentarism due to the COVID-19 home confinement on neuromuscular, cardiovascular and metabolic health: Physiological and pathophysiological implications and recommendations for physical and nutritional countermeasures. Eur J Sport Sci. 2021;21:614–35.
Welch C, Greig C, Masud T, Wilson D, Jackson TA. COVID-19 and acute sarcopenia. Aging Dis. 2020;11:1345–51.
Huang NX, Yuan Q, Fang F, Yan BP, Sanderson JE. Systematic review and meta-analysis of the clinical outcomes of ACEI/ARB in East-Asian patients with COVID-19. PLoS ONE. 2023;18:e0280280.
Moonen HPFX, van Zanten FJL, Driessen L, de Smet V, Slingerland-Boot R, Mensink M, et al. Association of bioelectric impedance analysis body composition and disease severity in COVID-19 hospital ward and ICU patients: The BIAC-19 study. Clin Nutr. 2021;40:2328–36.
de Blasio F, Scalfi L, Castellucci B, Sacco AM, Berlingieri GM, Capitelli L, et al. Poor nutritional status and dynapenia are highly prevalent in post-acute COVID-19. Front Nutr. 2022;9:888485.
Cornejo-Pareja I, Vegas-Aguilar IM, Lukaski H, Talluri A, Bellido-Guerrero D, Tinahones FJ, et al. Overhydration assessed using bioelectrical impedance vector analysis adversely affects 90-day clinical outcome among SARS-CoV2 patients: a new approach. Nutrients. 2022;14:2726.
Jeong YJ, Wi YM, Park H, Lee JE, Kim S-H, Lee KS. Current and Emerging Knowledge in COVID-19. Radiology. Radiol Soc North Am. 2023;222462.
Zhao X, Li Y, Ge Y, Shi Y, Lv P, Zhang J, et al. Evaluation of nutrition risk and its association with mortality risk in severely and critically Ill COVID-19 patients. JPEN J Parenter Enteral Nutr. 2021;45:32–42.
Lukaski HC, Kyle UG, Kondrup J. Assessment of adult malnutrition and prognosis with bioelectrical impedance analysis: Phase angle and impedance ratio. Curr Opin Clin Nutr Metab Care. 2017;20:330–9.
Lukaski HC, Vega Diaz N, Talluri A, Nescolarde L. Classification of hydration in clinical conditions: Indirect and direct approaches using bioimpedance. Nutrients. 2019;11.
Garlini LM, Alves FD, Ceretta LB, Perry IS, Souza GC, Clausell NO. Phase angle and mortality: a systematic review. Eur J Clin Nutr. 2019;73:495–508.
Cornejo-Pareja I, Vegas-Aguilar IM, García-Almeida JM, Bellido-Guerrero D, Talluri A, Lukaski H, et al. Phase angle and standardized phase angle from bioelectrical impedance measurements as a prognostic factor for mortality at 90 days in patients with COVID-19: a longitudinal cohort study. Clin Nutr. 2022;41:3106–14.
Moonen HPFX, Bos AE, Hermans AJH, Stikkelman E, van Zanten FJL, van Zanten ARH. Bioelectric impedance body composition and phase angle in relation to 90-day adverse outcome in hospitalized COVID-19 ward and ICU patients: The prospective BIAC-19 study. Clinical Nutrition ESPEN. 2021;46:185–92.
Paiva SI, Borges LR, Halpern-Silveira D, Assunção MCF, Barros AJD, Gonzalez MC. Standardized phase angle from bioelectrical impedance analysis as prognostic factor for survival in patients with cancer. Support Care Cancer. 2010;19:187–92.
Del Giorno R, Quarenghi M, Stefanelli K, Rigamonti A, Stanglini C, De Vecchi V, et al. Phase angle is associated with length of hospital stay, readmissions, mortality, and falls in patients hospitalized in internal-medicine wards: a retrospective cohort study. Nutrition. 2021;85:111068.
do Amaral Paes TC, de Oliveira KCC, de Carvalho Padilha P, Peres WAF. Phase angle assessment in critically ill cancer patients: Relationship with the nutritional status, prognostic factors and death. J Crit Care. 2018;44:430–5.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. Br Med J Publ Group. 2021;372:n71.
Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336:1106–10.
Cumpston MS, McKenzie JE, Thomas J, Brennan SE. The use of ‘PICO for synthesis’ and methods for synthesis without meta-analysis: Protocol for a survey of current practice in systematic reviews of health interventions. F1000Res. 2021;9:678.
Kavanagh BP. The GRADE system for rating clinical guidelines. PLoS Med. 2009;6:e1000094.
Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso-Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64:1311–6.
Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, et al. Bioelectrical impedance analysis-part II: Utilization in clinical practice. Clin Nutr. 2004;23:1430–53.
García Almeida JM, García García C, Vegas Aguilar IM, Bellido Castañeda V, Bellido GD. Morphofunctional assessment of patient´s nutritional status: a global approach. Nutr Hosp. 2021;38:592–600.
Cornejo-Pareja I, Soler-Beunza AG, Vegas-Aguilar IM, Fernández-Jiménez R, Tinahones FJ, García-Almeida JM. Predictors of sarcopenia in outpatients with post-critical SARS-CoV2 disease. Nutritional ultrasound of rectus femoris muscle, a potential tool. Nutrients. 2022;14:4988.
Fernández-Jiménez R, Dalla-Rovere L, García-Olivares M, Abuín-Fernández J, Sánchez-Torralvo FJ, Doulatram-Gamgaram VK, et al. Phase angle and handgrip strength as a predictor of disease-related malnutrition in admitted patients: 12-month mortality. Nutrients. 2022;14:1851.
da Silva BR, Gonzalez MC, Cereda E, Prado CM. Exploring the potential role of phase angle as a marker of oxidative stress: a narrative review. Nutrition. 2022;93:111493.
Cardinal TR, Wazlawik E, Bastos JL, Nakazora LM, Scheunemann L. Standardized phase angle indicates nutritional status in hospitalized preoperative patients. Nutr Res. 2010;30:594–600.
Piccoli A, Nigrelli S, Caberlotto A, Bottazzo S, Rossi B, Pillon L, et al. Bivariate normal values of the bioelectrical impedance vector in adult and elderly populations [Internet]. Am J Clin Nutr. 1995 [cited 2020 Jun 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/7840061/?from_single_result=.+Piccoli+A%2C+Nigrelli+S%2C+Caberlotto+A%2C+Bottazzo+S%2C+Rossi+B%2C+Pillon+L%2C+et+al.+Bivariate+normal+values+of+the+bioelectrical+impedance+vector+in+adult+and+elderly+populations.+Am+J+Clin+Nutr.+1995%3B61%3A+269%E2%80%93270.
Piccoli A, Rossi B, Pillon L, Bucciante G. A new method for monitoring body fluid variation by bioimpedance analysis: The RXc graph. Kidney Int. 1994;46:534–9.
Rosas-Carrasco O, Núñez-Fritsche G, López-Teros MT, Acosta-Méndez P, Cruz-Oñate JC, Navarrete-Cendejas AY, et al. Low muscle strength and low phase angle predicts greater risk to mortality than severity scales (APACHE, SOFA, and CURB-65) in adults hospitalized for SARS-CoV-2 pneumonia. Front Nutr. 2022;9:965356.
Reyes-Torres CA, Flores-López A, Osuna-Padilla IA, Hernández-Cárdenas CM, Serralde-Zúñiga AE. Phase angle and overhydration are associated with post-extubating dysphagia in patients with COVID-19 discharged from the ICU. Nut in Clin Prac. 2022;37:110–6.
Osuna-Padilla IA, Rodríguez-Moguel NC, Rodríguez-Llamazares S, Aguilar-Vargas A, Casas-Aparicio GA, Ríos-Ayala MA, et al. Low phase angle is associated with 60-day mortality in critically ill patients with COVID-19. J Parenter Enteral Nutr. 2022;46:828–35.
Del Giorno R, Quarenghi M, Stefanelli K, Capelli S, Giagulli A, Quarleri L, et al. Nutritional risk screening and body composition in COVID-19 patients hospitalized in an internal medicine ward. IJGM. 2020;13:1643–51.
Da Porto A, Tascini C, Peghin M, Sozio E, Colussi G, Casarsa V, et al. Prognostic role of malnutrition diagnosed by bioelectrical impedance vector analysis in older adults hospitalized with COVID-19 pneumonia: a prospective study. Nutrients. 2021;13:4085.
Alves EAS, Salazar TCDN, Silvino VO, Cardoso GA, Dos Santos MAP. Association between phase angle and adverse clinical outcomes in hospitalized patients with COVID‐19: a systematic review. Nut Clin Prac. 2022;37:1105–16.
Schwenk A, Beisenherz A, Römer K, Kremer G, Salzberger B, Elia M. Phase angle from bioelectrical impedance analysis remains an independent predictive marker in HIV-infected patients in the era of highly active antiretroviral treatment. Am J Clin Nutr. 2000;72:496–501.
Shah S, Whalen C, Kotler DP, Mayanja H, Namale A, Melikian G, et al. Severity of human immunodeficiency virus infection is associated with decreased phase angle, fat mass and body cell mass in adults with pulmonary tuberculosis infection in Uganda. J Nutr. 2001;131:2843–7.
de Blasio F, Scalfi L, Di Gregorio A, Alicante P, Bianco A, Tantucci C, et al. Raw bioelectrical impedance analysis variables are independent predictors of early all-cause mortality in patients with COPD. Chest. 2019;155:1148–57.
Lee Y, Kwon O, Shin CS, Lee SM. Use of bioelectrical impedance analysis for the assessment of nutritional status in critically ill patients. Clin Nutr Res. 2015;4:32–40.
Suzuki Y, Kushimoto Y, Ishizawa H, Kawai H, Ito A, Matsuda Y, et al. The phase angle as a predictor of postoperative complications in patients undergoing lung cancer surgery. Surg Today. 2022.
Pagano AP, Sicchieri JMF, Schiavoni IL, Barbeiro D, Manca CS, da Silva BR, et al. Phase angle as a severity indicator for liver diseases. Nutrition. 2020;70:110607.
Valtueña J, Ruiz-Sánchez D, Volo V, Manchado-López P, Garayar-Cantero M. Acral edema during the COVID-19 pandemic. Int J Dermatol. 2020;59:1155–7.
Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving sepsis campaign: Guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020;46:854–87.
Scicchitano P, Massari F. The role of bioelectrical phase angle in patients with heart failure. Rev Endocr Metab Disord. 2022;1–13.
Han B-G, Lee JY, Kim J-S, Yang J-W. Clinical significance of phase angle in non-dialysis CKD stage 5 and peritoneal dialysis patients. Nutrients. 2018;10:1331.
González-Salazar LE, Guevara-Cruz M, Hernández-Gómez KG, Serralde Zúñiga AE. Nutritional management of the critically ill inpatient with COVID-19. A narrative review. Nutr Hosp. 2020;34:622–30.
Cornejo-Pareja IM, Gómez-Pérez AM, Fernández-García JC, Barahona San Millan R, Aguilera Luque A, de Hollanda A, et al. Coronavirus disease 2019 (COVID-19) and obesity. Impact of obesity and its main comorbidities in the evolution of the disease. Eur Eat Disord Rev. 2020;28:799–815.
Cava E, Carbone S. Coronavirus disease 2019 pandemic and alterations of body composition. Curr Opin Clin Nutr Metab Care. 2021;24:229–35.
Barbosa-Silva MCG, Barros AJD, Wang J, Heymsfield SB, Pierson RN. Bioelectrical impedance analysis: population reference values for phase angle by age and sex. Am J Clin Nutr. 2005;82:49–52.
Gonzalez MC, Heymsfield SB. Bioelectrical impedance analysis for diagnosing sarcopenia and cachexia: What are we really estimating? J Cachexia Sarcopenia Muscle. 2017;8:187–9.
Funding
Funding for open access publishing: Universidad Málaga/CBUA. I.C.-P. was the recipient of a postdoctoral grant (Río Hortega CM 17/00169) and is now the recipient of a postdoctoral grant (Juan Rodes JR 19/00054) from the Instituto de Salud Carlos III and co-funded by Fondo Europeo de Desarrollo Regional-FEDER. Funding for open access charge: Universidad de Málaga / CBUA.
Author information
Authors and Affiliations
Contributions
The authors I.C.-P., J.M.G.-A., D.B.-G., F.J.T., I.M.V.-A., R.F.-J. and C.G.-G. contributed to the design, search, sorting and data 483 extraction and analysis procedure. I.C.-P., I.M.V.-A., R.F.-J. and C.G.-G. I. drafted the manuscript. I.C.-P., J.M.G.-A., D.B.-G., and F.J.T., critically revised 211 the manuscript for important intellectual content. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cornejo-Pareja, I., Vegas-Aguilar, I.M., Fernández-Jiménez, R. et al. Phase angle and COVID-19: A systematic review with meta-analysis. Rev Endocr Metab Disord 24, 525–542 (2023). https://doi.org/10.1007/s11154-023-09793-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-023-09793-6